Abstract
In response to DNA damage, the Rad6/Rad18 ubiquitin-conjugating complex monoubiquitinates the replication clamp proliferating cell nuclear antigen (PCNA) at Lys-164. Although ubiquitination of PCNA is recognized as an essential step in initiating postreplication repair, the mechanistic relevance of this modification has remained elusive. Here, we describe a robust in vitro system that ubiquitinates yeast PCNA specifically on Lys-164. Significantly, only those PCNA clamps that are appropriately loaded around effector DNA by its loader, replication factor C, are ubiquitinated. This observation suggests that, in vitro, only PCNA present at stalled replication forks is ubiquitinated. Ubiquitinated PCNA displays the same replicative functions as unmodified PCNA. These functions include loading onto DNA by replication factor C, as well as Okazaki fragment synthesis and maturation by the PCNA-coordinated actions of DNA polymerase δ, the flap endonuclease FEN1, and DNA ligase I. However, whereas the activity of DNA polymerase ζ remains unaffected by ubiquitination of PCNA, ubiquitinated PCNA specifically activates two key enzymes in translesion synthesis: DNA polymerase η, the yeast Xeroderma pigmentosum ortholog, and Rev1, a deoxycytidyl transferase that functions in organizing the mutagenic DNA replication machinery. We propose that ubiquitination of PCNA increases its functionality as a sliding clamp to promote mutagenic DNA replication.
Keywords: DNA replication, postreplication repair, translesion synthesis, ubiquitination, yeast
Modification of the replication clamp proliferating cell nuclear antigen (PCNA) has recently emerged as an important regulatory switch during DNA replication and DNA damage response. The critical site of modification on PCNA is Lys-164, and either sumoylation or ubiquitination at this residue can occur (1, 2). Although sumoylation at Lys-164 is proposed to be associated with normal replicative functions, monoubiquitination of this residue by the Rad6/Rad18 ubiquitin E2/E3 complex channels DNA damage into the postreplication DNA repair (PRR) pathway (1, 3, 4). The PRR pathway is comprised of two error-prone branches involving translesion synthesis (TLS) by error-prone DNA polymerases, and an error-free damage avoidance branch that proceeds by fork regression and template switching (5, 6). The latter branch depends on further modification of monoubiquitinated PCNA involving Rad5 and Mms2/Ubc13 E2/E3 catalyzed formation of polyubiquitin chains via an unusual Lys-63 ubiquitin linkage (1).
Replicative DNA polymerases of the B-family possess a very restrictive active site, which promotes high-fidelity DNA replication but inhibits bypass synthesis of many DNA lesions (5, 7, 8). On the other hand, Y-family DNA polymerases are particularly adapted to the bypass of DNA lesions because of a more open active site. For instance, DNA polymerase η (Pol η), the ortholog of the human Xeroderma pigmentosum protein, can accommodate a cis-syn pyrimidine dimer in its active site allowing facile damage bypass (9). TLS in yeast comprises a bifurcated pathway in which cis-syn pyrimidine dimers are primarily bypassed by Pol η (10, 11). Most other forms of DNA damage are bypassed by a more complex pathway involving the participation of three DNA polymerases, Pol δ, Pol ζ, and Rev1, and requiring additional activation by the Cdc7/Dbf4 protein kinase that normally functions in cell cycle progression (5, 12). It is this complex pathway that mainly contributes to DNA damage induced mutagenesis in eukaryotic cells. Although normally involved in lagging strand DNA replication, the high-fidelity Pol δ is also required for damage-induced mutagenesis. The functions of Pol ζ and Rev1 appear to be uniquely confined to mutagenesis. Pol ζ is an error-prone DNA polymerase that can bypass damage (13). Rev1 is a deoxycytidyl transferase that shows the highest catalytic activity opposite template guanines and abasic sites (14, 15). Rev1 is primarily responsible for inserting dC residues opposite abasic sites during mutagenesis (16–18). Several models have been proposed in which one DNA polymerase carries out the insertion step across the lesion, whereas a second polymerase extends from the lesion (19, 20).
Although it is clear that ubiquitination of PCNA is an initiating step in postreplication DNA repair, the precise function of ubiquitinated PCNA (PCNAUbi) has remained obscure. Several possible models for PCNAUbi can be envisaged. PCNAUbi may function in fork remodeling during preparation for TLS, e.g., it may inhibit further progression of the replicative DNA polymerases. Secondly, PCNAUbi may recruit translesion DNA polymerases to sites of DNA damage. Additionally, PCNAUbi could function as a specialized processivity factor for translesion DNA polymerases. Currently, these models have remained unexamined for lack of a biochemical source of PCNAUbi.
Studies in human cells show colocalization of PCNAUbi with Pol η at DNA damage foci that are consistent with, but do not prove, a model in which PCNAUbi recruits Pol η to sites of DNA damage (21, 22). Furthermore, although ubiquitination of PCNA is an essential step in mutagenesis, there is no evidence that PCNAUbi can actually function as a processivity factor for DNA polymerases in a manner similar to PCNA. The simple prediction would be that if PCNAUbi would retain this ability, it would function specifically in a complex with one or more translesion DNA polymerases (5). However, several studies have already demonstrated that efficient in vitro TLS of damaged DNA can be accomplished by a Y-class DNA polymerase in a complex with unmodified PCNA. These PCNA-stimulated error-prone polymerases include yeast and human Pol η, and human Pol ι and Pol κ (23–26). In addition, the mutagenic Pol ζ is also stimulated by PCNA (27). Therefore, data indicating that a TLS polymerase functions specifically with PCNAUbi remain unclear.
To determine the biochemical function of PCNAUbi, we have developed a robust assay for in vitro PCNA ubiquitination and have prepared and purified PCNAUbi on a scale permitting biochemical analysis. We report that ubiquitination does not appear to affect normal replicative functions of the replication clamp. However, surprisingly, PCNAUbi is a specific activator for two DNA polymerases, Pol η and Rev1, that function in the two defined branches of TLS.
Materials and Methods
DNA Substrates. Bluescript SKII+ ssDNA, prepared from Escherichia coli by using phagemid technology, was primed with ten 30-mer primers, roughly equally spaced along the circle (DECAprimed DNA). Bluescript ssDNA primed with a single RNA–DNA primer was used in the Okazaki fragment maturation assays (28). mp18 ssDNA primed with a single primer was used in the processivity assays (29). All oligonucleotide substrates were prepared as described (27). The linear oligonucleotide templates contained a biotin at both ends, to which a 2-fold molar excess of streptavidin was added. They are V9, 5′-Bio-CCT T TGCGA AT TCT25GCGGCTCCCT TCT TCTCCTCCCTCTCCCTTCCCT30-Bio; V9AP1, 5′-Bio-CCTTTGCGAATTCT25GCG0CTCCCTTCTTCTCCTCCCTCTCCCTTCCCT30-Bio; and V9AP2, 5′-Bio-CCTTTGCGAATTCT25GC0GCTCCCTTCTTCTCCTCCCTCTCCCTTCCCT30-Bio (where 0 indicates a tetrahydrofuran moiety and subscripts indicate homopolymeric runs). The primers are C12, 5′-AGGGAAGGGAGAGGGAGGAGAAGAAGGGAG; and C12–4, 5′-AGGGAAGGGAGAGGGAGGAGAAGAAG.
Enzymes. Replication protein A (RPA), replication factor C (RFC), PCNA, and mutant PCNAs, Pol δ, Pol ζ, and Pol η, were purified as described in refs. 27 and 30. Overexpression and purification of His6-ubiquitin, which also contained an S-affinity tag, was as described (31). Uba1, Rad6/Rad18, and Rev1 were purified as described (14, 32).
Ubiquitination of PCNA. The 20-μl standard assay contained 25 mM Hepes 7.6, 50 mM NaCl, 0.1 mg/ml BSA, 8 mM MgAc2, 1 mM ATP, 50 fmol of DECAprimed-SKII ssDNA, 2 pmol of RPA, 1 pmol of 32P-labeled PCNA (29), 600 fmol of RFC, 4 pmol of Rad6/Rad18, 1 pmol of Uba1, and 20 pmol of His6-ubiquitin at 30°C for 30 min. Samples were run on a 11% SDS/polyacrylamide gel, followed by STORM PhosphorImager (Molecular Dynamics) analysis.
Preparative ubiquitination of untagged, nonradioactive PCNA was carried out by directly scaling up the above assay to 20 ml, except that 1.6 mM ATP was used, and the incubation was for 45 min. At that time, ≈60–90% of the PCNA monomers were ubiquitinated by SDS/PAGE analysis (three preparations). Ubiquitinated intermediates of Uba1 and Rad6/Rad18 were discharged with 10 mM DTT for 10 min at 30°C, and the reaction was diluted 2-fold and adjusted to 250 mM NaCl. PCNAUbi was batch-bound to a 1-ml Ni/agarose column in buffer A (25 mM Hepes·NaOH, pH 7.6/250 mM NaCl) containing 5 mM imidazole. The column was washed with 5 ml of this buffer and step-eluted with buffer A containing 250 mM imidazole. The eluate was diluted with 1.5 vol of buffer B (30 mM Hepes·NaOH, pH 7.6/1 mM EDTA/1 mM DTT/0.02% E10C12 (a nonionic detergent)/5% glycerol/5 mM NaHSO3), loaded on a 0.8-ml MiniQ column in B plus 100 mM NaCl, and eluted by using a 100 to 500 mM NaCl gradient in buffer B. PCNAUbi eluted at ≈260 mM NaCl before unmodified PCNA.
DNA Replication and Damage Bypass Assays. Standard 5-min assays contained 40 mM Tris·HCl (pH 7.8), 0.2 mg/ml BSA, 8 mM MgAc2, 100 μM each dNTPs, 0.1 mM ATP, 100 mM NaCl, 100 fmol of DNA substrate (the primer was 5′ labeled with 32P), 1 pmol of RPA, 200 fmol of RFC, and 300 fmol of PCNA or PCNAUbi unless otherwise indicated. After preincubation with RFC at 30°C for 30 sec to allow clamp loading, reactions were started by the addition of the appropriate DNA polymerase (200 fmol). Reaction products were analyzed on a 12% polyacrylamide/7 M urea gel. Gels were quantitated by using a STORM PhosphorImager and imagequant software (Molecular Dynamics).
Results and Discussion
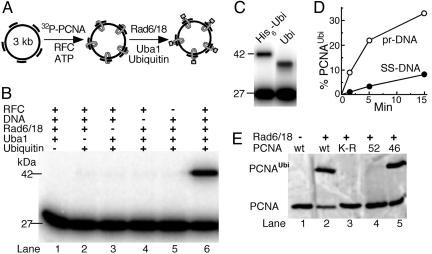
Ubiquitination of PCNA Requires That It Encircle the DNA. Previous in vitro ubiquitination studies of human PCNA showed that limited monoubiquitination could be achieved in the absence of effector DNA (22). However, the observation that only a small fraction of the cellular PCNA pool is actually ubiquitinated suggested to us that perhaps only PCNA bound at stalled replication forks is ubiquitinated (1). Indeed, in our in vitro ubiquitination assay, significant PCNA ubiquitination was only detected when PCNA encircled the effector DNA (Fig. 1 A and B). DNA-independent ubiquitination proceeded at 1–2% of the DNA-dependent rate and was not further investigated. Western blot analysis with antibodies against ubiquitin showed that the 42-kDa species contained ubiquitin (data not shown). In our studies, we used a ≈13-kDa derivative of yeast ubiquitin that contains a 4.5-kDa N-terminal fusion including the His6 purification tag (designated in Materials and Methods as His6-ubiquitin). Such N-terminal ubiquitin fusions retain full functionality in vivo (33). The observed shift in mobility from 27 to 42 kDa is consistent with the addition of a single His6-ubiquitin moiety. When human ubiquitin (8.5 kDa) was used in the ubiquitination assay, an expectedly smaller shift of mobility, from 27 to 37 kDa, was observed (Fig. 1C). Efficient linkage of human ubiquitin to yeast PCNA also requires that PCNA encircles the DNA (data not shown). Only one ubiquitin residue was added per PCNA monomer because no slower migrating species were observed.
Fig. 1.
DNA-bound PCNA is ubiquitinated at Lys-164. (A) Scheme of the assay. (B) Factor requirement for efficient PCNA ubiquitination. (C) Human ubiquitin is linked to PCNA. The standard PCNA ubiquitination assay (described in Materials and Methods) contained either the tagged yeast ubiquitin (His6-Ubi, left lane) or human ubiquitin (right lane). (D) Time course of ubiquitination. Assays were carried out on unprimed or DECAprimed ssDNA. (E) Ubiquitination of mutant PCNAs. Standard assays contained unlabeled PCNA or the indicated mutant PCNA. Detection was by Western blot analysis with rabbit antibodies to PCNA.
Our complete assay includes a multiple primed ssDNA circle, the yeast ssDNA-binding protein RPA, His6-ubiquitin, ubiquitin activating enzyme (Uba1), Rad6/Rad18, PCNA, and RFC, the loader of PCNA onto DNA. The Rad18 subunit of Rad6/Rad18 is a DNA-binding protein with a binding preference for ssDNA (34). Rad18 also interacts with PCNA (1). However, Rad6/18 bound to DNA is ineffective in ubiquitinating PCNA that is not loaded onto the DNA by RFC (Fig. 1B, compare lane 5 with lane 6). As expected, because RFC loads PCNA preferentially onto template–primer junctions, unprimed ssDNA was a poor effector for ubiquitination (Fig. 1D).
Studies in yeast and humans have found detectable ubiquitination only on Lys-164 (1, 21). Similarly, in our in vitro assay, addition of ubiquitin occurred uniquely at Lys-164, because a PCNA mutant protein with a K164R mutation was not ubiquitinated (Fig. 1E, lane 3). In replication assays, this mutant PCNA functioned as a fully active processivity factor (data not shown). A PCNA mutant (pcna-52, S115P) that is a monomer rather than a trimer, and is not stably loaded onto DNA (30), was not ubiquitinated, supporting our conclusion that only PCNA encircling the DNA is subject to ubiquitination (Fig. 1E, lane 4). In vivo, the pol30–52 allele shows defects in DNA replication, DNA repair, and mutagenesis (30). The damage avoidance pathway of postreplication DNA repair is activated by Lys-63-linked polyubiquitination of monoubiquitinated PCNA in a Rad5/Mms2/Ubc13-dependent manner (1). We have previously identified a PCNA mutant (pol30–46) that is still functional for TLS but is defective for the damage avoidance branch (35). As expected, this mutant PCNA was still monoubiquitinated like wild type (Fig. 1E, lane 5).
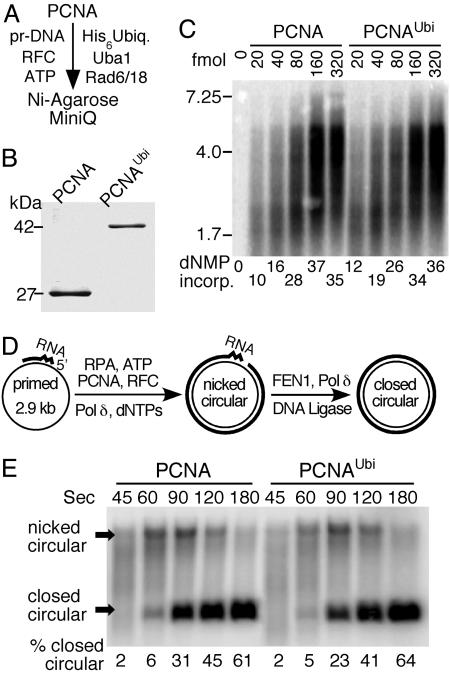
PCNA and PCNAUbi Show Similar Functional Interactions with Replication Factors. Having established a robust system for PCNA ubiquitination, we scaled up the assay 1,000-fold. Surprisingly, although we observed maximally 30–40% ubiquitination of input PCNA in our analytical assays (Fig. 1B), the large-scale reactions went to 60–90% completion (three independent 20-ml preparations). We observed that, in the analytical assays, both Rad6/Rad18 and RFC are prone to rapid inactivation, and we attributed this inactivation to air oxidation. Because thiol reagents such as DTT inhibit ubiquitination by discharging the thiolated intermediates, we tried to exclude oxidation by degassing buffers and inclusion of argon but with minor success. Therefore, we do not understand yet how in the large-scale assays the enzymes retain activity during extended incubations. After two-column chromatographic steps, we obtained ≈50 μg of pure PCNAUbi (Fig. 2 A and B). Mass spectrometry analysis (MALDI-TOF) confirmed that the purified PCNAUbi was monoubiquitinated (data not shown).
Fig. 2.
Biochemical properties of PCNAUbi in DNA replication. (A) Purification scheme. Pr-DNA is DECAprimed Bluescript SKII ssDNA. (B) Gel analysis (11% SDS/PAGE) of PCNA and purified PCNAUbi. Staining was with colloidal Coomassie blue. Scanning of the gel showed that ≈3% nonubiquitinated PCNA remained in the purified preparation. (C) Replication of RPA-coated singly primed mp18 ssDNA (60-fmol circles) by Pol δ (120 fmol) and RFC (120 fmol) with increasing PCNA or PCNAUbi was performed essentially as described (38). [α-32P]dTTP was used as radioactive tracer. The PCNA was loaded by RFC for 1 min at 30°C, Pol δ was added, and incubation continued for an additional 90 sec. Products were analyzed on a 1% alkaline agarose gel. (D) Scheme for measuring Okazaki fragment maturation kinetics. (E) Replication and maturation assays of RPA-coated circular ssDNA (100 fmol), primed with an RNA8DNA22 primer, by RFC (200 fmol), PCNA or PCNAUbi (300 fmol), Pol δ (200 fmol), FEN1 (200 fmol), and DNA ligase I (500 fmol) were exactly as described (28). Replication products after the indicated times were separated on a 1% agarose gel in the presence of 0.5 μg/ml ethidium bromide. This method separates covalently closed circular DNA from nicked circular DNA. [α-32P]dTTP was used as radioactive tracer.
Whether PCNAUbi is only functional in TLS as a fully ubiquitinated trimer, i.e., with one ubiquitin on each monomer, or whether a PCNA trimer with only one monomer ubiquitinated is active for TLS still remains to be determined. In vivo, the ubiquitinated form of human PCNA that is found to be associated with Pol η in response to DNA damage contains ubiquitin on all three monomers (21). Because our preparation of PCNAUbi is ubiquitinated on each PCNA monomer, the effect of this modification on PCNA function can be unambiguously determined.
PCNA is loaded onto template-primer DNA by RFC in an ATP-dependent manner. RFC has a low DNA-dependent ATPase activity, which is strongly stimulated by PCNA, presumably as a result of PCNA loading onto the template-primer DNA (36). PCNA and PCNAUbi stimulated the ATPase activity of RFC with identical stoichiometry and kinetics, suggesting that loading of PCNA was not affected upon ubiquitination (data not shown).
PCNA functions as a processivity factor in both leading- and lagging-strand DNA replication (reviewed in ref. 37). We determined that PCNA ubiquitination did not alter its properties as a processivity factor for DNA polymerase δ (Pol δ). The PCNA–Pol δ complex replicates DNA at a rate of ≈50 nt/sec at 30°C (38). During a 90-sec incubation, the average complex replicates ≈4–5 kb of the 7.3-kb mp18 template used in this study. Neither the stoichiometry of complex formation nor the rate or processivity of replication was affected by ubiquitination of PCNA, as shown by titrating increasing levels of either clamp into the replication assay (Fig. 2C). We observed that the putative leading strand DNA polymerase, Pol ε, was also stimulated similarly by PCNA and PCNAUbi (data not shown).
Lagging-strand DNA replication is a complex process requiring the interplay of many enzymes. The elongation and maturation phases of this process depend on the proper coordination between Pol δ, the flap endonuclease FEN1, and DNA ligase I (39, 40). Each of these three enzymes carries out its respective function (elongation, RNA primer removal, and nick closure) in a complex with PCNA (28, 41, 42). We previously established a model assay system that allows us to study Okazaki fragment elongation and maturation (Fig. 2D) (28). When PCNAUbi replaced PCNA in this assay, we observed similar kinetics of Okazaki fragment elongation and maturation, as demonstrated by the formation of covalently closed DNA (Fig. 2E). Therefore, having determined that ubiquitination of PCNA was not associated with loss of replicative function, we investigated whether ubiquitination endowed an additional function upon PCNA.
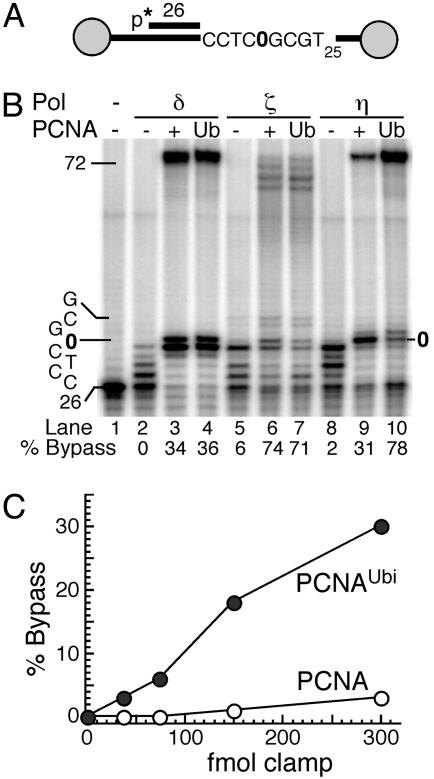
PCNAUbi Activates TLS Polymerases. TLS by error-prone DNA polymerases consists of two subpathways (5). Pol η is largely responsible for the relatively error-free bypass of cis-syn pyrimidine dimers. Most other types of damage are bypassed by the replicative Pol δ in combination with the error-prone Pol ζ and the Rev1 deoxycytidyl transferase, and this bypass forms the molecular basis for damage-induced mutagenesis in the cell. Notably, three of these four enzymes, Pol δ, Pol η, and Pol ζ, carry out both processive DNA synthesis and damage bypass with unmodified PCNA as sliding clamp (27, 43). We determined the effect of PCNA ubiquitination on TLS by these three DNA polymerases and by Rev1. A linear oligonucleotide-based system was used in which the template strand contained a model abasic site at a well defined position in the template. To prevent PCNA from sliding off the DNA, it was anchored onto the linear DNA template by biotin-streptavidin termini (Fig. 3A) (27). In the standard assay, the DNA was coated with the yeast ssDNA-binding protein, RPA. Subsequently, PCNA or PCNAUbi was loaded by RFC, and then DNA synthesis was initiated at t = 0 by the addition of the DNA polymerase.
Fig. 3.
PCNAUbi activates TLS of abasic sites by Pol η.(A) Scheme of substrate V9AP1/C12-4. The gray circles indicate the biotin-streptavidin blocks. The abasic site is shown as 0. (B) Stimulation of bypass by PCNA or PCNAUbi. Standard assays (described in Materials and Methods) were for 5 min with the indicated polymerase and the indicated form of PCNA. The template sequence of interest is shown at left. (C) PCNAUbi stimulates abasic bypass by Pol η. Standard assays with increasing clamp levels were carried out for 60 sec at 30°C. Full-length replication products as the percentage of total primer extension products were quantitated.
Replication of undamaged DNA by Pol δ, Pol ζ, or by Pol η was stimulated by PCNA. Essentially the same degree of stimulation was achieved when PCNA was substituted by PCNAUbi (data not shown). However, when the template contained an abasic site, the results were distinctly different. We still observed no significant difference between PCNA and PCNAUbi as the processivity factor for TLS by Pol δ or by Pol ζ (Fig. 3B). In contrast, Pol η was substantially more efficient in TLS with PCNAUbi as the clamp than with PCNA (78% vs. 31% bypass after 5 min; Fig. 3B). After 60 sec of synthesis, when rates of bypass were linear, even a 3-fold molar excess of unmodified PCNA was ineffective in mediating TLS compared with PCNAUbi (Fig. 3C).
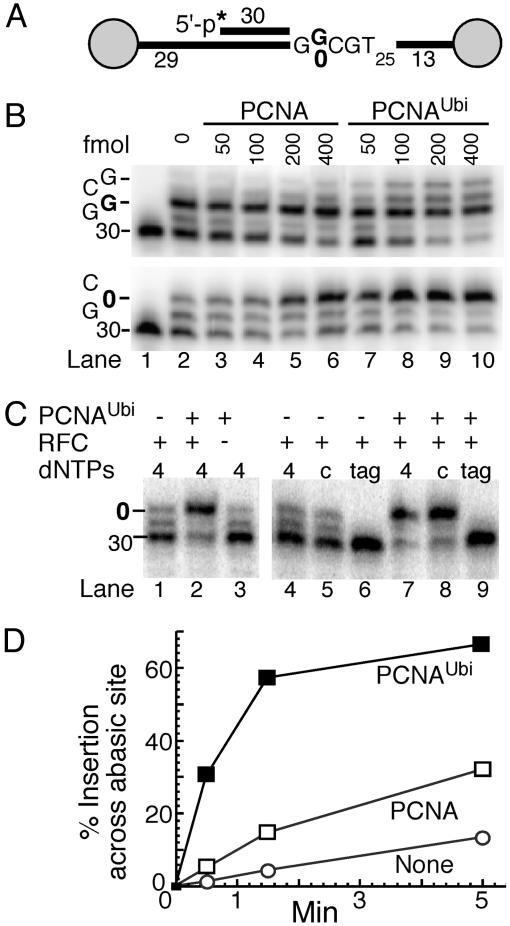
Insertion of cytosine residues opposite abasic sites by Rev1 constitutes over one-half of the bypass events in yeast (16–18). Therefore, we next investigated whether Rev1 was responsive to the modified clamp. Rev1 is primarily active opposite template guanine residues and abasic sites, and it has very low catalytic activity and low processivity (14, 15). Compared with PCNA, PCNAUbi very efficiently activated DNA synthesis by Rev1 on a G-rich template, and a similar increase in synthesis was observed across an abasic site (Fig. 4 A and B). These dose–response experiments displayed saturation behavior for both PCNA and PCNAUbi when the clamp concentration exceeded the DNA concentration, suggesting that the clamp needed to be loaded onto the DNA to exert its stimulatory effect. Indeed, control experiments showed that activation of Rev1 activity by PCNAUbi required that it was loaded around the DNA by RFC (Fig. 4C, lanes 2 and 3). Furthermore, the catalytic properties of Rev1 were not altered by PCNAUbi. Rev1 still exclusively inserted dCTP residues, as no incorporation was observed when only dATP, dGTP, and dTTP were included in the assay (lanes 6 and 9). From the observed rates of insertion across the abasic site, we conclude that PCNA and PCNAUbi activate Rev1 ≈2.5- and ≈15-fold, respectively (Fig. 4D).
Fig. 4.
PCNAUbi activates TLS of abasic sites by Rev1. (A) Scheme of substrates V9/C12 and V9AP2/C12. The abasic site is shown as 0.(B) PCNAUbi stimulates Rev1. Standard assays (described in Materials and Methods) contained either the normal template (Upper) or the abasic site template (Lower) and the indicated levels of clamp. The template sequence of interest is shown at left. (C) Control assays for PCNAUbi-mediated Rev1 activity. Either PCNAUbi (lane 1) or RFC (lane 3) was omitted from the standard assay with Rev1 (lane 2). Standard assays without PCNA (lanes 4–6) or with PCNAUbi (lanes 7–9) contained all four dNTPs (4, lanes 4 and 7), only dCTP (c, lanes 5 and 8), or dTTP, dATP, and dGTP (tag, lanes 6 and 9). (D) Time-course assays of PCNAUbi-mediated Rev1 activity at the abasic site. Standard assays were followed with time and insertion opposite the abasic site as percentage of total products was quantitated by using PhosphorImager analysis.
In the experiment in Fig. 4, Rev1 was required to insert one nucleotide opposite a template G before reaching the abasic site (“running start”). However, during DNA replication, the replicative DNA polymerase is expected to replicate up to the abasic site, and Rev1 may be required to act only across the abasic site. Therefore, we also measured TLS by Rev1 when the abasic site was positioned directly after the primer terminus (“standing start”). PCNAUbi, but not PCNA, was effective in accelerating insertion across the abasic site (Fig. 5). In three independent experiments, PCNA stimulated Rev1 activity 1.1- to 1.5-fold, and PCNAUbi stimulated it 3- to 7-fold. DNA synthesis by Rev1 beyond the abasic site was not observed under any condition, even when a preferred guanine template base was available directly after the abasic site (Fig. 5B).
Fig. 5.
PCNAUbi activates Rev1 during “standing start” TLS. (A) Scheme of substrate V9AP1/C12. The abasic site is shown as 0.(B) Time course of PCNAUbi-mediated Rev1 activity at an abasic site. Standard assays were used (described in Materials and Methods), and insertion opposite the abasic site (% TLS) was quantitated.
These TLS data show that PCNAUbi has acquired an increased functional interaction with two specific TLS DNA polymerases, Pol η and Rev1. In contrast, attesting to the specificity of this interaction, neither TLS nor normal replication by Pol δ was affected by PCNA modification (Figs. 2 C and E and 3B). Surprisingly, and contrary to general prediction, although Pol ζ is stimulated by PCNA, its ubiquitination does not affect this stimulation at an abasic site or at sites of UV damage (Fig. 3B and data not shown) (5, 27). The binding domain(s) responsible for the functional interactions of PCNAUbi with TLS polymerases remains to be elucidated. Possibly, such a domain could overlap with the motif responsible for interaction with unmodified PCNA. However, although Pol η has a close match to the PCNA consensus-binding motif, QxxLxxFF, neither Pol ζ nor Rev1 appear to possess this consensus-binding motif.
Does PCNAUbi Form an Organizing Center for TLS? Our studies suggest a unique function for PCNAUbi in TLS. The biochemical observation that only PCNA trimers encircling the DNA are ubiquitinated suggests that in the cell only the PCNA present at replication forks is modified. It remains to be established whether the ubiquitination machinery is specifically targeted to stalled replication forks, thereby restricting TLS, by means of PCNA ubiquitination, to damaged regions only. If this targeting mechanism is to work efficiently, it is essential that the replication fork actually stalls at sites of DNA damage, thereby providing a window of opportunity for Rad6/Rad18-dependent ubiquitination. Stalling may be ensured when abasic damage occurs on the leading strand because this lesion completely blocks bypass replication by the putative leading-strand polymerase, Pol ε, regardless of the modification state of PCNA (data not shown). Abasic site damage on the lagging strand does not form a complete block for Pol δ (Fig. 3B). However, the prolonged stalling observed may be of sufficient duration to initiate the ubiquitination response. UV damage forms a more permanent block for Pol δ that is neither overcome by the presence of PCNA or PCNAUbi (data not shown) (27). Interestingly, PCNAUbi acts on both branches of TLS through its function as a specialized processivity factor for Pol η and for Rev1. Of these two branches, the one with Rev1 requires the participation of multiple DNA polymerases.
Recent advances in the study of TLS have led to the proposal of the two-polymerase model for damage bypass (19, 20, 44). In this model, insertion opposite the site of damage is carried out by one DNA polymerase while a second DNA polymerase is responsible for extension past the lesion. Mammalian Rev1 occupies a unique position in TLS because its C terminus interacts with several Y-family DNA polymerases as well as with Pol ζ (45). The analogous interactions in yeast still remain to be demonstrated. We propose that PCNAUbi may serve as a loading pad for Rev1. Through its bifunctional properties, interaction with PCNAUbi on one hand and with Pol ζ on the other hand, Rev1 may serve to initiate mutagenic translesion synthesis at stalled replication forks (45). In support of the importance of Rev1-mediated protein–protein interactions in TLS are studies showing that TLS is severely crippled by REV1 mutations that abrogate the BRCT domain but retain catalytic activity; in contrast, a double-point mutation that only inactivates the 2-prime-deoxycytidine 5-prime-triphosphate (dCMP) transferase activity has less severe consequences for TLS (18, 46).
Acknowledgments
We thank Drs. Christopher Lawrence, Satya and Louise Prakash, Dorota Skowyra, and Linda Hicke for expression plasmids; Alaji Bah for initial studies with pcna-K164R; Carrie Stith for expert technical assistance; and John Majors for critical discussions. This work was supported in part by National Institutes of Health Grant GM32431 (to P.M.B.) and a Kauffman Foundation Fellowship (to P.G.).
Author contributions: P.G. and P.M.B. designed research, performed research, analyzed data, and wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PCNA, proliferating cell nuclear antigen; PCNAUbi, ubiquitinated PCNA; RFC, replication factor C; RPA, replication protein A; TLS, translesion synthesis.
References
- 1.Hoege, C., Pfander, B., Moldovan, G. L., Pyrowolakis, G. & Jentsch, S. (2002) Nature 419, 135–141. [DOI] [PubMed] [Google Scholar]
- 2.Papouli, E., Chen, S., Davies, A. A., Huttner, D., Krejci, L., Sung, P. & Ulrich, H. D. (2005) Mol. Cell 19, 123–133. [DOI] [PubMed] [Google Scholar]
- 3.Stelter, P. & Ulrich, H. D. (2003) Nature 425, 188–191. [DOI] [PubMed] [Google Scholar]
- 4.Haracska, L., Torres-Ramos, C. A., Johnson, R. E., Prakash, S. & Prakash, L. (2004) Mol. Cell. Biol. 24, 4267–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakash, S., Johnson, R. E. & Prakash, L. (2005) Annu. Rev. Biochem. 74, 317–353. [DOI] [PubMed] [Google Scholar]
- 6.Smirnova, M. & Klein, H. L. (2003) Mutat. Res. 532, 117–135. [DOI] [PubMed] [Google Scholar]
- 7.Franklin, M. C., Wang, J. & Steitz, T. A. (2001) Cell 105, 657–667. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel, T. A. (2004) J. Biol. Chem. 279, 16895–16898. [DOI] [PubMed] [Google Scholar]
- 9.Trincao, J., Johnson, R. E., Escalante, C. R., Prakash, S., Prakash, L. & Aggarwal, A. K. (2001) Mol. Cell 8, 417–426. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, R. E., Prakash, S. & Prakash, L. (1999) Science 283, 1001–1004. [DOI] [PubMed] [Google Scholar]
- 11.Masutani, C., Araki, M., Yamada, A., Kusumoto, R., Nogimori, T., Maekawa, T., Iwai, S. & Hanaoka, F. (1999) EMBO J. 18, 3491–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pessoa-Brandao, L. & Sclafani, R. A. (2004) Genetics 167, 1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson, J. R., Lawrence, C. W. & Hinkle, D. C. (1996) Science 272, 1646–1649. [DOI] [PubMed] [Google Scholar]
- 14.Nelson, J. R., Lawrence, C. W. & Hinkle, D. C. (1996) Nature 382, 729–731. [DOI] [PubMed] [Google Scholar]
- 15.Haracska, L., Prakash, S. & Prakash, L. (2002) J. Biol. Chem. 277, 15546–15551. [DOI] [PubMed] [Google Scholar]
- 16.Zhao, B., Xie, Z., Shen, H. & Wang, Z. (2004) Nucleic Acids Res. 32, 3984–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs, P. E., McDonald, J., Woodgate, R. & Lawrence, C. W. (2005) Genetics 169, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otsuka, C., Kunitomi, N., Iwai, S., Loakes, D. & Negishi, K. (2005) Mutat. Res. 578, 79–87. [DOI] [PubMed] [Google Scholar]
- 19.Prakash, S. & Prakash, L. (2002) Genes Dev. 16, 1872–1883. [DOI] [PubMed] [Google Scholar]
- 20.Friedberg, E. C., Lehmann, A. R. & Fuchs, R. P. (2005) Mol. Cell 18, 499–505. [DOI] [PubMed] [Google Scholar]
- 21.Kannouche, P. L., Wing, J. & Lehmann, A. R. (2004) Mol. Cell 14, 491–500. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe, K., Tateishi, S., Kawasuji, M., Tsurimoto, T., Inoue, H. & Yamaizumi, M. (2004) EMBO J. 23, 3886–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haracska, L., Kondratick, C. M., Unk, I., Prakash, S. & Prakash, L. (2001) Mol. Cell 8, 407–415. [DOI] [PubMed] [Google Scholar]
- 24.Haracska, L., Johnson, R. E., Unk, I., Phillips, B. B., Hurwitz, J., Prakash, L. & Prakash, S. (2001) Proc. Natl. Acad. Sci. USA 98, 14256–14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haracska, L., Unk, I., Johnson, R. E., Phillips, B. B., Hurwitz, J., Prakash, L. & Prakash, S. (2002) Mol. Cell. Biol. 22, 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidal, A. E., Kannouche, P. P., Podust, V. N., Yang, W., Lehmann, A. R. & Woodgate, R. (2004) J. Biol. Chem. 279, 48360–48368. [DOI] [PubMed] [Google Scholar]
- 27.Garg, P., Stith, C. M., Majka, J. & Burgers, P. M. (2005) J. Biol. Chem. 280, 23446–23450. [DOI] [PubMed] [Google Scholar]
- 28.Ayyagari, R., Gomes, X. V., Gordenin, D. A. & Burgers, P. M. (2003) J. Biol. Chem. 278, 1618–1625. [DOI] [PubMed] [Google Scholar]
- 29.Eissenberg, J. C., Ayyagari, R., Gomes, X. V. & Burgers, P. M. (1997) Mol. Cell. Biol. 17, 6367–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayyagari, R., Impellizzeri, K. J., Yoder, B. L., Gary, S. L. & Burgers, P. M. (1995) Mol. Cell. Biol. 15, 4420–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beal, R., Deveraux, Q., Xia, G., Rechsteiner, M. & Pickart, C. (1996) Proc. Natl. Acad. Sci. USA 93, 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailly, V., Lauder, S., Prakash, S. & Prakash, L. (1997) J. Biol. Chem. 272, 23360–23365. [DOI] [PubMed] [Google Scholar]
- 33.Ling, R., Colon, E., Dahmus, M. E. & Callis, J. (2000) Anal. Biochem. 282, 54–64. [DOI] [PubMed] [Google Scholar]
- 34.Bailly, V., Lamb, J., Sung, P., Prakash, S. & Prakash, L. (1994) Genes Dev. 8, 811–820. [DOI] [PubMed] [Google Scholar]
- 35.Torresramos, C., Yoder, B. L., Burgers, P. M., Prakash, S. & Prakash, L. (1996) Proc. Natl. Acad. Sci. USA 93, 9676–9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes, X. V., Schmidt, S. L. & Burgers, P. M. (2001) J. Biol. Chem. 276, 34776–34783. [DOI] [PubMed] [Google Scholar]
- 37.Garg, P. & Burgers, P. M. (2005) Crit. Rev. Biochem. Mol. Biol. 40, 115–128. [DOI] [PubMed] [Google Scholar]
- 38.Burgers, P. M. & Gerik, K. J. (1998) J. Biol. Chem. 273, 19756–19762. [DOI] [PubMed] [Google Scholar]
- 39.Hubscher, U. & Seo, Y. S. (2001) Mol. Cells 12, 149–157. [PubMed] [Google Scholar]
- 40.Kao, H. I. & Bambara, R. A. (2003) Crit. Rev. Biochem. Mol. Biol. 38, 433–452. [DOI] [PubMed] [Google Scholar]
- 41.Maga, G., Villani, G., Tillement, V., Stucki, M., Locatelli, G. A., Frouin, I., Spadari, S. & Hubscher, U. (2001) Proc. Natl. Acad. Sci. USA 98, 14298–14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin, D. S., McKenna, A. E., Motycka, T. A., Matsumoto, Y. & Tomkinson, A. E. (2000) Curr. Biol. 10, 919–922. [DOI] [PubMed] [Google Scholar]
- 43.Haracska, L., Johnson, R. E., Unk, I., Phillips, B., Hurwitz, J., Prakash, L. & Prakash, S. (2001) Mol. Cell. Biol. 21, 7199–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodgate, R. (2001) Mutat. Res. 485, 83–92. [DOI] [PubMed] [Google Scholar]
- 45.Guo, C., Fischhaber, P. L., Luk-Paszyc, M. J., Masuda, Y., Zhou, J., Kamiya, K., Kisker, C. & Friedberg, E. C. (2003) EMBO J. 22, 6621–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson, J. R., Gibbs, P. E., Nowicka, A. M., Hinkle, D. C. & Lawrence, C. W. (2000) Mol. Microbiol. 37, 549–554. [DOI] [PubMed] [Google Scholar]