Abstract
We report electrophysiological evidence that a simple odotopy, the spatial mapping of different odorants, is maintained above the level of the olfactory bulb (OB). Three classes of biologically relevant odorants for fish are processed in distinct regions of the forebrain (FB) in the channel catfish. Feeding cues, mainly amino acids and nucleotides, are represented in lateral, pallial portions of the FB, equivalent to the olfactory cortex of amniote vertebrates, whereas social signals mediated by bile salts are represented in medial FB centers, possibly homologous to portions of the amygdala. As in the OB, the different odorant classes map onto different territories; however, the response properties of units of the olfactory areas of the FB do not simply mirror those of the OB. For some units, distinctive response properties emerged, because the FB is the first center where odors subserving a common behavioral function (i.e., food function) converge.
Keywords: fish, odotopy, amygdala, piriform cortex
In the primary olfactory centers of both vertebrates (the olfactory bulb, OB) and invertebrates (e.g., the antennal lobe, AL), odor quality is represented in a spatial map within the structure (1). Both the OB and AL are organized into an array of glomeruli, which are round areas of neuropil in which the receptor cell axons terminate. In all systems studied to date, each glomerulus serves as a target for receptor cells expressing a common odorant receptor. Thus, the chemospecificity of a glomerular network is related to the chemospecificity of a particular odorant receptor molecule. The proposed function of an odotopic map across the glomerular array is to enhance both the detection and discrimination of odorants by means of lateral inhibitory interactions that sharpen the response specificity of the glomerular output neurons (2, 3).
Currently, a major question is whether odotopic maps occur in olfactory brain centers superior to the OB/AL and, if so, how odors represented there compare with the OB/AL. That is, is the odotopic map of the OB/AL maintained intact, altered, or eliminated? Recent anatomical studies in rodents indicate that the output neurons of single glomeruli project widely to downstream forebrain (FB) targets and evidence considerable overlap (4), a logic clearly different from the odotopic organization within the OB (5, 6). This organizational pattern suggests that, in vertebrates, third-order neurons in the olfactory pathway integrate odor information arriving from multiple OB glomeruli, possibly encoding features of odorant quality more directly related to the odor's behavioral significance (e.g., food or social signal) (4, 7, 8). A recent report relying on expression of the transcription factor c-FOS as a marker of neuronal activity showed that, in mice, single odorants evoke repeatable, complex patterns of activation spread across the olfactory cortex (9). Such complex patterns are quite different from the simple odotopy of the OB. Further, the odorants selected for study were not of particular biological significance to the animals. Investigation of the neural representation of odors in vertebrates is complicated by the immense number of odorant receptors, glomeruli, and potential odorants for most mammals. In fish, however, the odorant receptor repertoire and extent of odorants is limited. Fish express ≈100 receptors (10) and respond to a few classes of well defined odorants: amino acids, nucleotides, and bile salts. Of these, the first two are related to feeding behavior, whereas bile salts serve in a social context in the identification of conspecifics (11). Within the OB of catfish, the three classes of odorant are represented in different regions, confirming the principle of OB odotopy in this species (12) as in other fishes (13-15). Further, the OB output in catfish is well known, targeting only four or five terminal areas in the FB (16, 17) and thus enabling a targeted approach of physiological characterization of these target areas. We have used single-cell electrophysiology in vivo to address the question of how odors are represented within the FB targets of the OB.
Methods
Experimental Animals. Channel catfish, Ictalurus punctatus (15- to 20-cm total length), obtained from a local hatchery were maintained in floating cages held in ponds at the Louisiana State University Aquaculture Center facility. The fish were fed weekly with floating commercial fish chow. Each week, catfish were transferred to an aerated, 250-liter polyethylene aquarium filled with charcoal-filtered city tap water (CFTW) at the Louisiana State University Animal Care Facility and maintained on a 12-h:12-h light/dark regime. The temperature was held above 27°C during the spring and summer and below 20°C during the fall and winter to inhibit growth of the pathogenic bacterium, Edwardsiella ictaluri, which causes enteric septicemia and destroys chemosensory epithelia (18). The fish were used experimentally within a 1-week holding time and were not fed during this period.
Animal Immobilization and Anesthesia. The preparation of the animals was the same as that described in ref. 19. Each catfish was initially immobilized with an intramuscular injection of the neuromuscular blocking agent Flaxedil (gallamine triethiodide, 0.03 mg/100 g). During the experiments, additional injections were applied as needed by means of a hypodermic needle embedded in the flank musculature. The immobilized fish was wrapped in a wet Kim-Wipe, placed into a Plexiglas container, and stabilized by using a pair of orbital ridge clamps. The gills were irrigated by using an orally inserted glass tube supplying a constant flow of aerated CFTW that initially contained the anesthetic MS-222 (ethyl-m-aminobenzoate methane sulfonic acid, 50 mg/liter). Surgical wounds were also bathed with 3% tetracaine. Once surgery was completed, the gill irrigation water was replaced with CFTW not containing MS-222.
Surgical Preparation. Access to the olfactory organ was achieved by removing skin and connective tissue between the incurrent and excurrent nares, superficial to the olfactory organ (Fig. 1A). The right FB was exposed by removing ≈1 cm2 of skin at the midline at the top of the skull immediately caudal to the position of the eyes. After the removal of the underlying bone and cartilage, the open space was filled with freshwater teleost Ringer's solution.
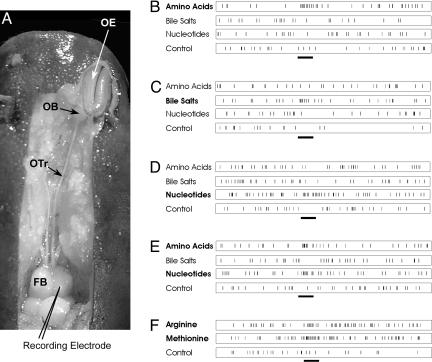
Fig. 1.
Organization of the olfactory system and typical olfactory responses in the forebrain of the channel catfish. (A) Dorsal view of the front part of the head of a channel catfish dissected to reveal the FB, olfactory tract (OTr), and OE. Unit responses were recorded from the FB while odorant stimuli were presented by means of a constant flow of water passing across the OE. The EOG (data not shown) was recorded from the OE to monitor the onset of olfactory receptor responses while a microelectrode recorded responses of single FB neurons. Lighting highlights were reduced or eliminated from the figure by digital replacement with adjacent pixels. (B-F) Examples of odor responses of five different FB units are shown. Excitatory responses were obtained to mixtures (see Methods) of amino acids (B), bile salts (C), nucleotides (D), amino acids and nucleotides (E), and Arg and Met (F). All odorants shown were tested at 10-5 M. (Time bar, 0.8 s.)
Odorant Stimuli and Delivery. The chemical stimuli (amino acids, bile salts, and nucleotides) were obtained commercially (Sigma) and were the purest available. Stock solutions (10-3 M) of l-methionine (Met), a representative neutral amino acid, and l-arginine (Arg), a basic amino acid, and their binary mixture was prepared weekly in CFTW; logarithmic step dilutions in CFTW to 10-5 M were made daily. In a previous study, the majority (77%) of the amino acid-responsive OB units responded with excitation to one of these amino acids (19). Stock solutions (10-2 M) of a ternary mixture of nucleotides previously shown to be stimulatory to olfactory receptor neurons of channel catfish (19) (ATP, ITP, and IMP) dissolved in CFTW were prepared individually; 1 ml of each stock solution was placed into cryovials and frozen at -20°C. Logarithmic step dilutions of nucleotides to 10-6 M in CFTW were made daily. Stock solutions (10-4 M) of a ternary mixture of bile salts [Na+ salts of taurocholic (TCA), taurolithocholic (TLC), and lithocholic (LCA)] were prepared weekly. TLC and LCA were indicated to activate olfactory receptor neurons of catfish (Michel, W. C. & Caprio, J., unpublished data); TCA was also included because of its known stimulatory action on olfactory receptor neurons in goldfish (Sorensen, P. W., personal communication). TCA and TLC (both water soluble) were prepared weekly and logarithmic step dilutions to 10-6 M in CFTW were made daily; 10-3 M LCA was prepared in ethanol weekly, and logarithmic step dilutions in CFTW were made daily. The concentration of methanol to water was <1:10,000, below the olfactory threshold for this compound (20). Each stimulus at each tested concentration was applied two to three times for each FB unit examined. Control solutions included (i) CFTW obtained from the same water source as that used to prepare the test solutions and (ii) ethanol at the appropriate dilution for testing LCA. Interstimulus intervals were at least 2 min.
Stimulus delivery simultaneously to both olfactory organs was by means of a “gravity-feed” system employing a spring-loaded valve (Model 5301, Rheodyne, Cotati, CA) driven by a pneumatic actuator (Model 5300) at 40 psi. Stimulus solutions and the CFTW used to bathe the olfactory mucosae between stimuli were delivered through separate Teflon tubes (0.79-mm diameter) at a rate of 4-5 ml/min. The olfactory cavities were continuously perfused with CFTW to (i) facilitate stimulus delivery, (ii) protect the mucosa from desiccation, (iii) avoid the introduction of mechanical artifacts associated with stimulus presentation, and (iv) thoroughly rinse the olfactory organ between stimuli (3- to 5-min interstimulus intervals). A foot switch connected to an electronic timer (Model 645, Dimco-Gray, Centerville, OH) triggered the valve to introduce the odorants for a 0.8-s stimulus duration without a change in either pressure or temperature and without dilution (21).
Recording Techniques. The electroolfactogram (EOG). The underwater EOG is an odorant-induced, slow negative potential measured in the water immediately above the olfactory mucosa, which is thought to reflect summated olfactory receptor generator potentials (22, 23). The EOG was recorded in vivo with sintered Ag/AgCl electrodes by means of Ringer-agar-filled capillary pipettes. The EOG signal was amplified (P-18 dc amplifier, Grass Instruments, Quincy, MA), digitized, and stored on a video channel of a hi-fi VCR recorder. The EOG signal served as an indicator of both the viability of the preparation and the response onset to the tested odorants.
FB unit recordings. Unit/few unit activity (generally 75- to 300-μV peak-to-peak amplitude) was recorded extracellularly from the medial, middle, and lateral portions of the rostral, intermediate, and caudal portions of the dorsal and ventral FB (generally 3-3.5 mm in length and 1.8-2.2 mm in width at its midregion). Each of these nine FB regions was ≈600-700 μm in width and 1,000-1,200 μm in length, depending on the size of the fish. The electrode, a low-impedance (2-5 MΩ), platinum and gold-plated, metal-filled, glass micropipette (glass tip, 1.5-2.0 μm; ball diameter, 2-3 μm), was mounted on a hydraulic microdrive attached to a stereotaxic micromanipulator and advanced vertically downward from the dorsal surface of the OB. The x, y, and z FB coordinates were recorded for each recording electrode position. The reference “zero” position (i) for the x coordinate was the midline between the right and left FB, (ii) for the y coordinate was the midline at the rostral end of the right and left FB or rostral extension of the cerebellar corpus, and (iii) for the z coordinate was the surface of the FB. Vertical electrode tracks were spaced 100 μm apart. Odor application began once a spontaneously active unit was encountered and was clearly isolated by fine-positioning the recording electrode by means of the remote fluid-filled microdrive. For each of the 49 units obtained by using search paradigm 1, the test odors were applied bilaterally to the olfactory organs with at least a 2-min interstimulus interval. In paradigm 2, which included a nucleotide mixture, 25 units were obtained. Initially, a moderate (10-6 M) concentration of each of the three odor mixtures (amino acids, bile salts, and nucleotides) and the amino acids, Arg and Met, presented individually were tested. For any odorant that resulted in an apparent increase in activity, a logarithmic unit lower concentration was also tested. If no apparent change in unit activity occurred to any of the moderate concentrations of the test odor, a logarithmic unit higher concentration of the respective odor was tested. The neural activity was amplified (P511k, Grass Instruments) (bandpass 30-10,000 Hz), observed with an oscilloscope, and stored on an audio channel of a hi-fi VCR.
Histological Verification of Recording Sites. In 12 fish, the recording electrode position was marked by making a small lesion (5-μA, 80-ms duration pulses; 10 Hz for 8 s) at the locus of unit activity for each of the stimulus classes: bile salts, nucleotides, and amino acids. The lesioned animals were permitted to survive at least 1 h after the last lesion and then anesthetized and perfused transcardially with buffered 4% paraformaldehyde. The skulls then were opened, and the brain was permitted to fix in situ a minimum of 24 h. The brains then were removed, cryoprotected in 20% buffered sucrose, and sectioned serially at 18 μm on a cryostat. The unstained sections were examined for potential lesion sites and, after photography, were counterstained with thionin (0.25% in acetate buffer). The sections then were dehydrated, cleared, and coverslipped. Importantly, the FB locations for the recorded units having different odorant specificities that were obtained from external (x and y) and micromanipulator (z) coordinates were consistent with the position of those units confirmed by histological analysis (Fig. 3).
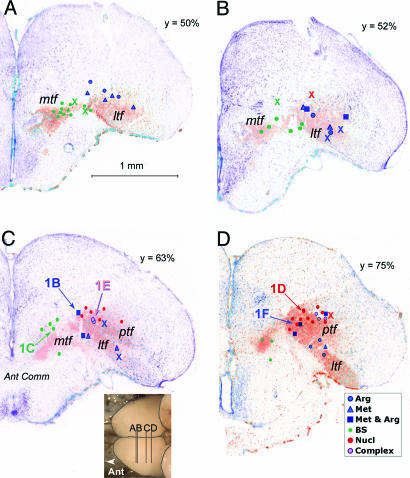
Fig. 3.
Representative transverse sections from anterior (A) to posterior (D) through the FB of the channel catfish in which the olfactory tracts had been labeled by dextran-amine (orange-red shading) and counterstained for Nissl (purple-blue staining). The sections correspond to key areas of the olfactory terminal fields; levels of the sections (A-D) are indicated in the Inset below panel C and by the y value reflecting the relative antero-posterior distance from front to back of the FB. Plotted onto these sections are the electrode positions determined by the coordinate values (dots) or by reconstruction of lesions (X). The sites labeled 1B-F refer to locations where the respective recordings shown in Fig. 1B-F were obtained. Note the close correspondence between positions determined by coordinates and those from anatomical reconstructions. The color coding corresponds to that in Fig. 2. Complex units are indicated by lavender mtf. Medial terminal field; ptf, posterior terminal field; ltf, lateral terminal field.
The approximate degree of shrinkage could be estimated by comparing the mediolateral distance measured on the microscope slides to the coordinates recorded at the time of lesioning. We used this distance as the metric because other lesion coordinates were relative to landmarks that were difficult to assess in the histological specimens: e.g., distance from the rostral tip of the cerebellum or distance from the surface of the FB to the underlying bone (the coordinate used to determine relative depth of the electrode placements). The histological coordinates for the electrodes were ≈80-90% of the values determined at the time of recording, giving a value of ≈10-20% shrinkage due to histological processing. This value was similar to that determined by measuring the antero-posterior distance between lesion sites and comparing these values with those measured by the manipulators at the time of recording.
For comparison across all cases, the relative coordinates for all electrode placements were calculated as a percentage distance of the total length, width, and depth of the FB as measured at the time of recording. These percentages then were projected onto dorsal or frontal views, which were then projected respectively onto a dorsal view of the brain (Fig. 2) or onto representative transverse (frontal) sections through the appropriate levels of the FB. The sections selected for mapping (Fig. 3) were taken from one of two fish in which the olfactory tracts were labeled with 3K-rhodamine dextran (Molecular Probes) 3 or 4 days before fixation in 4% paraformaldehyde. The sections were counterstained with fluorescent green Nissl stain (NeuroTrace Green, Molecular Probes) and photographed. Thus, the electrode positions are plotted in relation to olfactory terminal fields from similar-sized fish of the same population.
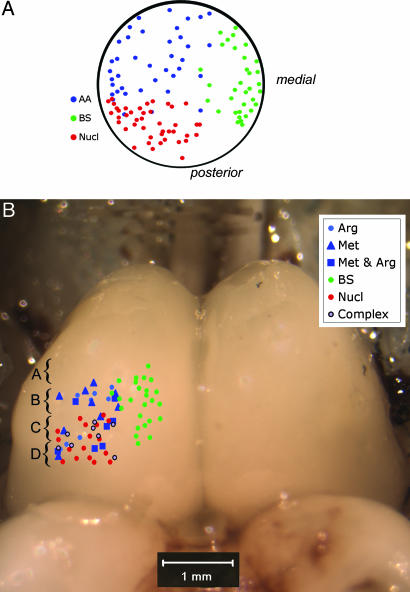
Fig. 2.
Schematic diagram showing odotopic representation in the dorsal OB (A) and the FB (B). Amino acid-responsive units are indicated by blue symbols, bile salt-responsive units are indicated in green, and nucleotide-responsive units are shown in red. Data for the OB were replotted from ref. 12. The points shown in B represent all of the units recorded and mapped in the present study. Electrode positions were plotted in Fig. 3 onto representative sections through key levels of the olfactory areas of the FB as indicated by the brackets and respective lettering.
Nomenclature. Previous studies on olfactory connections in catfish have used different terms to describe similar areas in closely related ictalurid species (16, 17). We have slightly modified the nomenclature of Finger (16) for describing the terminal field areas in the channel catfish as revealed by dextran-amine tracing.
Data Acquisition and Analysis. All recorded data from both the olfactory lamellae and FB were digitized at 32 kHz and analyzed offline with discovery software (brainwave systems discovery 5.0 with Autocut, DataWave Technologies, Longmont, CO) and printed. Some of the waveform parameters that were used by the software to identify and discriminate extracellularly recorded action potentials were peak amplitude, valley amplitude, spike height, spike width, spike time, and time between spikes. Spike events, EOG signals, and experimental parameters (i.e., beginning of a recording period, onset of stimulation, and end of the recording period) were time-stamped with a 32-bit, 100-μs resolution value and saved in a data file. The brainwave data files were displayed on a computer screen and viewed with neuroexplorer software (Nex Technologies, Littleton, MA).
Responses of single FB neurons to each of the three odor mixtures were classified as excitatory, suppressive, or null, based on the interrupted time-series analysis (24-26). The interrupted time-series analysis was conducted on the number of action potentials occurring within successive 250-ms time bins for 1.5 s before and subsequent to the initial onset of the odor-induced EOG.
Results
FB Odotopy. We recorded from 108 single FB units in a total of 41 catfish (Fig. 1 A) while introducing different odorants into a continuous stream of water directed across the olfactory epithelium (OE) (Fig. 1 A). Seventy-four (69%) FB neurons were identified that were excited by at least one of the tested odorant solutions, 21 (19%) were suppressed by all test stimuli, and 13 (12%) exhibited a changed temporal response with no significant change in response frequency. In general, the excitatory responses of the units fell into one of three broad categories: amino acid responsive, nucleotide responsive, and bile salt responsive (Fig. 1 B-D). Similar to what was previously shown for the OB (Fig. 2 A), each of these response types was localized predominantly within a restricted area of the telencephalon (Fig. 2B). Twenty-four neurons located in the lateral portion of the FB were excited selectively by amino acids, and 25 units located primarily in the medial FB were excited selectively by bile salts (Fig. 2B). Nucleotide-responsive units were situated in the lateral half of the telencephalon near the amino acid-response zone but generally lay more dorsal, caudal, and medial than the amino acid units. The 21 neurons that exhibited only suppression to the tested odorants were not located in any specific region of the FB but were scattered throughout the sampled FB areas.
The olfactory terminal fields in the FB form a continuous arc within the ventral half of the FB, extending from near the medial wall to the extreme ventrolateral edge of the pars dorsalis (pallium). As described by Finger (16), this projection area is divisible into three (or four) principal fields: medial terminal field (mtf, Fig. 3), posterior (and dorsal) terminal field (ptf, Fig. 3), and lateral terminal field (ltf, Fig. 3). Histological analysis of the electrode placements (Fig. 3) indicates that the amino acid-responsive zone is situated in the ventrolateral telencephalon, in areas Dpc and extending into the ventral part of DC-3 of Bass (17) and the lateral terminal fields (both rostral and caudal parts) of Finger (16) (Fig. 3). The nucleotide-responsive area, although still in the lateral half of the telencephalon, lies more dorsally and more posteriorly (Fig. 3 B-D) [including dorsal medial portions of DC-3 of Bass (17) and posterior terminal field and perhaps extending into the central terminal field of Finger (16)]. In contrast, the bile salt-response area is more anterior and in the medial part of the FB, lateral to area Vdd of Bass and the mtf described by Finger (16) (Fig. 3).
Unit Response Properties. In subsequent tests, 8 of the 24 amino acid units were highly selective for the basic amino acid Arg, 9 were highly selective for the neutral amino acid Met, and 7 were excited by both amino acids. Seventeen units located in more posterior regions of the lateral FB were excited selectively by the nucleotide mixture, and eight units were excited by both nucleotides and amino acids; two of these units were excited by both Met and the nucleotide mixture; two were excited by Arg and the nucleotide mixture, and four units (”complex” units) were excited by both Arg and Met and the nucleotide mixture (Figs. 1E and 3B). Units responding to both amino acids and nucleotides were never encountered in the OB (19). The complex and mixed amino acid units lie at the junction of the posterior, lateral, and medial terminal fields, perhaps corresponding to the central terminal field of Finger (16).
The units excited selectively by Arg and Met, respectively, were not grouped together within the amino acid zone but were mixed in both rostrocaudal and dorsoventral FB axes (Fig. 3), which is similar to the situation within the OB (Fig. 2 A) (12). The amino acid units excited by both Arg and Met (Figs. 1F, 2B, and 3C), a unit type never encountered within the OB, were also scattered within the lateral FB but tended to be located in more caudal regions of the FB mixed in with neurons that were excited by nucleotides alone or by nucleotides and amino acids. The units excited by bile salts were located more medially in the FB and generally at depths that were similar to amino acid-responsive units in the lateral FB (Figs. 2B and 3).
Discussion
Our results provide what we believe is the first direct physiological evidence that a simple odotopy, the spatial mapping of different odorants, is maintained above the level of the OB. We find that the three classes of biologically relevant odorants for catfish are processed in distinct regions of the FB. Social signals, mediated by bile salts, are represented in medial FB centers, whereas the feeding cues, amino acids, and nucleotides are represented in more lateral, pallial portions of the FB. Determining the exact equivalents of these areas in mammals is hampered by the peculiar development and organization of the FB in teleosts relative to other vertebrates. In all vertebrates except ray-finned fishes (class Osteichthyes, subclass Actinopterygii), the telencephalon invaginates to form the lateral ventricles. The dorsal parts of the FB form the pallium (including all cortices), of which the lateral pallium is the primary olfactory cortex in mammals. For the Actinopterygii, however, the FB develops as paired eversions that expand laterally and ventrally without an underlying ventricle (27). Because of this developmental difference, controversy exists as to the locations of the different components of the pallium. Recent molecular evidence from the FB of zebrafish indicates that only dorsal and medial regions of the pallium are everted and that the lateral pallial division, which receives the densest olfactory input from the OB, is not everted and is located laterally, similar to its location in the majority of vertebrates (28, 29). Thus, the amino acid-responsive, lateral terminal field in catfish appears homologous to olfactory cortex and perhaps olfactory tubercle.
The bile salt-responsive zone in catfish, the mtf, lies along the boundary between pallium and subpallium. Although this area, termed Vd, is conventionally assigned to the subpallium, recent molecular data suggest that this region may be pallial in nature. Our findings, coupled with other hodological and histochemical studies, suggest that the mtf in catfish may be homologous to portions of the amygdala in other vertebrates (29). In a functional context, both the amygdala of mammals (30) and the mtf in catfish deal with socially relevant olfactory signals.
The projection from the OB into the FB in catfish and some other teleosts occurs by means of distinct medial olfactory tracts (MOTs) and lateral olfactory tracts (LOTs). In general, for both channel and bullhead catfishes, the LOT projects predominantly to the ventrolateral wall of the telencephalon and extends dorsally and caudally into the FB, whereas the MOT projects medially, rostral to the anterior commissure; however, the lateral and medial termination zones are continuous, and both receive input from both the LOT and MOT. Thus, considerable overlap of LOT and MOT fibers occurs within the FB. In addition, fibers of both the LOT and MOT cross to the contralateral hemisphere by means of the anterior and/or habenular commissures. Nonetheless, the different olfactory tracts largely convey different types of odorant information and may represent separate pathways for the processing of different odorant streams. In a carp and codfish, the LOT primarily conveys information about feeding cues, whereas the MOT carries social information relevant to fright reactions and mating (31-34). This separation of olfactory information in the tracts appears to be largely maintained in the central targets of this system.
Odor Processing Within the Catfish OB. In the channel catfish, bile salts are processed in the medial OB, both dorsally and ventrally; nucleotide odorants are processed by the dorsolateral portion, and amino acids are processed by the majority of the remaining lateral OB (Fig. 2A) (12, 35). Because the bulbar efferents are organized in a similar topographic fashion, the medial tract predominantly transmits information of a pheromonal or social nature (13) to the medial FB areas (32, 36-39), whereas the lateral tract processes food-related odors (13) relayed through the lateral, basal FB (37, 40). The medial-lateral distinction in odotopy in the OBs of channel catfish (12) and other fishes (41, 42) is consistent with mitral cell axons of the medial and lateral OB projecting into the MOT and LOT, respectively (43-45). Thus, the mitral cell activities on one side of the OB in fish are not greatly influenced by those in the opposite side and may be explained by limited dendritic fields of neurons in each part of the bulb (45). This bulbar organization in fish is quite different from that in rodents, where functionally connected, mirror-symmetric (46) odotopic maps (47-49) occur in medial and lateral OB.
Odor Processing Within the Catfish FB and Relation to Other Model Systems. Similar to what we report for the telencephalon and OB (12) of the channel catfish, recent studies of the spatial representation of odor information in the Drosophila brain [i.e., the protocerebrum (50, 51) and mushroom body (52)], regions receiving direct AL input (analogous to the vertebrate OB), indicate a preservation of an odotopic map. These studies in Drosophila are also similar to those reported in the olfactory cortex of mice (7), in that projections from second-order neurons within the OB/AL to higher brain centers are extensive and often interdigitate with projections from different glomeruli. However, despite this extensive arborization of input fibers seen in higher brain centers in both invertebrates and vertebrates, there still remains considerable odorant specificity of single neurons within the FB of the channel catfish. Both major types of amino acid-responsive neurons of the catfish OB (Arg and Met units) (19) were commonly observed within the FB.
Intriguingly, the response properties of units of the olfactory areas of the FB do not simply mirror those of the OB. Rather, unique response properties emerge. One of the advantages that a topographically organized system confers is ready lateral interactions between coding modules. Whereas the olfactory tract input to the FB carries distinct streams of Arg- and Met-responsive bulbar units, amino acid-responsive FB units can be excited by both Arg and Met. Not only do odorants of a similar chemical class (amino acids) converge onto single units, but odorants serving a common function (feeding) (i.e., the “complex” units of the FB) respond to both amino acids and nucleotides, whereas units of the OB are excited only by one or the other of these odorant types. Thus, the FB is the first center where odors subserving a common behavioral (i.e., food) function converge. Both examples of convergence (i.e., between separate amino acid pathways and between amino acid and nucleotide pathways) are restricted to integration of olfactory information related to food odors (12, 37, 53). We find no convergence of socially relevant odor information with food-related responses.
In summary, we provide evidence that odors are represented within distinct FB areas according to odorant type. Further, higher order processes within the FB permit the confluence of odorant streams conveying information about different odorant types serving similar behavioral functions (e.g., feeding) while maintaining separation of odorant streams conveying disparate behavioral signals.
Acknowledgments
We thank S. Rolen and B. Böttger for technical assistance. This work was supported by grants from the National Science Foundation (to J.C.) and the National Institute of Deafness and Other Communication Disorders [to J.C., T.E.F., and D. Restrepo (University of Colorado Health Sciences Center)].
Author contributions: A.A.N. and J.C. designed research; A.A.N. and T.E.F. performed research; A.A.N., T.E.F., and J.C. analyzed data; and T.E.F. and J.C. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OB, olfactory bulb; FB, forebrain; AL, antennal lobe; CFTW, charcoal-filtered city tap water; EOG, electroolfactogram; OE, olfactory epithelium; mtf, medial terminal field; MOT, medial olfactory tract; LOT, lateral olfactory tract.
References
- 1.Christensen, T. A. & White, J. (2000) in Representation of Olfactory Information in the Brain, eds. Finger, T. E., Silver, W. L. & Restrepo, D. (Wiley-Liss, New York), pp. 202-232.
- 2.Yokoi, M., Mori, K. & Nakanishi, S. (1995) Proc. Natl. Acad. Sci. USA 92, 3371-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu, F., Greer, C. A. & Shepherd, G. M. (2000) J. Comp. Neurol. 422, 489-495. [DOI] [PubMed] [Google Scholar]
- 4.Haberly, L. B. (2001) Chem. Senses 26, 551-576. [DOI] [PubMed] [Google Scholar]
- 5.Ressler, K. J., Sullivan, S. L. & Buck, L. B. (1993) Cell 73, 597-609. [DOI] [PubMed] [Google Scholar]
- 6.Vassar, R., Ngai, J. & Axel, R. (1993) Cell 74, 309-318. [DOI] [PubMed] [Google Scholar]
- 7.Zou, Z., Horowitz, L. F., Montmayeur, J.-P., Snapper, S. & Buck, L. B. (2001) Nature 414, 173-179. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, D. M. G., Illig, K. R., Behan, M. & Haberly, L. B. (2000) J. Neurosci. 20, 6974-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou, Z., Li, F. & Buck, L. B. (2005) Proc. Natl. Acad. Sci. USA 102, 7724-7729. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Ngai, J., Dowling, M. M., Buck, L., Axel, R. & Chess, A. (1993) Cell 72, 657-666. [DOI] [PubMed] [Google Scholar]
- 11.Li, W., Scott, A. P., Siefkes, M. J., Yan, H., Liu, Q., Yun, S.-S. & Gage, D. A. (2002) Science 296, 138-141. [DOI] [PubMed] [Google Scholar]
- 12.Nikonov, A. A. & Caprio, J. (2001) J. Neurophysiol. 86, 1869-1876. [DOI] [PubMed] [Google Scholar]
- 13.Døving, K. B., Selset, R. & Thommesen, G. (1980) Acta Physiol. Scand. 108, 123-131. [DOI] [PubMed] [Google Scholar]
- 14.Hara, T. J. & Zhang, C. (1996) Neurosci. Res. 26, 65-74. [DOI] [PubMed] [Google Scholar]
- 15.Sato, Y., Miyasaka, N. & Yoshihara, Y. (2005) J. Neurosci. 25, 4889-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finger, T. E. (1975) J. Comp. Neurol. 161, 125-142. [DOI] [PubMed] [Google Scholar]
- 17.Bass, A. H. (1981) J. Morphol. 169, 91-111. [DOI] [PubMed] [Google Scholar]
- 18.Morrison, E. E. & Plumb, J. A. (1994) J. Aquat. Anim. Health 6, 101-109. [Google Scholar]
- 19.Nikonov, A. A. & Caprio, J. (2004) J. Neurophysiol. 92, 123-134. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen, P. W., Hara, T. J., Stacey, N. E. & Dulka, J. G. (1990) J. Comp. Physiol. A 166, 373-383. [Google Scholar]
- 21.Sveinsson, T. & Hara, T. J. (1990) Comp. Biochem. Physiol. A 97, 279-287. [Google Scholar]
- 22.Caprio, J. (1995) in Experimental Cell Biology of Taste and Olfaction (Current Techniques and Protocols), eds. Spielman, A. I. & Brand, J. G. (CRC, Boca Raton, FL), pp. 251-261.
- 23.Ottoson, D. (1971) in Handbook of Sensory Physiology, ed. Beidler, L. M. (Springer, Berlin), Vol. 4, Part 1, pp. 95-131. [Google Scholar]
- 24.Crosbie, J. (1993) J. Consult. Clin. Psychol. 61, 966-974. [DOI] [PubMed] [Google Scholar]
- 25.Hudson, W. W. (1977) Soc. Serv. Rev. 51, 311-326. [Google Scholar]
- 26.Kang, J. & Caprio, J. (1995) J. Neurophysiol. 74, 1421-1434. [DOI] [PubMed] [Google Scholar]
- 27.Liem, K. F., Bemis, W. E., Walker, W. F., Jr., & Grande, L. (2001) Functional Anatomy of the Vertebrates (Harcourt College Publishers, Fort Worth, TX).
- 28.Wullimann, M. F. & Rink, E. (2002) Brain Res. Bull. 57, 363-370. [DOI] [PubMed] [Google Scholar]
- 29.Wullimann, M. F. & Mueller, T. (2004) J. Comp. Neurol. 475, 143-162. [DOI] [PubMed] [Google Scholar]
- 30.Bevalier, J. (2000) in The Amygdala, ed. Aggleton, J. P. (Oxford Univ. Press, London), pp. 509-543.
- 31.Selset, R. & Døving, K. B. (1980) Acta Physiol. Scand. 108, 113-122. [DOI] [PubMed] [Google Scholar]
- 32.Hamdani, E. H., Stabell, O. B., Alexander, G. & Døving, K. B. (2000) Chem. Senses 25, 103-109. [DOI] [PubMed] [Google Scholar]
- 33.Hamdani, E. H., Alexander, G. & Døving, K. B. (2001) Chem. Senses 26, 1139-1144. [DOI] [PubMed] [Google Scholar]
- 34.Hamdani, E. H. & Døving, K. B. (2003) Chem. Senses 28, 181-189. [DOI] [PubMed] [Google Scholar]
- 35.Hansen, A., Rolen, S. H., Anderson, K., Morita, Y., Caprio, J. & Finger, T. E. (2003) J. Neurosci. 23, 9328-9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demski, L. S. & Dulka, J. G. (1984) Brain Res. 291, 241-247. [DOI] [PubMed] [Google Scholar]
- 37.Stacey, N. E. & Kyle, A. L. (1983) Physiol. Behav. 30, 621-628. [DOI] [PubMed] [Google Scholar]
- 38.Kyle, A. L., Sorensen, P. W., Stacey, N. & Dulka, J. G. (1987) Ann. N.Y. Acad. Sci. 519, 97-107. [Google Scholar]
- 39.Sorensen, P. W., Hara, T. J. & Stacey, N. E. (1991) Brain Res. 558, 343-347. [DOI] [PubMed] [Google Scholar]
- 40.Von Rekowski, C. & Zippel, H. P. (1993) Brain Res. 618, 338-340. [DOI] [PubMed] [Google Scholar]
- 41.Hara, T. J. & Zhang, C. (1998) Neuroscience 82, 301-313. [DOI] [PubMed] [Google Scholar]
- 42.Friedrich, R. W. & Korsching, S. I. (1998) J. Neurosci. 18, 9977-9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheldon, R. E. (1912) J. Comp. Neurol. 22, 177-339. [Google Scholar]
- 44.Dubois-Dauphin, M., Døving, K. B. & Holley, A. (1980) Chem. Senses 5, 159-169. [Google Scholar]
- 45.Satou, M. (1990) Prog. Neurobiol. 34, 115-142. [DOI] [PubMed] [Google Scholar]
- 46.Lodovichi, C., Belluscio, L. & Katz, L. C. (2003) Neuron 38, 265-276. [DOI] [PubMed] [Google Scholar]
- 47.Ressler, K. J., Sullivan, S. L. & Buck, L. B. (1994) Cell 79, 1245-1255. [DOI] [PubMed] [Google Scholar]
- 48.Vassar, R., Chao, S. K., Sitcheran, R., Nuñez, J. M., Vosshall, L. B. & Axel, R. (1994) Cell 79, 981-991. [DOI] [PubMed] [Google Scholar]
- 49.Mombaerts, P., Wang, F., Dulac, C., Chao, S. K., Nemes, A., Mendelsohn, M., Edmondson, J. & Axel, R. (1996) Cell 87, 675-686. [DOI] [PubMed] [Google Scholar]
- 50.Marin, E. C., Jefferis, G. S. X. E., Komiyama, T., Zhu, H. & Luo, L. (2002) Cell 109, 243-255. [DOI] [PubMed] [Google Scholar]
- 51.Wong, A. M., Wang, J. W. & Axel, R. (2002) Cell 109, 229-241. [DOI] [PubMed] [Google Scholar]
- 52.Wang, Y., Guo, H.-F., Pologruto, T. A., Hannan, F., Hakker, I., Svoboda, K. & Zhong, Y. (2004) J. Neurosci. 24, 6507-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamdani, E. H., Kasumyan, A. & Døving, K. B. (2001) Chem. Senses 26, 1133-1138. [DOI] [PubMed] [Google Scholar]