Abstract
Human mitochondrial NAD(P)+-dependent malate dehydrogenase (decarboxylating) (malic enzyme) can be specifically and allosterically activated by fumarate. X-ray crystal structures have revealed conformational changes in the enzyme in the absence and in the presence of fumarate. Previous studies have indicated that fumarate is bound to the allosteric pocket via Arg67 and Arg91. Mutation of these residues almost abolishes the activating effect of fumarate. However, these amino acid residues are conserved in some enzymes that are not activated by fumarate, suggesting that there may be additional factors controlling the activation mechanism. In the present study, we tried to delineate the detailed molecular mechanism of activation of the enzyme by fumarate. Site-directed mutagenesis was used to replace Asp102, which is one of the charged amino acids in the fumarate binding pocket and is not conserved in other decarboxylating malate dehydrogenases. In order to explore the charge effect of this residue, Asp102 was replaced by alanine, glutamate or lysine. Our experimental data clearly indicate the importance of Asp102 for activation by fumarate. Mutation of Asp102 to Ala or Lys significantly attenuated the activating effect of fumarate on the enzyme. Kinetic parameters indicate that the effect of fumarate was mainly to decrease the Km values for malate, Mg2+ and NAD+, but it did not notably elevate kcat. The apparent substrate Km values were reduced by increasing concentrations of fumarate. Furthermore, the greatest effect of fumarate activation was apparent at low malate, Mg2+ or NAD+ concentrations. The Kact values were reduced with increasing concentrations of malate, Mg2+ and NAD+. The Asp102 mutants, however, are much less sensitive to regulation by fumarate. Mutation of Asp102 leads to the desensitization of the co-operative effect between fumarate and substrates of the enzyme.
Keywords: allosteric activation, fumarate, malate dehydrogenase, malic enzyme, mutagenesis, enzyme regulation
Abbreviations: MDH, malate dehydrogenase (malic enzyme); c-NADP-MDH, cytosolic NADP+-dependent MDH; m-NADP-MDH, mitochondrial NADP+-dependent MDH; m-NAD-MDH, mitochondrial NAD(P)+-dependent MDH; WT, wild type
INTRODUCTION
MDH [malate dehydrogenase (decarboxylating); malic enzyme; EC 1.1.1.39] is a homotetrameric enzyme with a double dimer quaternary structure that catalyses the bivalent metal ion (Mn2+ or Mg2+)-dependent reversible oxidative decarboxylation of L-malate to yield CO2 and pyruvate, with concomitant reduction of NAD(P)+ to NAD(P)H [1–5]. The enzymes are widely distributed in Nature, with conserved sequences and similar overall structural topology among different species ([6–9]; see [10] for a review). In mammals, based on their nucleotide specificity, the enzymes are grouped into three isoforms: cytosolic NADP+-dependent (c-NADP-MDH), mitochondrial NADP+-dependent (m-NADP-MDH), and mitochondrial NAD(P)+-dependent (m-NAD-MDH) MDHs [11]. It has been shown that m-NAD-MDH can use either NAD+ or NADP+ as a cofactor, but prefers NAD+ under physiological conditions [3].
In tumour cells, glutamine and glutamate, rather than glucose, are the major energy sources [13,14]. Human m-NAD-MDH, via the NADH and pyruvate products, is considered to have an important role in the metabolism of glutamate in rapidly proliferating tissues, particularly in tumour cells [3,12,13,15–18]. Thus the possible involvement of m-NAD-MDH in neoplasia has attracted much attention. m-NAD-MDH is distinct from the other two mammalian isoforms in that it shows co-operative behaviour with respect to the substrate malate, as well as being an allosteric enzyme, which is activated by fumarate and inhibited by ATP [1,3,16,19]. The allosteric properties of this enzyme isoform are integrated into a rationale for its specific role in the pathways of malate and glutamine oxidation in tumour cell mitochondria [17,19].
Various crystal structures of the enzyme in complexes with substrate, metal ion, cofactor, regulator and inhibitor have been successfully resolved since 1999 [6–9,20–22]. There are a total of 14 crystal structures available in the Protein Data Bank. From the structural information, the enzymes are suggested to belong to a new class of oxidative decarboxylases [6,10]. The availability of these structures opens up a new avenue of research on the structure–function relationship of this important enzyme.
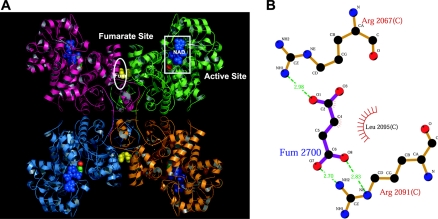
The catalytic activity of m-NAD-MDHs from both human and Ascaris suum is activated by fumarate [19,23–25], and the binding site for fumarate in human m-NAD-MDH has been identified [20]. In the A. suum m-NAD-MDH, the same position is occupied by a tartronate molecule, which could represent a comparable allosteric binding site [21]. Figure 1(A) shows the fumarate binding site at the dimer interface, which is approx. 30 Å (3 nm) from the active site, verifying that fumarate operates through an allosteric mechanism [20]. Structural and kinetic studies have revealed that Arg67 and Arg91 are essential amino acid residues for activation of the enzyme by fumarate. One carboxylate group of fumarate has bidentate ion-pair interactions with the side chain of Arg91, whereas the other is in a monodentate ion pair with Arg67 (Figure 1B). Mutation of Arg67 and Arg91 of human m-NAD-MDH leads to desensitization of the enzyme towards fumarate, confirming the importance of these residues in fumarate binding [20]. Both residues are conserved in A. suum m-NAD-MDH, as well as among many other decarboxylating MDHs that are insensitive to fumarate. Therefore additional amino acid residues may be involved in the mechanism of activation by fumarate in the m-NAD-MDHs from human and A. suum [20,25].
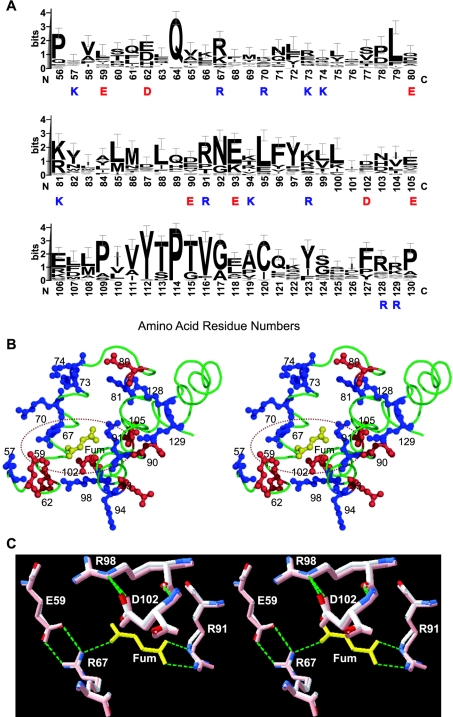
Figure 1. Crystal structure and fumarate binding pocket of human m-NAD-MDH.
(A) Crystal structure of the enzyme in complex with NAD+, pyruvate, Mn2+ and fumarate (PDB code 1pj3). The active- and fumarate-site regions are highlighted with white boxes. Blue denotes NAD+, green denotes pyruvate, and red denotes Mn2+ in the active site; yellow denotes fumarate in the dimer interface. This figure was generated with PyMOL (DeLano Scientific LLC, San Carlos, CA, U.S.A.). (B) Fumarate binding ligands of human m-NAD-MDH are shown as a LIGPLOT diagram [29]. The bold bonds belong to the fumarate, the thin bonds belong to the hydrogen-bonded residues from the enzyme, and the dashed lines represent the hydrogen bonds. Spoked arcs represent hydrophobic contacts.
Here we report a detailed kinetic analysis of the activation of human m-NAD-MDH by fumarate. We further explore the functional role of the anionic residue Asp102 in fumarate activation. We suggest that Asp102 is an essential residue in the mechanism of activation by fumarate, due to its contribution to the charge balance with other charged residues in the allosteric site of human m-NAD-MDH.
MATERIALS AND METHODS
Expression and purification of recombinant human m-NAD-MDH
The expression and purification steps were modified from earlier protocols [3,26]. m-NAD-MDH was overexpressed in Escherichia coli BL21 cells in the expression vector pRH281 under the control of the inducible trp promoter system [3], and purified by anion-exchange chromatography on DEAE-Sepharose (Amersham Biosciences), followed by ATP–agarose affinity chromatography (Sigma). The purified enzymes were exchanged and concentrated using a Centricon 30000 instrument (Amicon) with 30 mM Tris/HCl (pH 7.4). The enzyme purity was examined by SDS/PAGE, and protein concentrations were determined by the Bradford method [27].
Site-directed mutagenesis
Site-directed mutagenesis was carried out using the QuikChange™ kit (Stratagene). This mutagenesis method was performed using Pfu DNA polymerase, which replicated both plasmid strands with high fidelity in a 16–20-cycle PCR reaction. Primers including the mutation site are 25–45 mers, which is required for specific binding of template DNA. The synthetic oligonucleotides used as mutagenic primers were 5′ GAATACTGCAAGCTGACATTGAGAGTTTAATGCC 3′ for D102A, 5′ GAATACTGCAAGAAGACATTGAGAGTTTAATGCC 3′ for D102E, and 5′ GTTTTATAGAATACTGCAAAAAGACATTGAGAGTTTAATGCC 3′ for D102K (mutation positions are underlined). After completing the mutagenic reactions, plasmid constructs were sequenced to confirm the presence of the desired mutations.
Enzyme kinetics
For the m-NAD-MDH activity assay, the reaction mixture contained 50 mM Tris/HCl (pH 7.4), 15 mM malate (pH 7.4), 1.0 mM NAD+ and 10 mM MgCl2 in a total volume of 1 ml. After the enzyme was added to the reaction mixture, the absorbance at 340 nm at 30 °C was traced continuously in a Beckman DU 7500 spectrophotometer. In this process, one enzyme unit was defined as the amount of enzyme that can catalyse the production of 1 μmol of NADH per min. An absorption coefficient of 6220 M−1 for NADH was used in calculations. Apparent Michaelis constants for the substrate and cofactors were determined by varying the concentration of one substrate (or cofactor) around its Km value while maintaining other components constant at the saturation levels. Kact values for fumarate activation were obtained by fitting the saturation curves to the following equation:
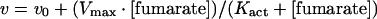 |
(1) |
All calculations were carried out with the Sigma Plot 8.0 program (Jandel, San Rafael, CA).
RESULTS
Kinetic properties of recombinant human m-NAD-MDH
The kinetic parameters for the WT (wild type) and mutant enzymes determined in the absence and presence of fumarate are shown in Table 1. There were no significant differences between the WT and mutant enzymes with regard to Km and kcat values; however, the extent of the decrease in Km and of the increase in kcat induced by fumarate was affected in the order: WT (D102)>D102E>D102A>D102K. The Km values of malate, Mg2+ and NAD+ for the WT enzyme were decreased 3–4-fold by fumarate, but for the D102K enzyme the Km values were reduced by only 1.5–2-fold.
Table 1. Kinetic parameters for the WT and D102 mutant human m-NAD-MDHs.
Fumarate was added at 3 mM where indicated.
| Enzyme | Fumarate added | Km,Malate (mM) | Km,Mg (mM) | Km,NAD (mM) | kcat (s−1) |
|---|---|---|---|---|---|
| WT (−) | No | 12.95±2.46 | 3.53±0.56 | 1.03±0.04 | 130.50±4.21 |
| WT (+) | Yes | 3.53±0.16 | 0.90±3.53 | 0.35±0.02 | 206.40±5.83 |
| D102E (−) | No | 13.01±1.28 | 2.55±0.39 | 0.80±0.06 | 44.14±1.23 |
| D102E (+) | Yes | 4.53±0.21 | 0.91±0.10 | 0.33±0.03 | 60.61±2.11 |
| D102A (−) | No | 9.12±0.51 | 1.21±0.10 | 0.40±0.03 | 114.72±5.95 |
| D102A (+) | Yes | 3.81±0.34 | 0.47±0.05 | 0.23±0.04 | 127.14±2.63 |
| D102K (−) | No | 7.86±0.25 | 1.04±0.09 | 0.44±0.05 | 99.06±0.59 |
| D102K (+) | Yes | 5.47±0.30 | 0.53±0.03 | 0.31±0.04 | 111.09±1.17 |
Correlation of fumarate activation with substrate concentration
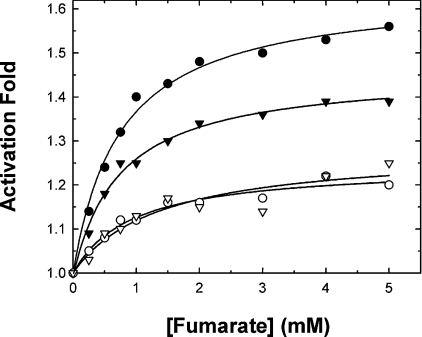
The activating effects of fumarate on the WT and Asp102 mutant enzymes were examined. When the enzymes were assayed under conditions of substrate concentrations around the Km, the maximal activation by fumarate of the WT enzyme was approx. 1.6-fold, and that for the D102E enzyme was 1.4-fold. The other mutants, D102A and D102K, could be activated only slightly by fumarate (Figure 2). These preliminary results indicated that the anionic side chain of Asp102 seems to have a significant effect on activation of the enzyme by fumarate.
Figure 2. Activation of human m-NAD-MDH by fumarate.
The assay mixture contained 15 mM malate, 10 mM MgCl2 and 1 mM NAD+. ●, WT enzyme; ▼, D102E; ○, D102A; ▽, D102K.
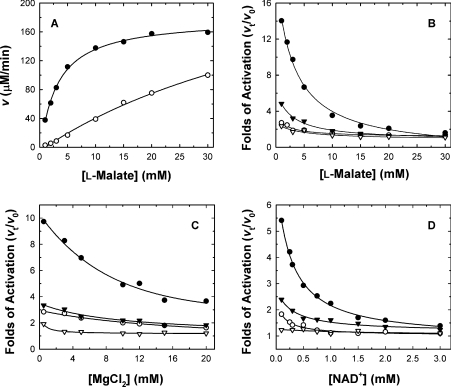
To investigate further the relationship between activation by fumarate and substrate concentration, a wide range of substrate concentrations were used in the assay mixture. The activating effect of fumarate was more pronounced at lower substrate concentrations. Figure 3(A) shows the initial rate of the WT enzyme with or without fumarate at various malate concentrations. The fold activation was obtained by measuring the rate in the presence of fumarate (vt) divided by each of the rates measured in the absence of fumarate (v0). The activation of WT enzyme activity ranged from 14- to1.6-fold with increasing concentrations of malate (Figure 3B). Not only malate, but also Mg2+ and NAD+, had significant effects on activation by fumarate. The activation gradually disappeared with increasing malate, Mg2+, or NAD+ concentrations (Figures 3B, 3C and 3D respectively). The WT enzyme could be activated by 5–15-fold by low concentrations of malate, Mg2+ and NAD+, but the activation diminished when saturating concentrations were used.
Figure 3. Substrate and cofactor concentration-dependence of the activation of human m-NAD-MDH by fumarate.
The fumarate concentration was fixed at 3 mM to achieve maximal activation of the enzyme under the indicated conditions. (A) Initial rate of the WT enzyme with (●; vt) or without (○; v0) 3 mM fumarate at various malate concentrations. The concentrations of MgCl2 and NAD+ were fixed at 10 mM and 1.0 mM respectively. In (B)–(D), each point was obtained from vt divided by v0 at various concentrations of malate, MgCl2 and NAD+ respectively. The non-varied substrate concentrations in (B)–(D) were 15, 10 and 1.0 mM for L-malate, MgCl2 and NAD+ respectively. ●, WT enzyme; ▼, D102E; ○, D102A; ▽, D102K.
Altering the charge properties of the side chain on residue 102 had a significant effect on activation of the enzyme by fumarate. The fumarate activation of the D102K mutant was much less sensitive to malate, Mg2+ or NAD+ concentration, and much lower per se. Similar phenomena were observed for D102A, but the activation of this mutant enzyme was slightly greater than for the D102K enzyme. The D102E mutant, in which the negative charge is preserved but the side chain is extended by one carbon, showed significantly decreased sensitivity to activation by fumarate compared with the WT enzyme. The activation of the D102E enzyme was slightly dependent on malate, Mg2+ or NAD+ concentration, but to a significantly lesser extent than that of the WT enzyme. The above results indicate that the allosteric regulation of m-NAD-MDH enzyme activity by fumarate is highly related to the active-site ligands, i.e. malate, Mg2+ and NAD+.
Activation constants for WT and D102 mutant human m-NAD-MDHs
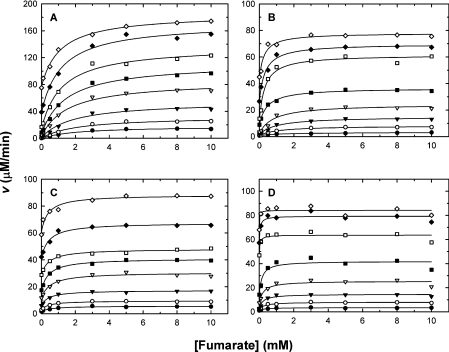
In order to delineate the mechanism of activation of m-NAD-MDH by fumarate, enzyme activities were assayed with a broad range of fumarate concentrations at different fixed concentrations of substrate (L-malate, Mg2+ or NAD+). Figure 4 shows the initial rates for the WT and D102 mutant enzymes with varying fumarate concentrations at each of the fixed malate concentrations. Similar concentration-dependent patterns were also observed for Mg2+ and NAD+ (results not shown). Each of the saturation curves plotted by the measured initial rates was fitted to eqn (1), and the activation constants (Kact) were obtained from these calculations.
Figure 4. Activation by fumarate of human m-NAD-MDH at various malate concentrations.
The initial velocities were measured at 50 mM Tris/HCl (pH 7.4), 10 mM MgCl2 and 1.0 mM NAD+. The malate concentrations used were, from top to bottom, 0.25, 0.5, 1, 2, 3, 5, 10 and 15 mM. (A) WT; (B) D102E; (C) D102A; (D) D102K.
The Kact,Fumarate values were greatly dependent on the malate, Mg2+ or NAD+ concentration. Kact,Fumarate decreased as the concentration of malate, Mg2+ or NAD+ increased. The Kact,Fumarate value with respect to malate for the WT enzyme was 2.1 mM at the lowest malate concentration (25 μM) and fell gradually to a plateau value of 1.1 mM, indicating that the enzyme was activated by fumarate to the greatest extent at low malate concentrations, but decreased its requirement for fumarate upon elevating the malate concentration.
The dependence of Kact,Fumarate on malate concentration for the D102E enzyme was similar to that of the WT enzyme, but the value was smaller than for the WT enzyme. The Kact,Fumarate value for D102E was 1.4 mM at 25 μM malate, and fell steadily to a plateau value of 0.27 mM as the malate concentration increased, indicating that the catalytic activity of the D102E enzyme was significantly influenced by fumarate at low malate concentrations and that, similar to the WT enzyme, this mutant was activated less by fumarate as the malate concentration increased. However, the D102E enzyme had a lower requirement for fumarate than did the WT enzyme, since a lower Kact,Fumarate was observed for this mutant, suggesting that the allosteric regulation of the D102E enzyme by fumarate is somewhat impaired.
The enzyme activities of D102A and D102K were not stimulated by fumarate (Figure 2), in agreement with the small and constant Kact,Fumarate values of these mutants compared with those of the WT and D102E enzymes. The Kact,Fumarate values of these two mutant enzymes in the presence of a broad range of malate concentrations were 0.52–0.39 and 0.23–0.01 mM respectively. The malate-concentration-independence of Kact,Fumarate for the D102A and D102K enzymes indicated that mutation of this residue to one with a neutral or positively charged side chain significantly diminished allosteric activation by fumarate.
The effects of the concentrations of Mg2+ and NAD+ on Kact,Fumarate for the WT and D102E enzyme were similar to those of malate. For the WT enzyme, the Kact,Fumarate value with respect to Mg2+ fell from 5.1 mM at the lowest Mg2+ concentration (0.5 mM) to a plateau value of 1.2 mM; that for NAD+ fell from 2.2 mM to 0.6 mM. For the D102E enzyme, the Kact,Fumarate value with respect to Mg2+ fell from 2.6 mM to a plateau value of 1.2 mM; that for NAD+ fell from 1.5 mM to 0.6 mM. For the other two mutant enzymes, D102A and D102K, Kact,Fumarate was almost independent of both Mg2+ and NAD+ concentrations, indicating the significance of the regulatory role of Asp102 for activation by fumarate. Among the WT and mutant enzymes, the Kact,Fumarate value of D102K showed a minimum dependence on all three ligands, implying that the balance of electrostatic interactions is essential for allosteric regulation by fumarate.
Apparent Km values on activation by fumarate
Fumarate decreased the apparent Km values for malate, Mg2+ and NAD+ of the recombinant WT and D102 mutant enzymes (Table 1). We examined the dependence of Km on fumarate concentration in the range 0–10 mM for the WT and D102 mutant enzymes. The apparent Km,Malate for the WT enzyme was 12.95 mM without fumarate, and it fell gradually to a plateau value of 4.41 mM at a saturating fumarate concentration. The apparent Km,Mg and Km,NAD values were 3.53 and 1.03 mM without fumarate, falling to plateau values of 0.69 and 0.22 mM respectively. The apparent value of Km,Malate was reduced by 2.9-fold, that of Km,Mg was reduced by 5.1-fold, and that of Km,NAD was reduced by 4.7-fold, indicating that activation of the enzyme by fumarate was due to the enhancement of the affinity of the enzyme for its ligands (malate, Mg2+ and NAD+) at the active site. The mutant enzymes showed a slightly smaller decrease in apparent Km values compared with the WT enzyme. For the D102E enzyme, the values of apparent Km,Malate and Km,Mg were reduced by 2.3-fold (13.01 to 5.60 mM) and 4.3-fold (2.55 to 0.59 mM) respectively, similar to the WT enzyme. The Km,NAD value of the D102E enzyme was reduced by only 2-fold (0.80 to 0.45 mM), less than the decrease observed for the WT enzyme (4.7-fold). For the D102A enzyme, the apparent Km values were reduced by only approx. 2-fold. The apparent Km,Malate, Km,Mg and Km,NAD values were 9.12, 1.21 and 0.40 mM respectively without fumarate, and decreased steadily to plateau values of 4.53, 0.50 and 0.21 mM respectively. For the D102K enzyme, the apparent Km values for malate and NAD+ were not changed significantly in the presence of fumarate. The apparent Km,Malate values were 7.86 and 6.87 mM, and Km,NAD values were 0.44 and 0.33 mM, without and with 10 mM fumarate respectively. The Km,Mg of the D102K enzyme was reduced by 1.9-fold (1.04 to 0.54 mM), similar to that of the D102A enzyme.
DISCUSSION
Binding site for the allosteric activator fumarate
Earlier studies have suggested that fumarate is an allosteric activator of human m-NAD-MDH, by decreasing the Km of malate [19,28]. The catalytic activity of A. suum m-NAD-MDH is also activated by fumarate [23,24]. The crystal structure of human m-NAD-MDH complexed with fumarate has been resolved, and the mode of binding of fumarate in human m-NAD-MDH has been identified [20]. The allosteric regulation of human m-NAD-MDH by fumarate is consistent with its role in glutamine metabolism for energy production, since fumarate is the product of the preceding step of this pathway [6]. The c-NADP-MDH isoform, which is involved in the generation of NADPH for fatty acid synthesis, is therefore not subject to regulation by fumarate.
Two arginine residues in the fumarate binding pocket, Arg67 and Arg91, have been identified as being essential for the binding of fumarate to the enzyme (Figure 1B). However, both Arg67 and Arg91 are conserved in many other decarboxylating MDHs (Figure 5A) that cannot be activated by fumarate. Glu59, which is ion-paired with Arg67 in the structure but is not conserved among the MDHs, has a profound influence on activation by fumarate: mutation of this residue made the enzyme completely insensitive to fumarate [20]. These results prompted us to look for additional factors governing activation by fumarate. According to the crystal structure, the fumarate binding pocket is highly hydrophilic [20], and there are many charged residues in this binding site (Figure 5B). We chose Asp102 because it is present only in the human and A. suum m-NAD-MDHs, but not in other decarboxylating MDHs (Figure 5A). Figure 5(B) shows that Asp102 is close to the Arg67–fumarate–Arg91 ion-pair network. Our kinetic data clearly demonstrate that Asp102 is an important amino acid for activation of the enzyme by fumarate. We verify that the negative charge of the side chain in Asp102 is involved in this activation. Altering the charge properties of this residue has a substantial impact on activation by fumarate (Figure 2) and sensitivity of the enzyme to substrates (Figure 3). Our experimental results suggest that the negative charge of Asp102 is crucial for activation by fumarate. Retaining the negative charge in the D102E enzyme conserved approx. 80–90% of the activation capacity of the WT enzyme (Figure 2), but activation was less sensitive to substrates. Asp102 may be ion-paired with another positively charged amino acid residue. Changing the length of the side chain may impede this ionic interaction, thus interfering with the electrostatic balance in this allosteric site that is crucial for fumarate activation.
Figure 5. Fumarate binding region of human m-NAD-MDH.
(A) Sequence alignments around the fumarate binding site of the 35 decarboxylating MDHs with amino acid sequences available. Amino acid sequences of the enzymes were searched by Blast [30] and alignments were generated by Clustal W [31]. The results are expressed by sequence logos with error bars [32]. The amino acid residues highlighted are charged residues in the fumarate binding region; blue for positively and red for negatively charged amino acids. (B) Local structural representation of the charged amino acid residues at the fumarate (Fum) binding region. Blue indicates basic residues (Lys and Arg) and red indicates acidic residues (Asp and Glu). This figure was generated with PyMOL (DeLano Scientific LLC). (C) Superimposition of the closed form I (PDB code 1do8; in pink) and closed form II (PDB code 1pj4) structures, indicating the location and conformation of Asp102 in the fumarate binding pocket. The green dashed lines representing hydrogen bonds between amino acid residues and fumarate (in yellow) were generated by Swiss-Pdb Viewer [33].
The Kact values of the WT enzyme decrease upon increasing the concentration of substrate (malate, Mg2+ or NAD+), indicating that the binding of fumarate and substrates is synergistic in order to optimize the catalytic machinery of the enzyme. Mutation of Asp102, however, desensitizes this co-operative effect (Figure 3). This result is corroborated by the allosteric binding of fumarate, since competition for the same binding site by fumarate and substrates would bring about an increase in the apparent Kact value. Thus fumarate may bind to the active centre as well, albeit with low affinity, and a high fumarate concentration (above 20 mM fumarate) inhibits enzyme activation (results not shown).
Significance of Asp102 in the fumarate binding site
Structural data have clearly revealed that human m-NAD-MDH without any active-site ligands (open form I) is not compatible with fumarate binding, since the side chain of Phe68 assumes a different conformation to block this binding site, and the guanidinium group of Arg91 is rotated away from the binding site [20]. Substrate binding (malate, Mg2+ and NAD+) induces a transition of the enzyme from open form I to closed form I, which is catalytically active to execute the chemical reaction. Open form II, in which fumarate is bound, may have a higher affinity for the substrates, since the Km values of the WT enzyme decrease as the fumarate concentration increases, demonstrating that binding of fumarate at the allosteric site enhances the binding of substrates at the active site, and thus the maximal activity of the enzyme can be achieved. On the other hand, closed form I (substrates bound) could be converted into closed form II after binding of fumarate to the enzyme [10]. However, the requirement for fumarate for this transition becomes negligible at high substrate concentrations. In agreement with this, activation was up to 10-fold at the lowest substrate concentrations, but was only 1.5-fold when saturating substrate was present (Figure 3), and decreases in the Kact values of the enzyme were observed as the concentration of substrate was elevated.
Figure 5(C) shows the superimposition of the fumarate binding pocket of closed form I (PDB code 1do8), which is bound with active-site ligands only, and closed form II (PDB code 1pj4), which is bound with active-site ligands and the allosteric ligand fumarate. In closed form I, even without a fumarate molecule, the allosteric site is available for fumarate binding; the side chains of Arg67 and Arg91, which are the fumarate binding ligands, display almost the same conformations as observed in closed form II. Asp102 is in close proximity to the Arg67–fumarate–Arg91 ion-pair network, and its side-chain conformation is the same in closed forms I and II. Asp102 does not interact directly with fumarate, and is hydrogen-bonded and may be ion-paired with Arg98. From our kinetic data, we suggest that the role of Asp102 is to maintain the electrostatic balance in the fumarate binding pocket. Mutation of Asp102 to Ala and Lys, however, abolishes the allosteric regulation of the enzyme. Asp102 is not conserved in all the enzymes. In pigeon c-NADP-MDH, which is not allosterically activated by fumarate, the corresponding amino acid residue 102 is Ser, which has a neutral side chain.
Conclusions
In summary, fumarate enhances the binding affinity of m-NAD-MDH for its ligands at the active site, since it decreases the apparent Km values of malate, Mg2+ and NAD+. The susceptibility of substrate Km values to allosteric activation is partially conserved in the D102E enzyme, but almost abolished in the D102A and D102K enzymes, suggesting that mutation of Asp102 causes the enzyme to be non-allosterically regulated by fumarate. The significance of Asp102 in the fumarate binding site is in the maintenance of the electrostatic balance that may be a critical factor governing the mechanism of regulation by fumarate.
Acknowledgments
This work was supported by the National Science Council, ROC (grants NSC 91-2320-B-005-004 and NSC-93-2311-B-005-018 to H.-C.H.).
References
- 1.Frenkel R. Regulation and physiological function of malic enzyme. Curr. Top. Cell. Regul. 1975;9:157–181. doi: 10.1016/b978-0-12-152809-6.50012-3. [DOI] [PubMed] [Google Scholar]
- 2.Hsu R. Y. Pigeon liver malic enzyme. Mol. Cell. Biochem. 1982;43:3–26. doi: 10.1007/BF00229535. [DOI] [PubMed] [Google Scholar]
- 3.Loeber G., Infante A. A., Maurer-Fogy I., Krystek E., Dworkin M. B. Human NAD+-dependent mitochondrial malic enzyme. J. Biol. Chem. 1991;266:3016–3021. [PubMed] [Google Scholar]
- 4.Rao G. S. J., Coleman D. E., Kulkarni G., Goldsmith E. J., Cook P. F., Harris B. G. NAD-malic enzyme from Ascaris suum: sequence and structural studies. Protein Pept. Lett. 2000;7:297–304. [Google Scholar]
- 5.Cleland W. W. Chemical mechanism of malic enzyme as determined by isotope effects and alternate substrates. Protein Pept. Lett. 2000;7:305–312. [Google Scholar]
- 6.Xu Y., Bhargava G., Wu H., Loeber G., Tong L. Crystal structure of human mitochondrial NAD(P)+-dependent malic enzyme: a new class of oxidative decarboxylases. Structure. 1999;7:877–889. [PubMed] [Google Scholar]
- 7.Yang Z., Floyd D. L., Loeber G., Tong L. Structure of a closed form of human malic enzyme and implications for catalytic mechanism. Nat. Struct. Biol. 2000;7:251–257. doi: 10.1038/73378. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z., Zhang H., Hung H. C., Kuo C. C., Tsai L. C., Yuan H. S., Chou W. Y., Chang G. G., Tong L. Structural studies of the pigeon cytosolic NADP+-dependent malic enzyme. Protein Sci. 2002;11:332–341. doi: 10.1110/ps.38002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman D. E., Rao G. S., Goldsmith E. J., Cook P. F., Harris B. G. Crystal structure of the malic enzyme from Ascaris suum complexed with nicotinamide adenine dinucleotide at 2.3 Å resolution. Biochemistry. 2002;41:6928–6938. doi: 10.1021/bi0255120. [DOI] [PubMed] [Google Scholar]
- 10.Chang G. G., Tong L. Structure and function of malic enzymes, a new class of oxidative decarboxylases. Biochemistry. 2003;42:12721–12733. doi: 10.1021/bi035251+. [DOI] [PubMed] [Google Scholar]
- 11.Loeber G., Dworkin M. B., Infante A., Ahorn H. Characterization of cytosolic malic enzyme in human tumor cells. FEBS Lett. 1994;344:181–186. doi: 10.1016/0014-5793(94)00386-6. [DOI] [PubMed] [Google Scholar]
- 12.Sauer L. A., Dauchy R. T., Nagel W. O., Morris H. P. Mitochondrial malic enzymes. Mitochondrial NAD(P)+-dependent malic enzyme activity and malate-dependent pyruvate formation are progression-linked in Morris hepatomas. J. Biol. Chem. 1980;255:3844–3848. [PubMed] [Google Scholar]
- 13.Baggetto L. G. Deviant energetic metabolism of glycolytic cancer cells. Biochimie. 1992;74:959–974. doi: 10.1016/0300-9084(92)90016-8. [DOI] [PubMed] [Google Scholar]
- 14.Sanz N., Diez-Fernandez D., Valverde A. M., Lorenzo M., Benito M., Cascales M. Malic enzyme and glucose 6-phosphate dehydrogenase gene expression increases in rat liver cirrhogenesis. Br. J. Cancer. 1997;75:487–492. doi: 10.1038/bjc.1997.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreadith R. W., Lehninger A. L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J. Biol. Chem. 1984;259:6215–6221. [PubMed] [Google Scholar]
- 16.Moreadith R. W., Lehninger A. L. Purification, kinetic behavior, and regulation of NAD(P)+ malic enzyme of tumor mitochondria. J. Biol. Chem. 1984;259:6222–6227. [PubMed] [Google Scholar]
- 17.Fahien L. A., Teller J. K. Glutamate-malate metabolism in liver mitochondria. J. Biol. Chem. 1992;267:10411–10422. [PubMed] [Google Scholar]
- 18.Teller J. K., Fahien L. A., Davis J. W. Kinetic and regulation of hepatoma mitochondrial NAD(P) malic enzyme. J. Biol. Chem. 1992;267:10423–10432. [PubMed] [Google Scholar]
- 19.Sauer L. A. An NAD- and NADP-dependent malic enzyme with regulatory properties in rat liver and adrenal cortex mitochondrial fractions. Biochem. Biophys. Res. Commun. 1973;50:524–531. doi: 10.1016/0006-291x(73)90871-1. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z., Lanks C. W., Tong L. Molecular mechanism for the regulation of human mitochondrial NAD(P)+-dependent malic enzyme by ATP and fumarate. Structure. 2002;10:951–960. doi: 10.1016/s0969-2126(02)00788-8. [DOI] [PubMed] [Google Scholar]
- 21.Rao G. S. J., Coleman D. E., Karsten W. E., Cook P. F., Harris B. G. Crystallographic studies on Ascaris suum NAD-malic enzyme bound to reduced cofactor and identification of an effector site. J. Biol. Chem. 2003;278:38051–38058. doi: 10.1074/jbc.M305145200. [DOI] [PubMed] [Google Scholar]
- 22.Tao X., Yang Z., Tong L. Crystal structures of substrate complexes of malic enzyme and insights into the catalytic mechanism. Structure. 2003;11:1141–1150. doi: 10.1016/s0969-2126(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 23.Landsperger W. J., Harris B. G. NAD+-malic enzyme. Regulatory properties of the enzyme from Ascaris suum. J. Biol. Chem. 1976;251:3599–3602. [PubMed] [Google Scholar]
- 24.Lai C. J., Harris B. G., Cook P. F. Mechanism of activation of the NAD-malic enzyme from Ascaris suum by fumarate. Arch. Biochem. Biophys. 1992;299:214–219. doi: 10.1016/0003-9861(92)90266-y. [DOI] [PubMed] [Google Scholar]
- 25.Karsten W. E., Pais J. E., Rao G. S., Harris B. G., Cook P. F. Ascaris suum NAD-malic enzyme is activated by L-malate and fumarate binding to separate allosteric site. Biochemistry. 2003;42:9712–9721. doi: 10.1021/bi034101w. [DOI] [PubMed] [Google Scholar]
- 26.Bhargava G., Mui S., Pav S., Wu H., Loeber G., Tong L. Preliminary crystallographic studies of human mitochondrial NAD(P)(+)-dependent malic enzyme. J. Struct. Biol. 1999;127:72–75. doi: 10.1006/jsbi.1999.4126. [DOI] [PubMed] [Google Scholar]
- 27.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein, utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Mandella R. D., Sauer L. A. T. The mitochondrial malic enzymes I. Submitochondrial localization and purification and properties of the NAD(P)+-dependent enzyme from adrenal cortex. J. Biol. Chem. 1975;250:5877–5884. [PubMed] [Google Scholar]
- 29.Wallace A. C., Laskowski R. A., Thornton J. M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Proten Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- 30.Altschul S. F., Boguski M. S., Gish W., Wootton J. C. Issues in searching molecular sequence databases. Nat. Genet. 1994;6:119–129. doi: 10.1038/ng0294-119. [DOI] [PubMed] [Google Scholar]
- 31.Higgins D., Thompson J., Gibson T., Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crooks G. E., Hon G., Chandonia J. M., Brenner S. E. WebLogo: A sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guex N., Peitsch M. C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]