Abstract
Cell entry of enveloped viruses requires a wide-fusion-pore mechanism, involving clustering of fusion-activated proteins and fluidization of the plasma membrane and viral envelope. In the present study, GL (glycyrrhizin) is reported to lower membrane fluidity, thus suppressing infection by HIV, influenza A virus and vesicular stomatitis virus, but not by poliovirus. GL-treated HIV-1 particles showed reduced infectivity. GL also inhibited cell-to-cell fusion induced by HIV-1 and HTLV-I (human T-cell leukaemia virus type I). However, when cells treated with 1 mg/ml GL were placed in GL-free medium, they showed increased susceptibility to HIV-1 infection and HTLV-I fusion due to enhancement of membrane fluidity. The membrane dependence of GL and GL removal experiments suggest that GL does affect the cell entry of viruses. HIVs with more gp120 were less dependent on temperature and less sensitive to GL treatment than those with less gp120, indicating that the existence of more gp120 molecules resulted in a higher probability of forming a cluster of fusion-activated proteins.
Keywords: enveloped virus, fusion pore, glycyrrhizin, HIV-1, infectivity, membrane fluidity
Abbreviations: 5-DSA, 5-doxyl stearic acid; FBS, fetal bovine serum; GL, glycyrrhizin; HSV-1, herpes simplex virus type 1; HTLV-I, human T-cell leukaemia virus type I; IF, immunofluorescence; SARS, severe acute respiratory syndrome; TCID50, 50% tissue culture infectious dose; VSV, vesicular stomatitis virus; VZV, varicella-zoster virus
INTRODUCTION
Following attachment and adsorption of viral particles to specific receptors on host cells, enveloped viruses must enter the host cell by making a fusion pore, fusing their own lipid bilayer envelope coat with that of the plasma cell membrane. Fusion occurs via at least two classes of viral proteins in the envelope [1]. The class I model pertains to influenza haemagglutinin, HIV-1 gp120 and the F-proteins from paramyxoviridae [2], and class II involves E proteins of the flaviviridae and the E1 protein of Semliki Forest virus [3]. An essential feature of class I proteins is the formation of a trimeric helical coiled-coil rod adjacent to the hydrophobic fusion peptide. Class II fusion proteins are predominantly non-helical β-sheet-type structures with an internal hydrophobic fusion loop. The loop inserts into the outer leaflet of the plasma membrane enabling trimer formation. In both cases, clustering of several trimers is thought to be involved in the formation of a wide fusion pore. The formation of ring-like assemblies of fusion proteins and their co-operation in the membrane-insertion process have been observed in studies of rabies [4], baculovirus [5], influenza virus [6] and Semliki Forest virus [3,7]. Cell-to- cell fusion, by co-cultivation of HIV-1- or HTLV-I (human T-cell leukaemia virus type I)-infected cells with target CD4-positive cells, also requires the recruitment of viral envelope proteins and receptors to the interface as a virological synapse [8,9]. For cellular fusion, actin-dependent energy transfer [10–12] may be associated with the assembly of the pre-fusion structures in the membrane, which probably facilitates or results in the lateral movement of molecules via fluidization of both plasma membranes.
The plasma membrane and the viral envelope are liquid or disordered under physiological conditions. The fluidity of the membrane results from anisotropic rotation of phopholipid acyl chains and flip-flop movement of membrane molecules [13,14]. We quantitatively measured the fluidity of live cell plasma membranes and viral particle envelopes by ESR using 5-DSA (5-doxyl stearic acid) [15,16]. A correlation between HIV-1 infectivity and fluidity was observed following heating and treatment with fluidity modulating detergent, xylocaine and phosphatidylcholine dipalmitoyl. A 5% decrease in fluidity suppressed HIV-1 infectivity by 56%, whereas a 5% increase enhanced infectivity 2.4-fold [15]. Fluidities of HIV-1 envelopes were always lower than those of plasma membranes, since cholesterol/protein ratios of viral envelopes were higher than those of plasma membranes [15,17,18]. Sterols such as cholesterol serve essential functions in eukaryotic plasma membranes. It has been proposed that excess cholesterol leads to the liquid-ordered (rigid) phase of the plasma membrane bilayer from its liquid-disordered phase [19]. Small changes in plasma membrane cholesterol levels near the physiological set point might evoke large biological responses [20,21]. Thus it is conceivable that cholesterol or cholesterol-like substances associated with plasma membrane and/or viral envelope may play a negative role in the formation of large clusters of fusion proteins to create the fusion pore.
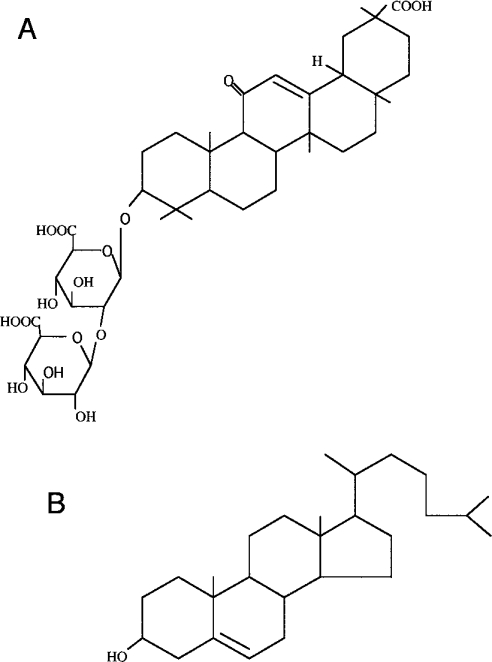
These findings and hypotheses form the basis of the present study. Since GL (glycyrrhizin), an active component of liquorice roots, has a similar molecular structure to that of cholesterol (Figure 1), its effects on the fluidity of the plasma membrane and viral envelope were investigated. Moreover, GL has been reported previously to show broad anti-viral activities against enveloped viruses such as vaccinia [22], HSV-1 (herpes simplex virus type 1) [22], Newcastle disease [22], VSV (vesicular stomatitis virus) [22], VZV (varicella-zoster virus) [23], measles [24], HIV-1 [25–27] and SARS (severe acute respiratory syndrome) [28] viruses. Here, inhibition of viral infection by GL is reported and was shown to be mostly due to suppression of the fluidity of the plasma membrane and viral envelope into which GL was incorporated. Its effects on fluidity were readily reversible when GL was withdrawn from the cultures. This study proposes a new anti-viral strategy to directly suppress the fluidity of lipid bilayer membranes, inhibiting the formation of fusion pores, as one of the host factors important for controlling enveloped viral entry. The availability of GL in clinical settings is also discussed.
Figure 1. Structures of GL (A) and cholesterol (B).
EXPERIMENTAL
Cells and culture
MOLT-4, MOLT-4/HIV-1C-2, MT-2, MT-4 and PM-1 CCR5 cells were cultured in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated FBS (fetal bovine serum) and antibiotics [29,30]. GHOST/CXCR4, GHOST/CCR5, FL and MDCK cells were cultured in Dulbecco's modified Eagle's medium with 10% (v/v) FBS and antibiotics as described previously [31,32].
Preparation of viruses
Plaque-cloned HIV-1 C-2 viruses from HIV-1LAI (X4) strain were obtained from 3-day-old culture supernatants of chronically infected MOLT-4/HIV-1C-2 cells [30]. JR-FL (R5 HIV-1) viruses [33] and a vaccine strain of type 1 poliovirus were propagated by infecting PM-1 CCR5 and FL cells respectively, and cultured for 4 days. After filtring through a 0.45 μm-pore-sized membrane, the supernatants were stored at −80 °C. NL43, JR-FL and VSV-envelope-pseudotyped viruses with the luciferase reporter gene (designated as NL43-luc, JR-FL-luc and VSV-G pseudoviruses respectively) were produced by the calcium phosphate transfection method [31–33]. HEK 293T cells were co-transfected with an envelope-deficient NL43 construct carrying the luciferase gene (pNL43-luc) and a pCXN2 vector expressing the envelope glycoprotein from pNL43. Pseudotyped viruses with JR-FL and VSV envelope glycoproteins were produced by co-transfecting pCXN/FLenv and pHCMV-G plasmids respectively. After the cells had been cultured for 2 days, supernatants were harvested, filtered and stored at −80 °C. Influenza A viruses (Influenza A/Aichi/2/68) were kindly provided by Dr T. Akaike (Department of Microbiology, Kumamoto University, Kumamoto, Japan).
Reagents
GL (20β-carboxy-11-oxo-30-norolean-12-en-3β-yl-2-O-β-D-glucopyranuronosyl-β-D-glucopyranosiduronic acid; Minophagen Pharmaceutical Co.) was dissolved in PBS at a concentration of 20 mg/ml (23.8 mM) and stored at 25 °C. 5-DSA (Sigma-Aldrich) was stored at a concentration of 20 mg/ml in ethanol at 4 °C. Verapamil (Sigma-Aldrich) was stored at a concentration of 1 mM in PBS at 4 °C. No cytotoxicity to MT-2 and MT-4 cells was observed at concentrations of up to 12 mM (10 mg/ml) GL and 10 μM verapamil. The expression levels of CD4 and CXCR4 on MT-2 cells were unaltered by treatment with 1 mg/ml GL or 10 μM verapamil.
ESR spectroscopy
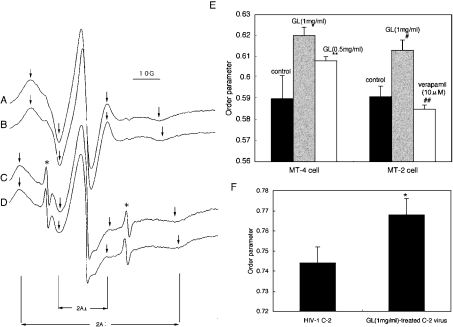
A 1 ml (7×106) volume of cells or 50 ml of culture supernatant containing 3 μg of p24 of HIV-1 was mixed with 1 ml of 60 μg/ml 5-DSA or 25 ml of 90 μg/ml 5-DSA in PBS respectively, to give a final concentration of 30 μg/ml, and then incubated at 37 °C for 20 min [14,15]. After removing the free spin label, pellets of cells or viruses were resuspended in 40 μl of PBS, with or without 1 mg/ml GL, and drawn into a capillary tube. The capillary tube was further placed into a quartz glass tube and spectra were recorded using a JES-RE1X ESR spectrometer (JEOL Ltd, Tokyo, Japan) at 37 °C. Instrument conditions were 2 min scan time, 5.0 mT sweep width, 0.1 mT field modulation width, 10 mW microwave power, and 0.3 s time constant. Figure 2 shows representative spectra, for which the outer hyperfine splitting indicates 2A∥, and the inner one denotes 2A⊥. The horizontal axis reflects varying magnetic fields and the vertical axis represents absorption of microwaves. The order parameter (S) was calculated as follows:
 |
Figure 2. Effects of GL on the fluidity of the plasma membrane and viral envelope.
ESR spectra of plasma membrane from intact MT-2 (A) and 1 mg/ml GL-treated MT-2 (B) and viral envelope from intact HIV-1 C-2 (C) and 1 mg/ml GL-treated HIV-1 C-2 (D) are shown. The outer and inner hyperfine splittings, 2A∥ and 2A⊥, were measured as shown by arrows. Asterisks show spectra of free 5-DSA remaining in each sample. Order parameters (S values) of (A), (B), (C) and (D) were 0.581, 0.615, 0.750 and 0.778 respectively. The scale of the horizontal axis (magnetic field) is shown as 10 G, equivalent to 1 mT. (E) MT-4 cells were treated with 1 and 0.5 mg/ml GL and membrane fluidity was assessed (control, n=6; 1 and 0.5 mg/ml GL, n=3 each). *P<0.01 compared with control and P<0.02 compared with 0.5 mg/ml GL. **P<0.05 compared with control. MT-2 cells were treated with 1 mg/ml GL and 10 μM verapamil (control, n=6; 1 mg/ml GL, n=3; 10 μM verapamil, n=3). #P<0.001 compared with controls and ##P<0.1 compared with controls. Bars show means±S.D. of repeated experiments. (F) HIV-1 C-2 were treated with 1 mg/ml GL and envelope fluidity was assessed. Values are means±S.D. for three independent experiments. * P<0.05 compared with HIV-1 C-2.
GL treatment
GL was mostly added to cultures during viral adsorption when cells were mixed with viruses in the absence or presence of 2-fold serially diluted GL at 37 °C for 1 h. Subsequently, cells were washed once and cultured at 37 °C until infectivity was assessed. In some experiments, cells or viruses were treated with serially diluted GL at 37 °C for 1 h and free GL was removed by centrifugation. GL-pretreated cells or viruses were then used for viral adsorption at 37 °C for 1 h. After washing once, cells were cultured at 37 °C.
Determination of viral infectivity
IF (immunofluorescence) method
A portion (5×105 cells) of MT-2 or MT-4 cells was mixed with 1 ml of HIV-1 C-2 in the absence or presence of 2-fold serially diluted GL and incubated at 37 °C for 1 h. After washing once, MT-2 or MT-4 cells were cultured at 37 °C for 3 days and 24 h respectively. Cells were smeared on to a glass slide and fixed with a 1:1 (v/v) mixture of cold methanol and acetone at −20 °C for 5 min. Infected cells were stained by an indirect IF method as described previously [29]. For assessing infectivity of HIV-1 JR-FL, 3×105 PM-1 CCR5 cells were used as target cells. More than 500 cells per sample were counted to calculate the percentage of HIV-1 JR-FL-positive cells. Percentage infection was calculated as (% positive cells in test×100/% positive cells in control).
Luciferase assay
GHOST/CXCR4 or GHOST/CCR5 cells (2.5×104 cells/well) that had been seeded the previous day on a flat 48-well plate were infected with 100 μl/well NL43-luc or JR-FL-luc respectively, in the absence or presence of serially diluted GL. Viral adsorption took place at 37 °C for 1 h. After washing once, the plate was incubated at 37 °C for 2 days, then infected cells were lysed with 100 μl/well luciferase assay buffer (Promega, Madison, WI, U.S.A.). Luciferase activity was measured as described previously [33]. FL cells at a concentration of 2.5×104 cells/well in a flat 48-well plate were used as target cells for VSV-G pseudovirus.
Haemadsorption test
MDCK cells (3.5×105 cells/well) were placed in a flat 12-well plate and cultured at 37 °C overnight. Cells were treated with 100 μl/well of a mixture of influenza A virus and 2-fold serially diluted GL at 37 °C for 1 h, then washed once and cultured at 37 °C for 24 h. After the plate was washed once with PBS, 0.5 ml/well 3% (v/v) red blood cells were added into each well and the plate was incubated at room temperature for 20 min before washing extensively three times with PBS. Haemadsorbed cells per well were counted under the microscope (×100).
TCID50 (50% tissue culture infectious dose)
FL cells (2.5×104 cells/well), that had been seeded the previous day on a flat 48-well plate, were infected with 100 μl/well 10-fold serially diluted poliovirus in the absence or presence of 2-fold serially diluted GL in hexade. Viral adsorption was carried out at 37 °C for 1 h and the plate was incubated at 37 °C for 5 days after washing once. Wells showing cytopathic effects were counted under a microscope. The TCID50 of each dilution of GL was calculated using the Reed–Muench's method.
Fusion assay
MOLT-4- and HTLV-I-transformed MT-2 cells were used as target and effector cells respectively, for the fusion assay. In the absence or presence of GL, 0.5 ml (3×105 cells) of MOLT-4 cells was mixed with the same amount of MT-2 cells. In some experiments, each cell type was treated separately with GL at 37 °C for 1 h and washed once before mixing. The mixture (1 ml/well) was placed in a flat 24-well plate and cultured at 37 °C for 24 h. Fused ballooning cells per one field under a microscope (×100) were counted.
Western blotting
Ultracentrifugation was used to pellet 200 ng of p24 of NL43-luc and JR-FL-luc pseudoviruses, which were then lysed by adding 30 μl of lysis buffer as described previously [34]. Viral lysates (5 μl each) were resolved on SDS/polyacrylamide gels and blotted on to a PVDF membrane (Atto Corporation, Tokyo, Japan). After blocking non-specific binding, the membrane was incubated with 1:100 diluted HIV-1-positive serum at 37 °C for 1 h. After washing three times, it was incubated with anti-human IgG conjugated with alkaline phosphatase (Sigma). The reaction was then detected chromatically using alkaline phosphatase-linked reagents according to standard techniques.
p24 assay
The amount of HIV-1 core p24 was assessed by using HIV-1 p24 antigen ELISA (Cellular Products, Buffalo, NY, U.S.A.) according to the manufacturer's protocol.
Statistical analysis
Statistical analyses were carried out using Student's unpaired t test, and a P value of <0.05 was considered indicative of statistical significance.
RESULTS
Suppression of fluidity by GL
Fluidization of the lipid-bilayer was examined by incorporating the 5-DSA spin probe into live cell membranes and viral particle envelopes. ESR spectra were recorded at 37 °C (Figures 2A–2D) and order parameters (S values) were calculated from the ESR spectra. The order parameter represents the anisotropic motion of the long molecular axis of the spin-labelled probe, thus inversely indicating the flexibility of the incorporated 5-DSA probe. Treatment of MT-4 and MT-2 cells with 1 mg/ml GL increased the order parameter by 5.1% and 3.7% respectively (Figure 2E). The fluidity of MT-4 membrane was dose dependently suppressed by GL. The order parameter (0.768) of 1 mg/ml GL-treated HIV-1 C-2 was also higher than that (0.744) of untreated HIV-1 C-2 (Figure 2F). Thus GL significantly suppressed the fluidity of both plasma membranes and viral envelopes when cells and viruses were incubated at 37 °C for 1 h with 1 mg/ml GL before being subjected to ESR.
Re-evaluation of the anti-viral activity of GL
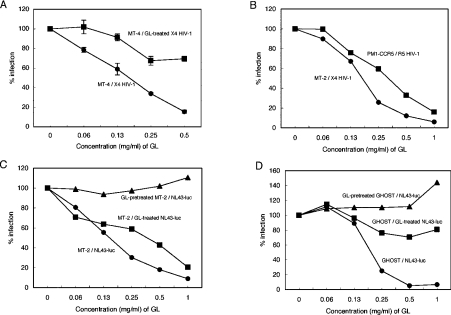
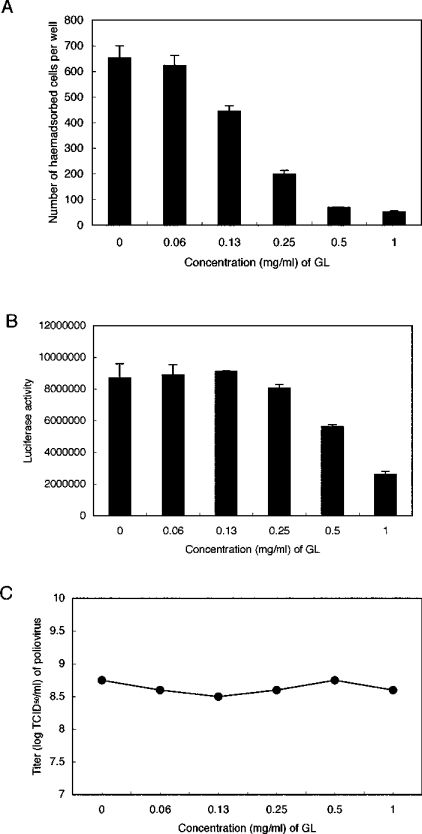
The effects of GL on HIV-1 infection were examined when target cells were incubated with viruses in the presence of 2-fold serially diluted GL during viral adsorption at 37 °C for 1 h. Figure 3(A) shows that the EC50 of GL was 0.2 mg/ml when MT-4 cells were infected with plaque-cloned C-2 virus from HIV-1 LAI (X4) strain and HIV-1-positive cells were stained and counted using the indirect IF method. Similarly to HSV-1 [22] and VZV [23], GL-treated C-2 viruses showed significantly decreased (30%) infectivity of MT-4 cells, indicating that GL affected not only cells, but also viral particles. Similar inhibitory effects of GL (EC50=0.2 mg/ml) were observed when MT-2 cells were used for C-2 virus infection instead of MT-4 cells, and an EC50 of 0.38 mg/ml GL was obtained by infection of PM-1 CCR5 cells with R5 strain JR-FL viruses (Figure 3B). Thus GL had equal effects on both X4 and R5 HIV-1. MT-2 (Figure 3C) or GHOST/CXCR4 (Figure 3D) cell infections were also investigated using replication-defective NL43-luciferase virus pseudotyped with NL43 (X4) envelope (NL43-luc virus). In this case, since the virus cannot replicate in the cell, luciferase activity correlated with the ability of the virus to penetrate target cells. Single-round infection experiments showed that the EC50 was 0.2 mg/ml when MT-2 or GHOST/CXCR4 cells were incubated concomitantly with NL43-luc virus and serially diluted GL during the adsorption period. The infectivity of GL-treated NL43-luc viruses for both MT-2 and GHOST/CXCR4 cells also decreased in a dose-dependent fashion. However, the effects seen with GHOST/CXCR4 cells were weaker than with MT-2 cells, implying that GL incorporation into pseudotyped virus particles could impede entrance into target cells. Unexpectedly, pretreating cells with GL had almost no effect on NL43-luc virus infection efficiency, but, in contrast with MT-2 cells, exhibited an enhancement of infectivity of 10% and 40% only in the cases of 1 mg/ml GL-pretreated MT-2 and GHOST/CXCR4 cells respectively. GL inhibited infection by influenza A virus and pseudotyped virus with VSV-G envelope at EC50 values of approx. 0.2 mg/ml and 0.7 mg/ml respectively (Figures 4A and 4B), but showed no effect on poliovirus infection (Figure 4C). No toxicity to MT-2 and MT-4 cells was observed up to 10 mg/ml (12 mM) GL and no changes in CD4 and CXCR4 expression on MT-2 cells was detected with 1 mg/ml GL (results not shown). Since viral production (p24 amount) by chronically infected MOLT-4/HIV-1C-2 cells was unaffected by the treatment with 1 mg/ml GL, the effect of GL on HIV-1 infectivity was not due to the level of transcription of proviral DNA.
Figure 3. Effects of GL on HIV-1 infection.
(A) MT-4 cells were infected with HIV-1 C-2 in the presence of increasing concentrations of GL (●) or infected with GL-treated HIV-1 C-2 (■). All experiments were carried out in triplicate and solid lines show means±S.D. (B) MT-2 cells were infected with HIV-1 C-2 in the presence of serially diluted GL (●). PM-1 CCR5 cells were infected with HIV-1 JR-FL in the presence of diluted GL (■). HIV-1-positive cells were detected by IF. (C) MT-2 cells were infected with NL43-luc pseudoviruses in the presence of increasing concentrations of GL (●) or infected with GL-treated NL43-luc viruses (■) and GL-pretreated MT-2 cells were infected with NL43-luc viruses (▲). (D) GHOST/CXCR4 cells were infected with NL43-luc pseudoviruses in the presence of increasing concentrations of GL (●) or infected with GL-treated NL43-luc viruses (■), and GL-treated GHOST/CXCR4 cells were infected with NL43-luc viruses (▲). All experiments (B, C and D) were carried out in triplicate and the mean S.D. of triplicate determinations was always less than 7%.
Figure 4. Effects of GL on infectivity of influenza A virus, VSV-G pseudovirus and poliovirus.
(A) MDCK cells were infected with influenza A viruses in the presence of increasing concentrations of GL. Haemadsorbed cells were counted. (B) FL cells were infected with VSV-G pseudoviruses in the presence of serially diluted GL. All experiments (A and B) were carried out in triplicate and values are means±S.D. (C) FL cells were infected with 10-fold serially diluted polioviral preparations in the presence of 2-fold serially diluted GL. The TCID50 of each dilution of GL was calculated by the Reed–Muenchs' method.
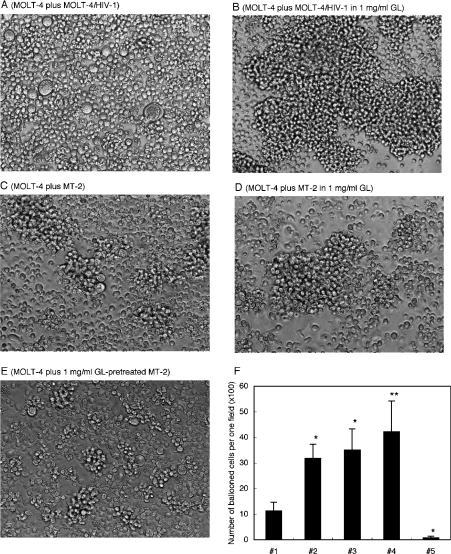
GL inhibited ballooning degeneration [26,27] of fused cells when GL was continuously present in the culture medium used to co-cultivate HIV-1-infected cells with target CD4-positive cells (Figures 5A and 5B). Figure 5 also shows that, when HTLV-I-transformed MT-2 cells were co-cultured with MOLT-4 cells, ballooned-fused cells were also observed (Figure 5C) but were almost completely absent in the presence of 1 mg/ml GL (Figure 5D). Thus cell-to-cell fusion induced by HTLV-I was also strongly inhibited by the continuous presence of 1 mg/ml GL. However, as with the cell-free HIV-1 infection system, GL pretreatment of effector MT-2 cells, target MOLT-4 cells or both enhanced fusion (Figure 5E). The number of ballooned-fused cells increased 3-fold when MT-2 or MOLT-4 cells were pretreated with 1 mg/ml GL (Figure 5F, lanes 2 and 3), and by 4 times when both cells were pretreated (Figure 5F, lane 4). All these enhancements of viral infectivity and cell fusion cannot be explained as being due only to the blocking of the attachment of viral ligand molecules to cell-surface receptors by covering and steric hindrance effects of GL.
Figure 5. Effects of GL on HIV-1- and HTLV-I-induced cell fusion.
MOLT-4 target cells were co-cultured with effector MOLT-4/HIV-1C-2 cells in the absence (A) or presence (B) of 1 mg/ml GL. MOLT-4 cells were also co-cultured with HTLV-I-transformed MT-2 cells in the absence (C) or presence (D) of 1 mg/ml GL, and were co-cultured with GL-pretreated MT-2 cells (E). After 24 h co-cultivation, photomicrographs were taken (magnification, ×100). (F) Results are shown as the number of ballooned cells per one field (magnification, ×100) for MT-2 cells co-cultured with MOLT-4 cells (#1), MT-2 cells with GL-pretreated MOLT-4 cells (#2), GL-pretreated MT-2 cells with MOLT-4 cells (#3), GL-pretreated MT-2 cells with GL-pretreated MOLT-4 cells (#4) and MT-2 cells co-cultured with MOLT-4 cells in the presence of 1 mg/ml GL (#5). Bars show means±S.D. of three independent experiments. *P<0.02 compared with MT-2 cells co-cultured with MOLT-4 cells (#1), **P<0.05 compared with MT-2 cells co-cultured with MOLT-4 cells (#1).
Kinetics of fluidity after GL treatment
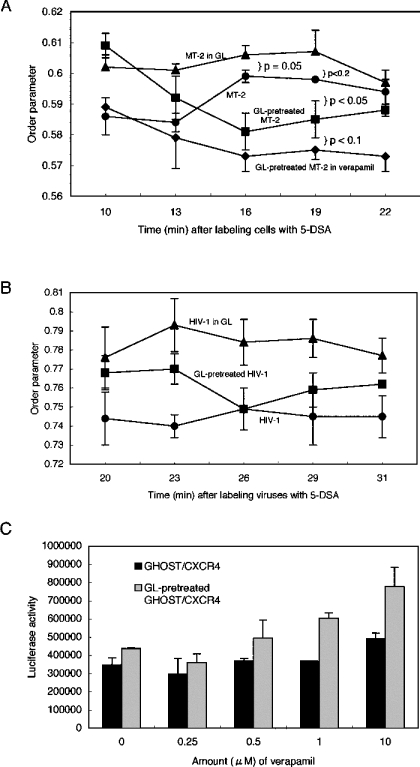
If the fluidity modification by GL is responsible for suppression or augmentation of HIV-1 infectivity and fusion, it should be hypothesized that fluidity can be altered when GL is removed from the medium. The kinetics of MT-2 membrane fluidity were examined with time after GL and 5-DSA were washed away from cells (Figure 6A). GL-untreated and 5-DSA-labelled MT-2 cells showed relatively stable fluidity between 0.584 and 0.599. MT-2 cells resuspended in 1 mg/ml GL in PBS after GL-washing (continuously GL-treated MT-2 cells) kept high order parameters between 0.595 and 0.607. In contrast, MT-2 cells resuspended in PBS after washing GL out (GL-pretreated MT-2 cells) exhibited an order parameter of 0.609 at 10 min after washing, which then rapidly decreased to 0.581 at 16 min after washing, and thereafter gradually increased and reached a value of 0.588. It should be noted that parameters of GL-pretreated MT-2 cells after 16 min were always lower than those of untreated control MT-2 cells. The kinetics indicated that GL readily diffused into the plasma membrane to obtain an equilibrium with the outside GL concentration, but quickly diffused out from the membrane when GL was removed from the medium.
Figure 6. Fluidity kinetics of the plasma membrane (A) and viral envelope (B) after labelling with 5-DSA, and effect of verapamil on HIV-1 infectivity (C).
(A) Order parameters were assessed with time after MT-2 (●) or GL-pretreated MT-2 (■) cells were labelled with 5-DSA. GL-pretreated MT-2 cells labelled with 5-DSA were also used for ESR analyses in the presence of 1 mg/ml GL (▲) or 10 μM verapamil (♦). (B) Order parameters were evaluated after HIV-1 C-2 (●) or GL-pretreated HIV-1 C-2 (■) were labelled with 5-DSA. GL-pretreated HIV-1 C-2 labelled with 5-DSA were also used for ESR in the presence of 1 mg/ml GL (▲). Values are means±S.D. of three independent experiments. (C) GHOST/CXCR4 (solid bar) or GL-pretreated GHOST/CXCR4 (grey bar) cells were incubated with NL43-luc pseudoviruses in the presence of 2-fold serially diluted verapamil at 37 °C for 1 h. After washing once, cells were cultured for 2 days in normal medium before assessing luciferase activity. Experiments were carried out in triplicate and values are means±S.D.
The kinetics of C-2 virus envelope fluidity (Figure 6B) were somewhat different from those of plasma membrane fluidity (Figure 6A). Untreated and continuously GL-treated C-2 viruses kept low and high levels of order parameters respectively. Order parameters of GL-pretreated C-2 envelope were also decreased at 26 min after GL and 5-DSA were washed out, but the parameters were always between those of continuously GL-treated and untreated C-2 viruses.
GL might equilibrate across the membrane by simple diffusion. Although GL movement may be passive as a consequence of its chemical hydrophilic and lipophilic properties, the possibility of flipping movements of P-glycoprotein or excretion of GL from the plasma membrane were examined. Verapamil, a flippase inhibitor, binds directly to P-glycoprotein and competitively inhibits drug transport [35]. Treatment of MT-2 cells with 10 μM verapamil resulted in a slight, but not significant, increase (1%) in membrane fluidity (Figure 2E), and consequently HIV-1 infectivity was enhanced by 40% (Figure 6C). No effect on HIV-1 infectivity to GHOST/CXCR4 cells was observed with less than 10 μM verapamil. However, when GL-pretreated GHOST/CXCR4 cells were treated with verapamil and used as target cells, the infectivity was additively enhanced (Figure 6C). The fluidities of GL-pretreated MT-2 cells treated with 10 μM verapamil increased greatly with time, showing lower parameters than those of GL-pretreated cells at any time points measured (Figure 6A). At the very least, verapamil did not block the effects induced by pretreatment with GL. Alternatively, these data may indicate that minimal changes of membrane fluidity induced by GL and verapamil had an influence on HIV-1 infectivity and that the mechanisms by which GL increases fluidity may be different from those of verapamil.
GL resistance of JR-FL-luc pseudovirus with high levels of gp120
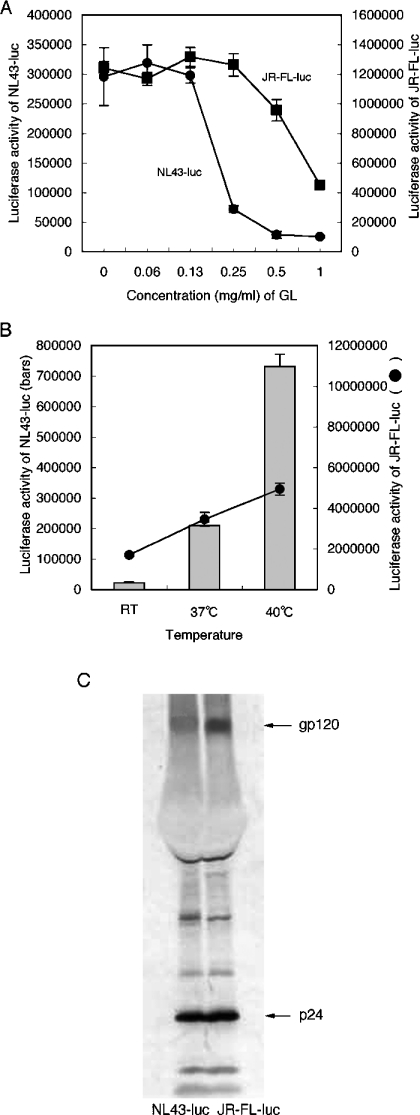
The EC50 values of NL43-luc and JR-FL-luc pseudovirus were 0.2 and 0.8 mg/ml respectively with GL (Figure 7A). Apparently, the JR-FL-luc viral preparation was more resistant to the effects of GL than NL43-luc. Dividing the luciferase activity (Figure 7A) by the amount of untreated NL43-luc or JR-FL-luc viruses' p24 allowed the viral infectivity of a single viral particle (assuming equal quanities of p24 per viral particle) to be calculated. The results indicated that the JR-FL-luc virus [1241,049/1.5 ng (827366)] had almost ten times higher infectivity per one viral particle than NL43-luc virus [295843/3.3 ng (89649)]. The effects of temperature, another fluidity-modifying factor, on infectivity of the JR-FL-luc and NL43-luc viruses (Figure 7B) was also examined. When viral adsorption was carried out at 25 °C, 37 °C or 40 °C for 1 h, viral infectivity was dependent on temperature. Infectivity of the NL43-luc virus increased by 9.5 times from 25 °C to 37 °C, and by 3.5 times from 37 °C to 40 °C. In contrast, the infectivity of JR-FL-luc was enhanced 2 times from 25 °C to 37 °C, and by 1.4 times from 37 °C to 40 °C. Thus the highly infectious JR-FL-luc viruses were more resistant to GL and less dependent on temperature than NL43-luc. Western blotting analysis showed that more gp120 per p24 was detected in JR-FL-luc viruses than in NL43-luc (Figure 7C). Augmentation of HIV-1 infectivity by increasing amounts of gp120 on virions has been reported [36–38], suggesting that multiple-site binding of gp120 with receptors is required to complete the infection. Theoretically, the existence of more gp120 molecules on a viral particle could result in a higher probability of forming a cluster of activated fusion peptides, due to multiple-site binding, regardless of the level of fluidity [39]. This could explain why the JR-FL-luc virus was more resistant to the effects of GL and less dependent on temperature than the NL43-luc virus.
Figure 7. GL resistance of JR-FL pseudovirus.
(A) Effects of GL were examined when GHOST/CXCR4 or GHOST/CCR5 cells were infected with NL43-luc (●) or JR-FL-luc (■) pseudoviruses respectively in triplicate. (B) Effects of temperature during viral adsorption were assessed using NL43-luc (bars) or JR-FL-luc (●) pseudoviruses in triplicate. Values are means±S.D. (C) Western blotting profile of NL43-luc and JR-FL-luc pseudoviruses.
DISCUSSION
GL is a broad anti-viral agent. It blocks infection by vaccinia [22], HSV-1 [22], Newcastle disease [22], VSV ([22] and Figure 4B), VZV [23], measles [24], HIV-1 ([25–27] and Figure 3), SARS [28] and influenza A (Figure 4A) viruses, but not by poliovirus ([22] and Figure 4C). The EC50 values of HIV-1, SARS virus, VSV and influenza A virus were approx. 0.2–0.7 mg/ml. GL may act in the lipid bilayer membrane, since firstly, a single-round infection system with pseudovirus was used and was effective; secondly, GL acted reversibly only for 1 h during viral adsorption; and thirdly, pretreatment of HIV-1 (Figure 3), HSV-1 [22] and VZV [23] particles with GL reduced their infectivity. In fact, GL decreased the fluidity of both the plasma membrane and viral envelope (Figure 2). Because minimal changes in membrane fluidity are logarithmically responsible for infectivity [15], it is plausible that a reduction of fluidity by GL is the primary mechanism causing inhibition of viral infectivity. It has been proposed that membrane and envelope fluidities play a pivotal role in the formation of the wide fusion pore of enveloped viruses, by clustering a substantial number of activated viral fusion proteins derived from multiple ligand–receptor complexes [39–43]. In this respect, GL mainly acts against enveloped viruses. In addition, reduced fluidity by GL could easily explain how GL inhibited cell-to-cell fusion induced by HIV-1 and HTLV-I ([26,27] and Figure 5) by suppressing the formation of virological synapses [8,9].
When GL-treated cells were washed once and the replacement medium was GL-free, the fluidity of the membrane increased with time (Figure 6A), and the cells showed enhanced susceptibility to infection (Figure 3) and fusion (Figure 5). Factors regulating the formation of a wide fusion pore are the number of receptors, number of gp120 molecules and the degree of fluidity. The cells are a mixture carrying a variety of receptor molecules in various amounts. Viral stocks also consist of a mixture of viruses bearing variable amounts of gp120. Cells with few receptors, or viruses with few gp120 molecules, could not form a cluster containing a substantial number of activated viral fusion proteins derived from multiple-site binding [39]. However, increased fluidity could promote sufficient multiple-site binding, thus enhancing the susceptibility to viral infection and fusion. Again, the degree of fluidity paralleled that of infectivity and fusion. What else could explain the observed anti- and pro-HIV-1 activities? Traditional interpretations can be ruled out: non-specific inhibition of viral adsorption such as that by other polyanionic compounds [44,45], reduction in cell signalling using pathways such as protein kinase C [26], induction of interferons in vivo [46] and production of nitrous oxide in macrophages [47] failed to account for GL activities, especially pro-HIV-1 action. One mechanism was found to fully explain the anti- and pro-HIV-1 phenomena: direct modulation of lipid bilayer membrane fluidity by GL. GL could prevent or promote rapid access of fusion proteins and their receptors to modify the pore widening for full fusion during virus entry. Alternatively, GL may impair the curvature of the membrane necessary for the formation of the fusion pore. However, this latter possibility has little to do with fluidity as such.
GL seems to diffuse easily across the lipid bilayer membrane. High concentrations of GL on the inside membrane restricted the anisotropic movement of acyl chains of 5-DSA and likewise that of phospholipids. Thus GL lowered fluidization of plasma membrane and viral envelope. In contrast, when cells were washed after GL-treatment, fluidization was increased, probably due to rapid reduction of GL content on the inside of the membrane. Rapid GL diffusion out of the membrane might induce the flexibility of acyl chains. The enhanced fluidity of GL-pretreated cells was not due to increased flip-flop movement of P-glycoproteins [13,35], because a flippase inhibitor, verapamil, did not block either enhanced fluidity or increased infectivity induced by GL pretreatment of cells. If anything, treatment with verapamil slightly enhanced both fluidity and infectivity and showed additive effects to those of GL pretreatment of cells, which confirmed further that fluidity may play a major role in HIV-1 infectivity.
Viruses with more gp120 were less sensitive to GL treatment than those with less gp120 (Figure 7). They were also less dependent on temperature, indicating that GL had the same effects on the membrane as temperature did. The existence of more gp120 molecules on a virus could result in a higher probability of forming a cluster of activated fusion peptides from multiple-site binding of gp120/receptors, regardless of the level of fluidity [39]. In this respect, GL is a virostatic agent, but not virocidal.
An advantage of GL is that it is a broad anti-viral agent with few side effects. Although data about the safety of long-term usage and high doses of GL still need to be collected, the best example of safety is a long history of safe use in clinical settings in Japan. A disadvantage of GL is that it has to be used in high concentrations to exhibit anti-viral activity and to be virostatic. HIV infection could be inhibited at pharmacologically achievable concentrations of GL with very low toxicity in clinical trials [25], but long-term use of the drug would be unrealistic except in cases of uncontrollable resistant viruses emerging against anti-HIV agents [48]. Rather, one would predict that topical application of high doses of GL would be invaluable for preventing sexually transmitted infections not only of HIV, but also of HSV. Based on the ability of GL to inhibit infections with influenza A (Figure 4A), SARS [28], vaccinia [22] viruses and VSV [22], it is suggested that the compound might be an appropriate drug for more accessible treatment of emerging acute lethal infections such as new types of influenza A, SARS, rabies and smallpox viruses. Since GL directly affected the viral envelope and reduced viral infectivity, the rate of nosocomial infections could be also reduced.
This approach is especially attractive, since GL exerted pharmacodynamic actions in the lipid bilayer membrane of the cell and virus and therefore has broad anti-viral potential with few side effects. The discovery of new modes of action for GL offers the additional opportunity for the systematic screening of accessible chemical compounds, such as glycolipids [33,49], as membrane-fluidity modulators. This might provide common therapeutic interventions against a wide range of viruses, with potential for new and highly effective applications.
Acknowledgments
I thank Y. Maeda, K. Yusa and K. Monde for their technical help and valuable encouragement. I also thank K. Hanadate at Minophagen Pharmaceutical Co., Ltd for providing GL and useful information. The studies described were funded by the Ministry of Education, Culture, Sport, Science and Technology, and the Ministry of Health, Labour and Welfare (Health Sciences Research Grants), Japan.
References
- 1.Jardetzky T. S., Lamb R. A. A class act. Nature (London) 2004;427:307–308. doi: 10.1038/427307a. [DOI] [PubMed] [Google Scholar]
- 2.Colman P. M., Lawrence M. C. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 2003;4:309–319. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons D. L., Vaney M., Roussel A., Vigouroux A., Reilly B., Lepault J., Kielian M., Rey F. A. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature (London) 2004;427:320–325. doi: 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]
- 4.Roche S., Gaudin Y. Characterization of the equilibrium between the native and fusion-inactive conformation of rabies virus glycoprotein indicates that the fusion complex is made of several trimers. Virology. 2002;297:128–135. doi: 10.1006/viro.2002.1429. [DOI] [PubMed] [Google Scholar]
- 5.Plonsky I., Zimmerberg J. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. J. Cell Biol. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markovic I., Leikina E., Zhukovsky M., Zimmerberg J., Chernomordik L. V. Synchronized activation and refolding of influenza haemagglutinin in multimeric fusion machines. J. Cell Biol. 2001;155:833–844. doi: 10.1083/jcb.200103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibbons D. L., Erk I., Reilly B., Navaza J., Kielian M., Rey F. A., Lepault J. Visualization of the target-membrane-inserted fusion protein of Semliki Forest virus by combined electron microscopy and crystallography. Cell (Cambridge, Mass.) 2003;114:573–583. doi: 10.1016/s0092-8674(03)00683-4. [DOI] [PubMed] [Google Scholar]
- 8.Igakura T., Stinchcombe J. C., Goon P. K., Taylor G. P., Weber J. N., Griffiths G. M., Tanaka Y., Osame M., Bangham C. R. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 9.McDonald D., Wu L., Bohks S. M., KewalRamani V. N., Unutmaz D., Hope T. J. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 10.del Real G., Jimenez-Baranda S., Mira E., Lacalle R. A., Lucas P., Gomez-Mouton C., Alegret M., Pena J. M., Rodriguez-Zapata M., Alvarez-Mon M., et al. Statins inhibit HIV-1 infection by down-regulating Rho activity. J. Exp. Med. 2004;200:541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolly C., Kashefi K., Hollinshead M., Sattentau Q. J. HIV-1 cell to cell transfer across an env-induced, actin-dependent synapse. J. Exp. Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontow S. E., Vander Heyden N., Wei S., Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of Rac during human immunodeficiency virus-induced cell fusion. J. Virol. 2004;78:7138–7174. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins C. F. Flip-flop: the transmembrane translocation of lipids. Cell (Cambridge, Mass.) 1994;79:393–396. doi: 10.1016/0092-8674(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 14.Sauerheber R. D., Zimmermann T. S., Esgate J. A., VanderLaan W. P., Gordon L. M. Effects of calcium, lanthanum, and temperature on the fluidity of spin-labelled human platelets. J. Membr. Biol. 1980;52:201–219. doi: 10.1007/BF01869190. [DOI] [PubMed] [Google Scholar]
- 15.Harada S., Yusa K., Monde K., Akaike T., Maeda Y. Influence of membrane fluidity on human immunodeficiency virus type 1 entry. Biochem. Biophys. Res. Commun. 2005;329:480–486. doi: 10.1016/j.bbrc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Harada S., Akaike T., Yusa K., Maeda Y. Adsorption and infectivity of human immunodeficiency virus type 1 are modified by the fluidity of the plasma membrane for multiple-site binding. Microbiol. Immunol. 2004;48:347–355. doi: 10.1111/j.1348-0421.2004.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 17.Aloia R. C., Jensen F. C., Curtain C. C., Mobley P. W., Gordon L. M. Lipid composition and fluidity of the human immunodeficiency virus. Proc. Natl. Acad. Sci. U.S.A. 1988;85:900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloia R. C., Tian H., Jensen F. C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host plasma membranes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bales B. L., Leon V. Magnetic resonance studies of eukaryotic cells. III. Spin labelled fatty acids in the plasma membrane. Biochim. Biophys. Acta. 1978;509:90–99. doi: 10.1016/0005-2736(78)90010-x. [DOI] [PubMed] [Google Scholar]
- 20.Kliewer S. Cholesterol detoxification by the nuclear pregnane X receptor. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2675–2676. doi: 10.1073/pnas.0500159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange Y., Ye J., Steck T. L. How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11664–11667. doi: 10.1073/pnas.0404766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pompei R., Flore O., Marccialis M. A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature (London) 1979;281:689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- 23.Baba M., Shigeta S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antiviral Res. 1987;7:99–107. doi: 10.1016/0166-3542(87)90025-8. [DOI] [PubMed] [Google Scholar]
- 24.Hosoya M., Shigeta S., Nakamura K., De Clercq E. Inhibitory effect of selected antiviral compounds on measles (SSPE) virus replication in vitro. Antiviral Res. 1989;12:87–98. doi: 10.1016/0166-3542(89)90072-7. [DOI] [PubMed] [Google Scholar]
- 25.Hattori T., Ikematsu S., Koito A., Matsushita S., Maeda Y., Hada M., Fujimaki M., Takatsuki K. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antiviral Res. 1989;11:255–262. doi: 10.1016/0166-3542(89)90035-1. [DOI] [PubMed] [Google Scholar]
- 26.Ito M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., Baba M., De Clercq E., Nakashima H., Yamamoto N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV) Antiviral Res. 1988;10:289–298. doi: 10.1016/0166-3542(88)90047-2. [DOI] [PubMed] [Google Scholar]
- 27.Ito M., Nakashima H., Baba M., Pauwels R., De Clercq E., Shigeta S., Yamamoto N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)] Antiviral Res. 1987;7:127–137. doi: 10.1016/0166-3542(87)90001-5. [DOI] [PubMed] [Google Scholar]
- 28.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 30.Masuda T., Matsushita S., Kuroda M. J., Kannagi M., Takatsuki K., Harada S. Generation of neutralization-resistant HIV-1 in vitro due to amino acid interchanges of third hypervariable env region. J. Immunol. 1990;145:3240–3246. [PubMed] [Google Scholar]
- 31.Maeda Y., Foda M., Matsushita S., Harada S. Involvement of both the V2 and V3 regions of the CCR5-tropic human immunodeficiency virus type 1 envelope in reduced sensitivity to macrophage inflammatory protein 1α. J. Virol. 2000;74:1787–1793. doi: 10.1128/jvi.74.4.1787-1793.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foda M., Harada S., Maeda Y. Role of V3 independent domains on a dualtropic human immunodeficiency virus type 1 (HIV-1) envelope gp120 in CCR5 coreceptor utilization and viral infectivity. Microbiol. Immunol. 2001;45:521–530. doi: 10.1111/j.1348-0421.2001.tb02653.x. [DOI] [PubMed] [Google Scholar]
- 33.Song W., Yahara S., Maeda Y., Yusa K., Tanaka Y., Harada S. Enhanced infection of an X4 strain of HIV-1 due to capping and colocalization of CD4 and CXCR4 induced by capsianoside G, a diterpene glycoside. Biochem. Biophys. Res. Commun. 2001;283:423–429. doi: 10.1006/bbrc.2001.4806. [DOI] [PubMed] [Google Scholar]
- 34.Morizono K., Harada S. Human immunodeficiency virus type 1 (HIV-1) infection and transcytosis activity of a HIV-1 susceptible clone from HeLa cell. Microbiol. Immunol. 1998;42:313–320. doi: 10.1111/j.1348-0421.1998.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 35.Yusa K., Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989;49:5002–5006. [PubMed] [Google Scholar]
- 36.Miller E. D., Duus K. M., Townsend M., Yi Y., Collman R., Reitz M., Su L. Human immunodeficiency virus type 1 IIIB selected for replication in vivo exhibits increased envelope glycoproteins in virions without alteration in coreceptor usage: separation of in vivo replication from macrophage tropism. J. Virol. 2001;75:8498–8506. doi: 10.1128/JVI.75.18.8498-8506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachrach E., Dreja H., Lin Y., Mettling C., Pinet V., Corbeau P., Piechaczyk M. Effects of virion surface gp120 density on infection by HIV-1 and viral production by infected cells. Virology. 2005;332:418–429. doi: 10.1016/j.virol.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 38.Yuste E., Reeves J. D., Doms R. W., Desrosiers R. C. Modulation of env content in virions of simian immunodeficiency virus: correlation with cell surface expression and virion infectivity. J. Virol. 2004;78:6775–6785. doi: 10.1128/JVI.78.13.6775-6785.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada S., Yusa K., Maeda Y. Heterogeneity of envelope molecules shown by different sensitivities to anti-V3 neutralizing antibody and CXCR4 antagonist regulates the formation of multiple-site binding of HIV-1. Microbiol. Immunol. 2004;48:357–365. doi: 10.1111/j.1348-0421.2004.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 40.Layne S. P., Merges M. J., Dembo M., Spouge J. L., Nara P. L. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature (London) 1990;346:277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- 41.Picard L., Simmons G., Power C. A., Meyer A., Weiss R. A., Clapham P. R. Multiple extracellular domains of CCR-5 contribute to human immunodeficiency virus type 1 entry and fusion. J. Virol. 1997;71:5003–5011. doi: 10.1128/jvi.71.7.5003-5011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt E. J., Wehrly K., Kuhmann S. E., Chesebro B., Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolate of human immunodeficiency virus type 1. J. Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steffens C. M., Hope T. J. Mobility of the human immunodeficiency virus (HIV) receptor CD4 and coreceptor CCR5 in living cells: implications for HIV fusion and entry events. J. Virol. 2004;78:9573–9578. doi: 10.1128/JVI.78.17.9573-9578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn K., Ou W., Silver J. Inhibition of certain strains of HIV-1 by cell surface polyanions in the form of cholesterol-labelled oligonucleotides. Virology. 2004;330:50–61. doi: 10.1016/j.virol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Ito M., Baba M., Sato A., Pauwels R., De Clercq E., Shigeta S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antiviral Res. 1987;7:361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- 46.Abe N., Ebina T., Ishida N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol. Immunol. 1982;26:535–539. doi: 10.1111/j.1348-0421.1982.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 47.Jeong H. G., Kim J. Y. Induction of inducible nitric oxide synthase expression by 18β-glycyrrhetinic acid in macrophages. FEBS Lett. 2002;513:208–212. doi: 10.1016/s0014-5793(02)02311-6. [DOI] [PubMed] [Google Scholar]
- 48.Markowitz M., Mohri H., Mehandru S., Shet A., Berry L., Kalyanaraman R., Kim A., Chung C., Jean-Pierre P., Horowitz A., et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report. Lancet. 2005;365:1031–1038. doi: 10.1016/S0140-6736(05)71139-9. [DOI] [PubMed] [Google Scholar]
- 49.Habib E. E., Yokomizo K., Nagao K., Harada S., Uyeda M. Antiviral activity of fattiviracin FV-8 against human immunodeficiency virus type 1 (HIV-1) Biosci., Biotechnol., Biochem. 2001;65:683–685. doi: 10.1271/bbb.65.683. [DOI] [PubMed] [Google Scholar]