Abstract
Plectin, a typical cytolinker protein, is essential for skin and skeletal muscle integrity. It stabilizes cells mechanically, regulates cytoskeleton dynamics, and serves as a scaffolding platform for signaling molecules. A variety of isoforms expressed in different tissues and cell types account for this versatility. To uncover the role of plectin 1, the major isoform expressed in tissues of mesenchymal origin, against the background of all other variants, we raised plectin isoform 1-specific antibodies and generated isoform-deficient mice. In contrast to plectin-null mice (lacking all plectin isoforms), which die shortly after birth because of severe skin blistering, plectin isoform 1-deficient mice were viable at birth, had a normal lifespan, and did not display the skin blistering phenotype. However, dermal fibroblasts isolated from plectin 1-deficient mice exhibited abnormalities in their actin cytoskeleton and impaired migration potential. Similarly, plectin 1-deficient T cells isolated from nymph nodes showed diminished chemotactic migration in vitro. Most strikingly, in vivo we found that leukocyte infiltration during wound healing was reduced in the mutant mice. These data show a specific role of a cytolinker protein in immune cell motility. Single isoform-deficient mice thus represent a powerful tool to unravel highly specific functions of plectin variants.
Keywords: epidermal dendritic cells, T cell migration, wound healing
Biological processes such as the generation of cell polarity, adhesion, migration, or organelle transport require crosstalk of different cytoskeletal network systems. An important element in this crosstalk is provided by a family of structurally related proteins, the plakins or cytolinkers, capable of binding to and crosslinking distinct cytoskeletal filament systems (1–3). Considered as a prototype cytolinker protein, plectin can interact with a number of different partners, including proteins of all three cytoskeletal filament systems, the subplasma membrane skeleton, and junctional complexes, such as focal adhesion contacts (FACs), desmosomes, and hemidesmosomes (4).
Plectin's role as a mechanical linker and stabilizer of structural elements became evident from the symptoms of patients harboring autosomal recessive or dominant plectin mutations. Patients with recessive mutations suffer from the skin blistering disease epidermolysis bullosa simplex (EBS) combined with late-onset muscular dystrophy (reviewed in ref. 5), whereas a dominant mutation leads to EBS without muscular dystrophy (6). Plectin-deficient mice, die 2–3 days after birth exhibiting severe skin blistering and abnormalities in heart and skeletal muscle (7). Beyond its linker function, plectin plays a role in cellular processes involving actin filament dynamics (8), and its role as a scaffolding platform for signaling molecules, such as nonreceptor tyrosine kinase Fer (9) and protein kinase C receptor proteins (10), is emerging as an intriguing new facet of its functional repertoire.
Plectin's versatility is due in part to a multitude of tissue and cell type-specific variants. In mice, this diversity is generated by alternative splicing of at least 11 different first exons (1–1j) into a common exon 2 (11). Ectopic overexpression of full-length plectin isoforms revealed that the different amino termini profoundly affected their subcellular localization (12). Furthermore, for two isoforms expressed in keratinocytes, plectin 1a and 1c, distinct localizations and functions within single cells have been demonstrated (13).
This report is focused on plectin isoform 1 (plectin 1), transcripts of which are expressed in a variety of tissues, predominantly in those of mesenchymal origin (11). Among all isoforms of plectin, plectin 1 contains the longest isoform-specific sequence (60 amino acid residues), increasing its potential to accommodate isoform-specific binding partners (14). We report here that plectin 1-deficient mice, lacking just this single isoform while expressing all others, provide a unique and powerful tool to identify isoform-specific functions in the context of the whole organism. These mice were viable and lacked the severe skin blistering phenotype characteristic of plectin-null mice. We found that primary dermal fibroblasts and T cells derived from this isoform-deficient mouse showed significantly impaired migration. In addition, skin wound healing experiments carried out in vivo revealed reduced recruitment of T cells and macrophages during the inflammatory phase of the healing process. This report shows the involvement of a cytolinker protein in the recruitment of immune-responsive cells.
Materials and Methods
Generation of Plectin Isoform 1-Specific Antibodies and Immunoblotting. Fusion proteins containing amino acid sequence 106–180 of plectin 1 (11) were used as immunogens for immunizing rabbits, and antibodies obtained were affinity-purified (unpublished data). Immunoblotting was performed as described in ref. 4, and transferred proteins were visualized by using anti-plectin 1 antibodies (1:2,000), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibodies (1:30,000; Jackson ImmunoResearch). Tubulin was visualized by using anti-tubulin B5-1-2 antibodies (1:4,000; Sigma), in combination with HRP-conjugated goat anti-mouse antibodies (1:12,500; Jackson ImmunoResearch), and the SuperSignal Chemiluminescence Detection System (Pierce).
Immunofluorescence Microscopy of Tissues and Cells. Tissues were shock frozen in isopentane (Fluka), fixed with acetone, cryosectioned (2 μm), and air-dried before incubation with antibodies. Epidermal sheets were prepared and analyzed as described in ref. 15. Fibroblasts grown on glass coverslips were fixed with methanol (4). T cells were seeded on ICAM-1 (R & D Systems)-coated chamber slides (Lab-Tek). Microscopy was performed by using a Zeiss LSM 510 confocal microscope. The following primary immunoreagents were used: affinity-purified anti-plectin 1 (1:1,000), anti-pan plectin (1:200; ref. 4), anti-actin AC40 (1:200; Sigma), anti-tubulin B5-1-2 (1:500; Sigma), anti-vimentin (1:500; ref. 16), anti-vinculin clone vin-11-5 (1:400; Sigma), anti-MHC II clone M5/114.15.2 (1:100; American Type Culture Collection), anti-Thy 1.2 (1:100; Becton Dickinson), and anti-CD4 (1:100; Pharmingen). As secondary antibodies we used: Texas red-conjugated goat anti-rabbit (1:500), goat anti-mouse (1:200), donkey anti-rabbit (1:100), and donkey anti-goat (1:3,000) (all from Jackson ImmunoResearch), Alexa 488-conjugated goat anti-mouse (1:1,000), donkey anti-goat (1:3,000), and goat anti-rat (1:2,000) antibodies (all from Molecular Probes), and Texas red Streptavidin (1:400; Amersham Pharmacia).
Generation of plectin 1-deficient mice, isolation of RNA and RT-PCR, isolation of primary cell cultures, adhesion and migration assays, and in vivo wound healing experiments are described in detail in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Results
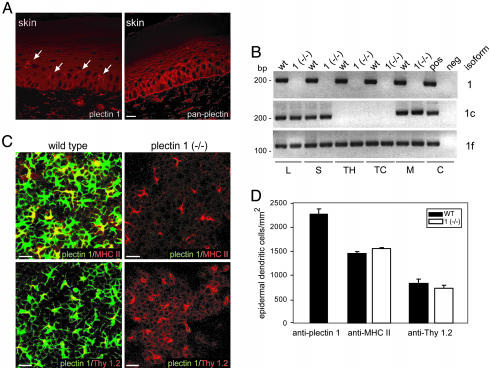
Plectin Isoform 1 Is Expressed in Connective Tissue and White Blood Cells. In previous studies, transcripts of plectin isoform 1, one of >10 different isoforms identified in mouse, were found to be most prominently expressed in organs with high connective tissue content (11). To monitor expression of plectin 1 on the protein level and to study its cellular localization, we raised isoform-specific antibodies. Rabbits were immunized with recombinant fusion proteins containing plectin 1-specific sequences, and antibodies highly specific for plectin 1 were obtained by affinity purification. Antibody specificity was confirmed among others by negative reaction with plectin 1-deficient tissues and cells (see Fig. 6D, which is published as supporting information on the PNAS web site). As assessed by immunofluorescence microscopy of cryosectioned mouse tissues, high level expression of plectin 1 was observed, as expected, in all specimens rich in connective tissue (data not shown). In skin, dermal fibroblasts were prominently stained, staining of basal epidermal keratinocytes was negligible, and upper epidermal layers were not stained at all. Interestingly, dendritic cells of the epidermis were strongly immunoreactive, whereas pan anti-plectin antibodies additionally stained epidermal keratinocytes and the basement membrane (Fig. 1A). In immunoblottings of cell lysates from primary T cells, macrophages, and fibroblasts, the antibodies specifically detected protein bands of >500 kDa, indicating that full-length versions of plectin 1 were expressed in these cells (Fig. 6D).
Fig. 1.
Immunolocalization of plectin isoform 1 in mouse skin and its distribution in epidermal dendritic cells and other cells of the immune system. (A) Immunofluorescence microscopy images of cryosectioned skin with anti-plectin 1 and anti-pan plectin antibodies. Note the isoform 1-specific immunoreactivity of dermal fibroblasts and epidermal dendritic cells (arrows) and partially overlapping expression patterns with pan anti-plectin antibodies. (Scale bar: 20 μm.) (B) RT-PCR of tissues and cells of immunological origin from mouse. cDNAs prepared from lymph nodes (L), spleen (S), thymus (TH), primary T cells (TC), and macrophages (M) of wild-type and plectin 1(–/–) mice and from control fibroblasts were subjected to nested PCRs by using plectin exon 1/1c/and 1f-specific primers to generate fragments of 218 bp, 231 bp, and 141 bp, respectively. Neg, negative control (H2Odest.). (C) Epidermal sheets from adult wild-type and plectin 1 (–/–) mice were exposed to the antibodies indicated and microscopically analyzed. Note that two populations of epidermal dendritic cells, MHC II-positive LCs and Thy 1.2-positive dendritic epidermal T cells, were immunoreactive with antibodies to plectin 1. (Scale bars: 20 μm.) (D) Numerical analysis of plectin 1-, MHC II-, and Thy1.2-positive cells in epidermal sheets derived from wild-type and plectin 1-deficient littermates. Thirty to 60 fields per sheet (n = 4) were randomly chosen, and the density of positive cells was determined and expressed as the number of cells (mean ± SD) per mm2 of skin surface. No difference in morphological appearance and cell number was observed.
The conspicuous occurrence of plectin 1 in dendritic cells of the epidermis prompted us to ask whether this isoform was expressed in other cell types of immunological origin. Indeed, RT-PCR revealed expression of transcripts in lymph nodes, spleen, thymus, primary T cells, and macrophages (Fig. 1B). An additional isoform expressed in these tissues was plectin isoform 1f, whereas another isoform tested, plectin 1c, showed a more restricted distribution.
Mice Deficient in Plectin Isoform 1 Are Viable Without Apparent Skin Defects. To investigate isoform-specific biological functions of plectin 1 in an animal model, we generated a mouse line that selectively lacked this isoform while expressing all others (details of generation and genotypic characterization are given in Materials and Methods and Fig. 6). The absence of plectin isoform 1 in mutant mice was confirmed on both RNA and protein levels (Figs. 1B and 6D). In addition, as assessed in at least one other case (plectin isoform 1d), the expression of other plectin isoforms seemed unaltered (data not shown). Contrary to plectin-null mice, which die shortly after birth (7), plectin 1-deficient mice were viable and showed no striking phenotype, in particular no skin blistering, when compared to heterozygous or wild-type littermates. Mutant mice had a normal lifespan, were fertile, and intercrossing them produced normal litter sizes.
The absence of skin blistering in plectin 1-deficient mice and the prominent expression of plectin 1 in nonepithelial cells of skin both argued for a role of plectin 1 different from mechanical stabilization of cells that is typical for other plectin isoforms (13). Hematoxylin/eosin staining of paraffin sections revealed no differences in skin architecture of plectin 1-deficient mice compared to wild-type littermates (data not shown), and immunostaining with antibodies to vimentin showed no differences in morphology or number of dermal fibroblasts (data not shown). To better visualize and enumerate dendritic cells, epidermal sheets were prepared. In wild-type mice, plectin 1 expression was found in MHC II-positive Langerhans cells (LCs) and Thy 1.2-expressing T cells, the two types of dendritic cells characteristic of the epidermis (Fig. 1C). In plectin 1-deficient sheets, LCs and dendritic epidermal T cells were indistinguishable in their distribution, morphology, and density when compared with wild-type controls, suggesting that their development was uncompromised (Fig. 1 C and D).
To investigate whether cytoarchitectural features of cells were affected by plectin deficiency, primary dermal fibroblasts derived from wild-type and plectin 1-deficient animals were subjected to immunofluorescence microscopy. Anti-plectin 1 antibodies revealed a filamentous staining pattern throughout the cytoplasm of wild type, contrary to mutant cells (see Fig. 7 a and f, which is published as supporting information on the PNAS web site). Apparently, plectin 1 was not the only isoform expressed in these cells, as anti-pan plectin antibodies (recognizing all known isoforms of plectin) in plectin 1-deficient cells generated filamentous staining patterns that were very similar to those visualized in wild-type cells with anti-plectin 1 antibodies (Fig. 7, compare a with g). Plectin 1-deficient primary fibroblasts showed no significant alterations in tubulin and vimentin network structures, whereas the actin stress fiber system was slightly more prominent in mutant cells (Fig. 7, compare c–e with h–j).
Plectin 1-Deficient Primary Dermal Fibroblasts Show Impaired Migration in Vitro. Using plectin-null fibroblasts, we had previously established a role of plectin in cellular processes involving actin filament dynamics, in particular cell migration (8). To investigate whether plectin 1 deficiency was responsible for a similar phenotype, primary dermal fibroblasts deficient in plectin 1 (plectin 1 –/–) and corresponding cells from wild-type littermates were plated for short time periods (2–3 h) onto culture dishes and then subjected to immunofluorescence microscopy by using antibodies to actin and vinculin. As shown in Fig. 2A, plectin 1-deficient fibroblasts showed a significant (cell size-independent) increase in the number of actin stress fibers and vinculin-positive FACs compared to wild-type cells. Similar to plectin-null fibroblasts, after prolonged time in culture (24 h) the abundance of actin stress fibers and FACs decreased in plectin 1 (–/–) cells, approaching the level of wild-type cells.
Fig. 2.
Delayed execution of actin cytoskeleton rearrangements and impaired cell migration of primary plectin 1-deficient fibroblasts. (A) Immunofluorescence microscopy of wild-type and plectin 1 (–/–) fibroblasts visualizing the actin cytoskeleton and vinculin-positive FACs. After an attachment period of 2 or 24 h, cells were fixed and stained with antibodies indicated. Note statistically significant (60 randomly chosen cells were analyzed) increases in actin stress fibers and FACs in plectin 1 (–/–) cells after 2 h of spreading, whereas after 24 h, hardly any differences compared to wild-type cells were noticeable. (Scale bar: 20 μm.) (B) In vitro wound healing assay to measure migration of primary wild-type and plectin 1 (–/–) fibroblasts. Values represent means ± SD of six measurements per time point in three independent experiments. (C and D) Chemotactic (C) and random (D) migration of wild-type and plectin 1 (–/–) fibroblasts assayed by using a Boyden chamber. Measurements in C were carried out after a 24 h exposure to the chemoattractant (means ± SD, n = 3). Note just slight differences between wild-type and plectin 1 (–/–) cell populations passing through the filter in random migration (without chemoattractant) compared to a 47% difference in PDGF-directed migration. (E) Growth curves of wild-type and plectin 1-deficient fibroblasts (±SD, n = 4).
To assess the migratory ability of primary plectin 1 (–/–) fibroblasts in an in vitro wound healing assay, a mechanical scratch was made in the cell monolayer and movement of cells into the empty area was followed over a period of 24 h by using video microscopy. As shown in Fig. 2B, migration of plectin 1 (–/–) cells was found to be considerably slower than that of wild-type cells. In a second assay, chemotactic migration of cells through the pores of a membrane toward PDGF was measured by using a Boyden chamber. Again, plectin 1 (–/–) cells showed a substantial delay in their response to the chemoattractant compared to their wild-type counterparts, with chemotaxis reduced to 60% (Fig. 2C), whereas the number of randomly migrating cells (no PDGF) was indistinguishable under similar conditions (Fig. 2D). No difference was found in the growth rates of plectin 1 (–/–) and wild-type cells when measured over a period of 4 days (Fig. 2E). This finding ruled out the possibility that compromised proliferative capacities of cells were a factor in these migration assays.
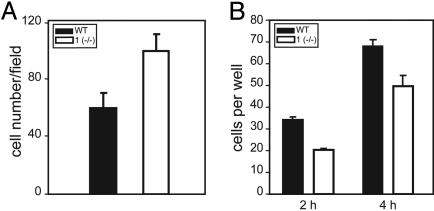
Lack of Plectin 1 Has No Effect on Polarization but on Adherence and Migration of Cultured T Cells. The observed reduced migratory ability of plectin 1-deficient dermal fibroblasts raised the question whether other cell types normally expressing this isoform showed a similar phenotype. T cells develop a polarized morphology when undergoing activation for cell–cell interaction and migration (17), and plectin previously has been shown (18) to redistribute to the trailing edge (uropod) during chemokine-induced polarization of human T cells. To assess whether uropod formation was affected by plectin 1 deficiency, CD 4-positive T cells were isolated from lymph nodes of wild-type and plectin 1-deficient mice, spread onto ICAM-1-coated surfaces, and uropod formation was induced with phorbolester. Immunofluorescence microscopy of stimulated T cells with antibodies to their major cytoskeletal constituents revealed no detectable differences between wild-type and plectin 1-deficient cells in cytoarchitecture (see Fig. 8, which is published as supporting information on the PNAS web site). In both cell types, densely packed microtubules and vimentin filaments were visualized all along the uropod structure, whereas microfilaments were found concentrated in lamellipodia at the cell front. Similar to plectin 1-deficient fibroblasts, T cells lacking plectin 1 did not show any significant morphological alterations, and their ability to polarize and form a uropod structure seemed unimpaired.
Interestingly, however, when the number of cells adhered to the ICAM-1-coated surfaces was statistically analyzed after 5 to 6 h of spreading, 38% more plectin 1 (–/–) T cells compared to wild-type cells were found attached (Fig. 3A). To assess whether this increased adhesion potential of plectin 1-deficient T cells affected their ability to migrate, they were subjected to a Boyden chamber assay where migration toward the chemokine SDF-1α was measured. Compared to wild-type cells, migration of plectin 1-deficient T cells was found, in fact, to be reduced by 41% after 2 h and 27% after 4 h of adhesion (Fig. 3B).
Fig. 3.
Statistical analyses of cell adhesion and migration assays of plectin 1-deficient T cells. (A) Equal numbers of stimulated T cells were plated onto ICAM-1-coated coverslips for 5–6 h, fixed, and immunostained by using antibodies to tubulin. Fifteen randomly chosen optical fields were counted in two independent experiments. Bars represent means ± SD. Note the higher number of adhered plectin 1-deficient T cells. (B) Migration of T cells in response to a chemokine measured after 2 and 4 h in a Boyden chamber. Bars represent means ± SD, n = 4. Note the significantly reduced migration of plectin 1-deficient T cells toward the chemokine.
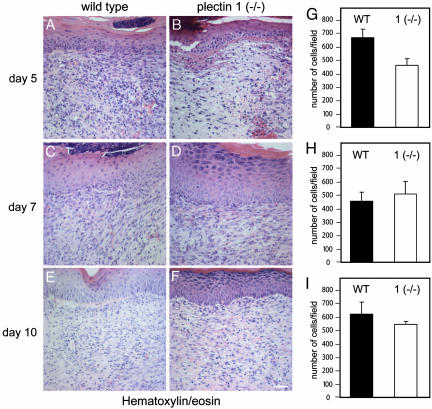
Plectin Isoform 1 Deficiency Affects Cellular Granulation Tissue Formation at Wound Sites in Vivo. To assess whether retardation of cell migration and altered adhesion properties of plectin 1 (–/–) cells observed in vitro would manifest themselves also in vivo in a process where cell motility and cell–cell interactions are known to play an important role, wound healing experiments were performed. For this reason, we analyzed full-thickness back skin wounds of plectin 1-deficient mice and control littermates at days 3, 5, 7, 10, and 14 after injury. After 2 weeks, the wounds of all experimental animals macroscopically appeared to be fully closed and scabs were discarded. At this level, no significant differences were seen between wild-type and plectin 1 (–/–) animals at any stage (data not shown). Differences became apparent, however, at the histological level (hematoxylin/eosin staining). By day 5 after wounding, wild-type wounds had generally established a thick, cell-rich granulation tissue (Fig. 4A), but in wounds of plectin 1 (–/–) mice, the equivalent tissue appeared less cellular (Fig. 4 B and G). However, by days 7 and 10, differences were hardly evident anymore (Fig. 4 C–I). By day 14, when wounds had fully closed, the cell densities in the connective tissues of the wounds of wild-type and plectin 1-deficient mice were indistinguishable and had returned to the level of unwounded epidermis (data not shown).
Fig. 4.
Histological analyses of in vivo wound healing experiments in plectin 1-deficient compared to wild-type mice. (A–F) Hematoxylin/eosin staining of paraffin sections of full-thickness wounds at days 5, 7, and 10 after wounding. By day 5 after wounding, in wild-type wounds a cell-dense granulation tissue had formed beneath the repairing epithelium (A), whereas the comparable plectin 1-deficient tissue was much less cellular (B). By days 7 (C and D) and 10 (E and F), the granulation tissues in wound areas of wild-type and mutant littermates became increasingly indistinguishable. At day 14 after wounding, wound closure was completed in both genotypes with regressing cell numbers (data not shown). (Scale bar: 50 μm.) (G–I) Statistical analyses (n = 6) revealing reduced cell numbers in the wound area of plectin 1 (–/–) mice at day 5 after wounding. At later time points, no significant differences were found.
The lower cell density observed at wound sites of plectin 1 (–/–) animals could have been due to reduced proliferation of fibroblasts. However, no significant differences were seen between wild-type and plectin 1 (–/–) mice when wound sites were immunostained by using antibodies to the proliferation marker Ki67 at days 5 and 7 after wounding (data not shown). The capacity of plectin 1 (–/–) fibroblasts to transform into α-smooth muscle actin-expressing myofibroblasts was monitored immunohistochemically at days 5, 7, and 10 after wounding. No apparent alterations in number and distribution of myofibroblasts in the granulation tissues were observed (data not shown). Both of these assays indirectly also ruled out that impaired migration of fibroblasts was a major cause for differences in granulation tissues.
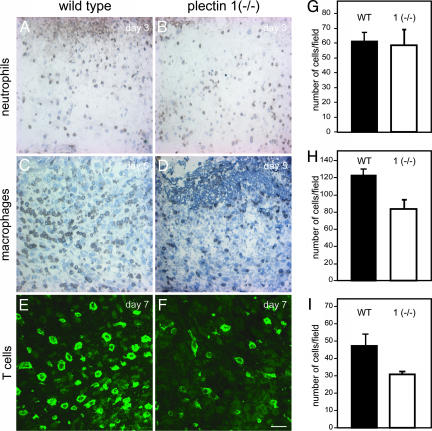
Reduced Recruitment of Macrophages and T Cells to Wound Sites of Plectin 1-Deficient Mice. During the inflammatory phase of the wound healing process, leukocytes, in particular neutrophils, macrophages, and T cells, migrate out of blood vessels and infiltrate the wound site (19). To assess the role of plectin 1 in the recruitment of neutrophils, skin wounds of plectin 1-deficient animals and of control littermates were processed for immunohistochemistry on day 3 after wounding by using antibodies to a neutrophil marker protein (Fig. 5 A and B). In both types of wounds, comparable numbers of immunoreactive cells were identified by statistical analysis (Fig. 5G), indicating unimpaired recruitment of neutrophils in plectin 1 (–/–) animals. When we similarly examined the number of infiltrating macrophages at the wound sites by using antibodies to a macrophage-specific marker (F4/80), we found that in wild-type animals, infiltration of macrophages reached its maximum at days 5 to 7 after injury (Fig. 5C). Interestingly, however, at this stage the number of F4/80-positive macrophages in plectin 1 (–/–) animals was found significantly (35%) reduced (Fig. 5 D and H).
Fig. 5.
Reduced recruitment of macrophages and T cells to the wound sites of plectin 1 (–/–) mice. Cryosections obtained from wild-type and mutant 1 (–/–) littermates were analyzed by immunohistochemistry at the indicated time points after wounding (A–D) or immunofluorescence microscopy (E and F) with antibodies to markers of neutrophils (anti-7/4; A and B), macrophages (anti-F4/80; C and D), and T cells (anti-CD 4; E and F), respectively. (Scale bar: 50 μm.) Statistical analyses (G–I; n = 4) revealed reduced infiltration of macrophages and T cells but not neutrophils into the wound areas of plectin 1-deficient animals.
Next we examined T cell infiltration at the wound sites by using antibodies specific for the T cell markers CD 4 and CD 8. CD 4- and CD 8-positive cells were observed in wild-type and plectin 1 (–/–) animals at days 3, 5, and 7 after wounding (data not shown). However, at days 5 and 7, the number of T cells in the wound area of plectin 1-deficient animals was significantly lower compared to normal littermates (Fig. 5 E, F, and I and data not shown). In contrast, in unwounded tissue (lymph nodes) of plectin 1-deficient mice, the overall number of T cells was not diminished (data not shown).
Discussion
The importance of cytolinker proteins in interlinking and anchoring cytoskeletal networks to cell junctions and, thereby, maintaining the structural integrity of cells and tissues has been established for epithelia and some nonepithelial tissues like muscle and neurons (for review, see ref. 1). However, little is known about their role in more motile cells, such as in those of the connective tissue and the immune system.
Using isoform-specific antibodies, we identified a variety of plectin 1-expressing cells in skin, including fibroblasts, T cells, and dendritic cells, all of which also expressed the intermediate filament protein vimentin. We find it particularly noteworthy that plectin 1 was found in dendritic cells, which among immune cells are the most potent ones in mediating rapid initiation of the primary immune response. A subset of dendritic cells is represented by highly motile LCs, which characteristically contain membrane-bound organelles called Birbeck granules (20). As the appearance of these granules and the expression of their major protein constituent, langerin, coincide with plectin 1 expression in LCs (21), it is conceivable that plectin 1 plays some role in the genesis or intracellular trafficking of these organelles. A role of plectin in the positioning of another organelle, the mitochondrium in skeletal muscle and heart previously has been proposed (22). It might be interesting to analyze plectin 1-deficient LCs on the ultrastructural level regarding the positioning of Birbeck granules.
We provide evidence that plectin 1 deficiency impairs the ability of cultured fibroblasts to rapidly rearrange their actin cytoskeleton, leading to reduced cell motility. This finding was not all that unexpected, considering that plectin-null fibroblasts exhibited a similar phenotype (8). A major contribution to the dynamics of actin filaments is made by actin-severing proteins (23), and at least in case of one of these proteins, gelsolin, ablation led to decreased motility and an increase in the number of actin stress fibers in fibroblasts (24). A related activity of plectin 1 could explain the actin stress fiber formation and temporary prevention of lamellipodia formation typical for plectin 1-deficient fibroblasts. A similar mechanism may be relevant for plectin 1-deficient T cells, highly motile cells forming only diffuse contacts with the substratum (25), but which, like fibroblasts, need lamellipodial structures at the leading edge for proper cell migration. In this context it would be interesting to analyze the status of typical actin severing proteins, such as gelsolin or cofilin, in plectin 1-deficient cells, because the cell may try to compensate for the loss of plectin 1 by generating more active forms of these proteins.
Previous results obtained from plectin-null fibroblasts showed a delayed response to stimuli of the Rho pathway, resulting in longer persistence of actin stress fibers (8). Because it is known that the small GTPase Rho A promotes formation of actin stress fibers and FACs in fibroblasts (26), one may expect that fibroblasts lacking plectin would show elevated levels of Rho A. In such a model, plectin 1 would act as a negative regulator downstream of the Rho signaling cascade. Depending on cell type, Rho A can have opposite effects. In leukocytes, Rho A is required for uropod retraction during transendothelial migration, and its inhibition leads to trapping of leukocytes between endothelial cells (27). Thus, the increased adherence and impaired cell migration of plectin 1-deficient T cells in vitro lead us to speculate that Rho A activity is decreased in these cells, implying that plectin 1 would act as a positive regulator of Rho activity in this cell type. Whether plectin 1 might indeed fulfill opposite roles in the regulation of Rho A activity in different cells remains an interesting question to be answered.
Wound healing requires rapid motile events and is characterized by four distinct phases: hemostasis, inflammation, proliferation, and remodeling (28). After hemostasis, which is triggered by the release of clotting factors and growth factors from platelets, the wound is invaded by neutrophils and macrophages, which immediately start phagocytosis of foreign material (19). Loss of plectin 1 appeared not to influence recruitment of neutrophils, because 3 days after wounding, no difference in neutrophil number was observed, although differences at earlier time points may have been missed. In contrast to neutrophil recruitment, invasion of macrophages and T cells was found to be reduced in wounds of plectin 1-deficient animals. The role of inflammatory cells in wound repair is controversial, spanning from reports claiming for a delay in reepithelialization (29) to others showing accelerated wound closure when reduced numbers of inflammatory cells were present at the wound site (30). However, a recent report by Martin et al. (31) questioned the validity of these studies, because wounds of PU.1-deficient mice, lacking macrophages and neutrophils, were found to heal without an inflammatory response in about the same time as those of normal mice.
Proliferation of resident dermal fibroblasts in the neighborhood of the wound and their migration into the wound is another requirement that has to be met during the healing process. Fibroblasts synthesize and remodel the extracellular matrix; furthermore, they transform into smooth muscle cell-resembling myofibroblasts, which have the ability to generate the strong contractile forces needed for proper wound contraction. Although migration of plectin 1-deficient fibroblasts was affected in vitro, no effects on fibroblast migration or myofibroblast transformation were observed in the wounds of plectin 1-deficient animals. This observation might be due to the transitory nature of the fibroblast phenotype (8) and its compensation in a longer time frame and more complex cellular environment.
In conclusion, our plectin 1-deficient mouse model revealed changes in immune cells of the skin, arguing for an important role of plectin isoform 1, specifically during the inflammatory phase of the wound healing process.
Supplementary Material
Acknowledgments
We thank M. Busslinger and T. Jenuwein (Institute of Molecular Pathology, Vienna), T. Decker (University of Vienna), M. Kraus (University of Cologne, Cologne, Germany), and P. Traub (University of Bonn, Bonn) for mouse lines, antibodies, and reagents; Z. Stojanovic for technical assistance and I. Sauer for help in cultivation of primary macrophages; G. Rezniczek for critically commenting on the manuscript; and T. Schweighoffer for helpful discussions. This work was supported by Austrian Science Research Fund Grants F006-11 (to G.W.) and P-14243-MED/P17078-B14 (to A.E-B.).
Author contributions: C.A., P.F., A.E.-B., and G.W. designed research; C.A., P.F., S.O.-M., I.F., F.P., and A.E.-B. performed research; C.A. and G.W. analyzed data; and C.A. and G.W. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FAC, focal adhesion contact; LC, Langerhans cell.
References
- 1.Jefferson, J. J., Leung, C. L. & Liem, R. K. (2004) Nat. Rev. Mol. Cell. Biol. 5, 542–553. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs, E. & Karakesisoglou, I. (2001) Genes Dev. 15, 1–14. [DOI] [PubMed] [Google Scholar]
- 3.Wiche, G. (1998) J. Cell Sci. 111, 2477–2486. [DOI] [PubMed] [Google Scholar]
- 4.Rezniczek, G. A., Janda, L. & Wiche, G. (2004) Methods Cell Biol. 78, 721–755. [PubMed] [Google Scholar]
- 5.Rouan, F., Pulkkinen, L., Meneguzzi, G., Laforgia, S., Hyde, P., Kim, D. U., Richard, G. & Uitto, J. (2000) J. Invest. Dermatol. 114, 381–387. [DOI] [PubMed] [Google Scholar]
- 6.Koss-Harnes, D., Hoyheim, B., Anton-Lamprecht, I., Gjesti, A., Jorgensen, R. S., Jahnsen, F. L., Olaisen, B., Wiche, G. & Gedde-Dahl, T., Jr. (2002) J. Invest. Dermatol. 118, 87–93. [DOI] [PubMed] [Google Scholar]
- 7.Andrä, K., Lassmann, H., Bittner, R., Shorny, S., Fässler, R., Propst, F. & Wiche, G. (1997) Genes Dev. 11, 3143–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrä, K., Nikolic, B., Stöcher, M., Drenckhahn, D. & Wiche, G. (1998) Genes Dev. 12, 3442–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunter, P. C. & Wiche, G. (2002) Biochem. Biophys. Res. Commun. 296, 904–910. [DOI] [PubMed] [Google Scholar]
- 10.Osmanagic-Myers, S. & Wiche, G. (2004) J. Biol. Chem. 279, 18701–18710. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs, P., Zörer, M., Rezniczek, G. A., Spazierer, D., Oehler, S., Castañón, M. J., Hauptmann, R. & Wiche, G. (1999) Hum. Mol. Genet. 8, 2461–2472. [DOI] [PubMed] [Google Scholar]
- 12.Rezniczek, G. A., Abrahamsberg, C., Fuchs, P., Spazierer, D. & Wiche, G. (2003) Hum. Mol. Genet. 12, 3181–3194. [DOI] [PubMed] [Google Scholar]
- 13.Andrä, K., Kornacker, I., Jörgl, A., Zörer, M., Spazierer, D., Fuchs, P., Fischer, I. & Wiche, G. (2003) J. Invest. Dermatol. 120, 189–197. [DOI] [PubMed] [Google Scholar]
- 14.House, C. M., Frew, I. J., Huang, H. L., Wiche, G., Traficante, N., Nice, E., Catimel, B. & Bowtell, D. D. (2003) Proc. Natl. Acad. Sci. USA 100, 3101–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang-Rodriguez, S., Hoetzenecker, W., Schwarzler, C., Biedermann, T., Saeland, S. & Elbe-Bürger, A. (2005) J. Leukocyte Biol. 77, 352–360. [DOI] [PubMed] [Google Scholar]
- 16.Giese, G. & Traub, P. (1986) Eur. J. Cell Biol. 40, 266–274. [PubMed] [Google Scholar]
- 17.Serrador, J. M., Nieto, M. & Sanchez-Madrid, F. (1999) Trends Cell Biol. 9, 228–233. [DOI] [PubMed] [Google Scholar]
- 18.Brown, M. J., Hallam, J. A., Liu, Y., Yamada, K. M. & Shaw, S. (2001) J. Immunol. 167, 641–645. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson, A. & Ferguson, M. W. (2003) Methods Mol. Biol. 225, 249–260. [DOI] [PubMed] [Google Scholar]
- 20.Valladeau, J., Dezutter-Dambuyant, C. & Saeland, S. (2003) Immuol. Res. 28, 93–107. [DOI] [PubMed] [Google Scholar]
- 21.Tripp, C. H., Chang-Rodriguez, S., Stoitzner, P., Holzmann, S., Stossel, H., Douillard, P., Saeland, S., Koch, F., Elbe-Bürger, A. & Romani, N. (2004) J. Invest. Dermatol. 122, 670–672. [DOI] [PubMed] [Google Scholar]
- 22.Reipert, S., Steinböck, F., Fischer, I., Bittner, R. E., Zeold, A. & Wiche, G. (1999) Exp. Cell Res. 252, 479–491. [DOI] [PubMed] [Google Scholar]
- 23.Condeelis, J. (2001) Trends Cell Biol. 11, 288–293. [DOI] [PubMed] [Google Scholar]
- 24.Witke, W., Sharpe, A. H., Hartwig, J. H., Azuma, T., Stossel, T. P. & Kwiatkowski, D. J. (1995) Cell 81, 41–51. [DOI] [PubMed] [Google Scholar]
- 25.Mitchison, T. J. & Cramer, L. P. (1996) Cell 84, 371–379. [DOI] [PubMed] [Google Scholar]
- 26.Ridley, A. J. & Hall, A. (1992) Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- 27.Worthylake, R. A., Lemoine, S., Watson, J. M. & Burridge, K. (2001) J. Cell Biol. 154, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, P. (1997) Science 276, 75–81. [DOI] [PubMed] [Google Scholar]
- 29.Mori, R., Kondo, T., Nishie, T., Ohshima, T. & Asano, M. (2004) Am. J. Pathol. 164, 1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida, Y., Kondo, T., Takayasu, T., Iwakura, Y. & Mukaida, N. (2004) J. Immunol. 172, 1848–1855. [DOI] [PubMed] [Google Scholar]
- 31.Martin, P., D'Souza, D., Martin, J., Grose, R., Cooper, L., Maki, R. & McKercher, S. R. (2003) Curr. Biol. 13, 1122–1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.