Abstract
Type 1 diabetes is a T cell-mediated autoimmune disease, and insulin is an important target of the autoimmune response associated with β cell destruction. The mechanism of destruction is still unknown. Here, we provide evidence for CD8 T cell autoreactivity associated with recurrent autoimmunity and loss of β cell function in type 1 diabetic islet transplant recipients. We first identified an insulin B chain peptide (insB10-18) with extraordinary binding affinity to HLA-A2(*0201) that is expressed by the majority of type 1 diabetes patients. We next demonstrated that this peptide is naturally processed by both constitutive and immuno proteasomes and translocated to the endoplasmic reticulum by the peptide transporter TAP1 to allow binding to HLA-A2 in the endoplasmic reticulum and cell surface presentation. Peripheral blood mononuclear cells from a healthy donor were primed in vitro with this peptide, and CD8 T cells were isolated that specifically recognize target cells expressing the insulin B chain peptide. HLA-A2insB10-18 tetramer staining revealed a strong association between detection of autoreactive CD8 T cells and recurrent autoimmunity after islet transplantation and graft failure in type 1 diabetic patients. We demonstrate that CD8 T cell autoreactivity is associated with β cell destruction in type 1 diabetes in humans.
Keywords: insulin, islet transplantation, cytotoxic T lymphocyte, tetramer
We and others (1–3) have demonstrated the presence of circulating islet autoreactive CD4+ T cells around the clinical onset of disease, but evidence for a role of these T cells in β cell destruction is lacking. The nonobese diabetic (NOD) mouse strain spontaneously develops diabetes in part owing to the activity of CD8+ T cells. This phenomenon was first demonstrated by the finding that MHC class I-deficient NOD mice were completely type 1 diabetes-resistant (4, 5). Subsequent analyses indicated that MHC class I-dependent T cell responses are an essential component of both the initiation and progression of pancreatic β cell destruction, ultimately leading to type 1 diabetes development in NOD mice (6, 7).
Transgenic expression of HLA-A*0201 significantly accelerates type 1 diabetes onset in NOD mice, with HLA-A*0201-restricted CD8 T cells appearing in early, prediabetic insulitic lesions. These results provide functional evidence that the HLA-A*0201 allele contributes to type 1 diabetes development (8). The HLA-A*0201 allele has been shown to confer additional risk to the development of type 1 diabetes in patients possessing the high-risk class II alleles HLA-DR3 and HLA-DR4 (9). HLA-A*0201 is one of the most prevalent class I alleles, with a frequency of >60% in type 1 diabetic patients (9, 10).
Autoreactive cytotoxic T cells recognize peptide epitopes displayed on the β cell surface in the context of HLA class I molecules. These epitopes are considered to be derived primarily from β cell proteins, but their identity in humans remains largely unknown (11, 12). Islet antigen-specific human CD8 T cells have been documented only twice, to a peptide from glutamic acid decarboxylase (12) and a peptide from islet amyloid polypeptide (or amylin) (11), but functional evidence for β cell cytotoxicity or association with the pathogenesis of type 1 diabetes is lacking. The identification of islet antigen-specific cytotoxic T lymphocytes (CTLs) is critical because they improve the prospects of immunodiagnosis and immunotherapy in type 1 diabetes.
In NOD mice, the majority of CD4+-infiltrating T cells recognize insulin, and of these T cells >90% react with amino acids 9–23 of the insulin B chain in the development of autoimmune diabetes in NOD mice (13, 14). This epitope appears to be critical in the pathogenesis (15). Similarly, T cell reactivity to the human equivalent of the insulin B chain was frequently detectable in diabetic patients (16, 17), and insulin was suggested to be a major target in T cells in pancreas-draining lymph nodes of longstanding type 1 diabetes patients (18). Because the insulin B chain is expressed only in pancreatic β cells, peptides derived from the insulin B chain should be a major target for cytotoxic T cells, causing β cell destruction. The present study identifies CD8 epitopes from the insulin B chain in type 1 diabetes for the class I allele HLA-A*0201. We demonstrate that the combination of computer-based epitope prediction and in vitro proteasomal processing enabled the identification of an HLA-A*0201-restricted epitope derived from the insulin B chain.
Materials and Methods
Epitope Prediction. Peptides of the human insulin B chain that potentially bind to HLA-A*0201 were predicted by using the HLA-A2 peptide binding motif and software as described (19).
Peptide Synthesis. Peptides were synthesized by using fluorenylmethoxycarbonyl amino acids and O-benzotriazol-1-yloxytris(pyrrolidino)phosphonium hexafluorophosphate/N-methylmorpholine chemistry. Synthetic peptides were analyzed by RP-HPLC (purity was at least 85%) and MALDI-TOF MS (expected masses were confirmed) (20).
Peptide-HLA Binding. Peptide binding was assessed as described (21). In short, a mixture of 100 fmol of fluorescein (FL)-labeled HLA-A*0201 binding peptide, 200 fmol of recombinant HLA-A*0201 heavy chain, 15 pmol of β2 microglobulin, and serial dilutions of the test peptides in a buffer of 100 mM sodium phosphate, 75 mM NaCl, and 1 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (100 μl end volume) was incubated 24 h at room temperature. HLA-bound and nonbound FL-labeled peptides were separated by HPLC gel permeation chromatography with fluorescence detection. The concentration at which the test peptide reduced the amount of HLA-bound FL-labeled peptide by 50% (IC50) was calculated by using nonlinear regression analysis.
Transporter Associated with Antigen Presentation (TAP) Translocation Assays. The TAP-dependent translocation assay was performed as described (22). In short, peptides of interest were tested for their ability to compete for TAP-dependent translocation of a 125I-iodinated model peptide in streptolysin O-permeabilized EL-4 cells.
Generation of HLA-A2-Peptide Tetramers. Tetrameric HLA-A2-peptide complexes were prepared essentially as described (23). Briefly, recombinant HLA-A2 and human β2 microglobulin, produced in Escherichia coli, were solubilized in urea and injected together with each synthetic peptide into a refolding buffer consisting of 100 mM Tris (pH 8.0), 400 mM arginine, 2 mM EDTA, 5 mM reduced glutathione, and 0.5 mM oxidized glutathione. Refolded complexes were purified by anion exchange chromatography using DE52 resin (Whatman) followed by gel filtration on a Superdex 75 column (Amersham Pharmacia Biotech). The refolded HLA-A*0201-peptide complexes were biotinylated by incubation for 16 h at 30°C with BirA enzyme (Avidity, Denver). Tetrameric HLA-peptide complexes were produced by the stepwise addition of extravidin-conjugated phycoerythrin (PE) (Sigma) to achieve a 1:4 molar ratio (extravidin-PE/biotinylated HLA class I).
In Vitro Proteasome-Mediated Digestions. 20S proteasomes were purified from a Epstein–Barr virus (immuno proteasome) and a HeLa cell line (constitutive proteasome) as described (24). Low molecular mass polypeptide 2 (LMP2) and LMP7 contents were confirmed by 2D immunoblotting (data not shown). To assess kinetics, digestions were performed during different incubation periods. The insulin B chain peptides (30-mer, 20 μg) were incubated with 1 μg of purified proteasome at 37°C for 2 and 20 h in 300 μl of proteasome digestion buffer (25). Trifluoroacetic acid (1%; 30 μl) was added to stop the digestion, and samples were stored at –20°C until MS analysis.
Generation and Isolation of Insulin B Chain10-18-Specific CD8+ T Cells. Human HLA-A2 homozygous peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient from HLA-typed buffy coats, obtained from healthy blood donors. Monocytes were cultured in RPMI medium 1640 (GIBCO/Life Technologies, Breda, The Netherlands) with 10% heat-inactivated FCS and penicillin/streptomycin supplemented with 1,000 units/ml of IL-4 (Strathmann Biotec, Hannover, Germany) and 800 units/ml of granulocyte–macrophage colony-stimulating factor (Leucomax, Novartis Pharma, Arnhem, The Netherlands) for 6 days. At day 6, the cells were cultured on CD40L cells for 48 h to induce dendritic cell maturation. Mature dendritic cells were pulsed with 10 μg/ml of insulin B chain 10-18 peptide for 2 h at 37°C in serum-free AIM-V medium (GIBCO/BRL). After washing, antigen-presenting cells and responder cells (CD4-depleted autologous PBMCs) (1:10) were cultured in 24-well culture plates. Culture medium was Iscove's modified Dulbecco's medium supplemented with penicillin/streptomycin, 10% human serum, and 20 units/ml of IL-2. The cells were kept at 37°C in a humidified, 5% CO2 air mixture. At day 5, 20 units/ml of IL-2 was added. From day 7 on, the T cell cultures were restimulated weekly with peptide-pulsed autologous dendritic cells. The T cell lines were expanded with 20 units/ml of IL-2-containing culture medium. At day 21, HLA-A2insB10-18 tetramer+ CD8+ T cells were sorted by FACS.
Cell Staining with MHC-Peptide Tetrameric Complexes. T cells (1 × 106) were stained with 1 μg/ml of phycoerythrin-labeled HLA-A2insB10-18 tetramers (in 500 μl of PBS with 10% FCS) and incubated for 20 min in the dark at room temperature. Cells were washed with 200 μl of PBS/1% FCS. After tetramer staining, cells were incubated with anti-CD8/antigen-presenting cell and anti-CD3/FITC antibodies (Becton Dickinson) in 200 μl of PBS/1% FCS for 30 min at 4°C. After washing twice, cells were resuspended in PBS/1% FCS and analyzed in a FACSCalibur (Becton Dickinson).
IFN-γ and Granzyme B Production. To determine specific excretion of the cytokines IFN-γ, granzyme B, and IL-10, HLA-A2insB10-18 tetramer+-sorted CD8 cells were harvested by gently rinsing the wells and washing the collected CD8 cells in a large volume of Iscove's modified Dulbecco's medium (IMDM). The insulin B10-18-specific CD8 cell line was stimulated with or without the peptide and subsequently plated on anticytokine (IFN-γ, granzyme B, and IL-10) antibody-precoated ELISA plates and cultured overnight in IMDM supplemented with 1% human serum at 37°C, 5% CO2. After lysis of the cells with ice-cold deionized water, the plates were washed with PBS/0.05% Tween-20 and incubated with biotinylated detector antibody for 1 h at 37°C, followed by a second incubation with gold-labeled antibiotin antibody (GABA) for 1 h at 37°C. All antibodies were diluted in PBS/1% BSA. After extensive washing with PBS/0.05% Tween-20, the plates were developed according to the manufacturer's protocol (U-CyTech, Utrecht, The Netherlands). Spots were counted on an Olympus microscope and analyzed with Olympus micro image 4.0 software (Paes Nederland, Zoeterwoude, The Netherlands). Results are expressed as means ± SD of triplicate wells.
Detection of Autoreactive CD8+ T Cells in Patients Transplanted with Islets of Langerhans. We tested the presence of insulin B10-18-specific CD8+ T cells with the HLA-A2insB10-18 tetramer staining in PBMCs of nine HLA-A*0201-positive patients that received human pancreatic islets from HLA-A*0201-positive pancreas donors (26, 27). Seventeen data points that consisted of one or more blood samples of patients at different time points after transplantation that were available for tetramer analysis were analyzed. Unfortunately, tetramer studies require several million PBMCs, and we had access to limited numbers of suitable cryopreserved blood samples of only a few time points in a small fraction of the patients that participate in our islet transplant clinical trial that were left over from our other extensive immunological studies on alloreactivity and autoreactivity as described (26, 27), and leukocyte numbers were often reduced because of the immune suppressive therapy that these patients received. HLA-A2-positive patients receiving HLA-A2-negative grafts would be very valuable for assessing the contribution of these CD8 T cells to islet allograft destruction, but we did not have any in the selected patients in our cohort fulfilling this criterion, because each patient was transplanted with islets from several donors that always contained at least one donor expressing HLA-A2. Patient and trial characteristics were as described (26, 27). All patients had C peptide-negative type 1 diabetes (>25 years) with a functioning renal allograft transplanted 2–6 years earlier. Patients were under different immunosuppressive regimens that always included cyclosporin and prednisolone. Three patients had received antithymocyte globulin as induction therapy for a previously implanted kidney allograft, and three other patients had azathioprin replaced by mycophenolate mofetil at the time of islet implantation. PBMCs (collected from blood samples 1–50 weeks after transplantation) were isolated by Ficoll density centrifugation and cryopreserved. Thawed cells were screened for the presence of insulin B10-18-specific CD8 T cells. The presence of these CTLs was compared with recurrent autoreactivity against other islet autoantigens, alloreactivity, and clinical outcome. The cut-off of positivity was set at 0.04%, as defined by the mean frequency of tetramer-binding CD8 T cells in six healthy controls and three A2-negative islet recipients plus three times the SD of the mean.
Statistical Analysis. Differences in IFN-γ and granzyme B response were compared by using the Student t test. P < 0.05 was considered to be statistically significant. All data are expressed as means ± SD. The Mann–Whitney rank sum test was used to determine the significance of the percentage of HLA-A2InsB10-18 tetramers in patients with recurrent autoimmunity versus patients with persistent β cell function or allograft rejection without signs of autoimmunity.
Results
Identification of HLA-A*0201 Binding Peptides from the Insulin B Chain. To select candidate HLA-A*0201 binding peptides from the insulin B chain, the molecule was screened for HLA-A*0201 binding motif-containing peptides with a combination of two known binding prediction algorithms (19). Peptides of 9–11 aa containing at least one anchor residue for HLA binding at position 2 or the N terminus were defined (28, 29). In total, 15 peptides (five 9-mer, five 10-mer, and five 11-mer) were synthesized and tested to determine their actual binding affinity for HLA-A*0201 by using a cellular-free binding assay (30). Ten high-affinity binding peptides were identified (IC50 < 6 μM), and two peptides bound with intermediate affinity (6 μM < IC50 < 15 μM), whereas the other peptides displayed a low (15 μM < IC50 < 100 μM) or undetectable binding capacity (IC50 > 100 μM) (Table 1).
Table 1. Binding affinity for HLA-A*0201 of 9-, 10-, and 11-mers derived from the insulin B chain.
| Peptide† | Sequence | IC50‡ |
|---|---|---|
| 10-18 | HLVEALYLV | <0.01 |
| 11-19 | LVEALYLVC | 3.60 |
| 4-12 | QHLCGSHLV | 9.12 |
| 9-17 | SHLVEALYL | 59.45 |
| 14-22 | ALYLVCGER | 90.24 |
| 5-14 | HLCGSHLVEA | 0.06 |
| 9-18 | SHLVEALYLV | 1.37 |
| 10-19 | HLVEALYLVC | 1.75 |
| 16-25 | YLVCGERGFF | 2.30 |
| 14-23 | ALYLVCGERG | >100 |
| 10-20 | HLVEALYLVCG | 0.19 |
| 5-15 | HLCGSGLVEAL | 0.44 |
| 14-24 | ALYLVCGERGF | 2.26 |
| 8-18 | GSHLVEALYLV | 3.73 |
| 16-26 | YLVCGERGFFY | 8.59 |
Position in the insulin B chain starting at the NH2-terminal amino acid of the peptide.
IC50 is the peptide concentration required to inhibit binding of fluorescein-labeled reference peptide for 50% (IC50 in μM).
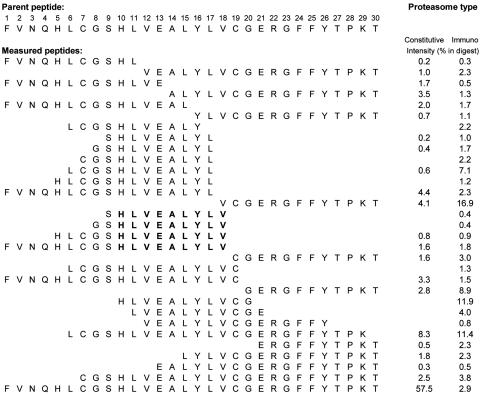
Proteasomal Cleavage and TAP Translocation. The insulin B chain was digested by immuno and constitutive proteasomes that were isolated from an Epstein–Barr virus-transformed B cell line and the human cervical cancer cell line HeLa, respectively. The peptide identity of the fragments present in the digests was identified by collision-induced dissociation MS. The digestion patterns obtained are shown in Fig. 1. The patterns from both types of proteasomes showed the presence of four peptides (18-, 14-, 11-, and 10-mer) that were processed with the exact C terminus at position 18, and two peptides (19- and 14-mer) with the exact C terminus at position 19 of the insulin B chain (Fig. 1). Because it is known that the generation of HLA class I epitopes requires cytosolic generation of the proper C terminus, these sequences were regarded as the most likely epitope precursors. Efficient translocation from cytosol to ER of three length variants (B8–18, B9–18, and B10-18) was demonstrated in a TAP translocation assay.
Fig. 1.
Proteasome digestion of the insulin B chain. In vitro proteasome-mediated digestions of the 30-mer insulin B chain peptide containing potential HLA-A*0201–restricted epitopes. 20S proteasomes isolated from an Epstein–Barr virus-transformed B cell line (immuno proteasome) and an HeLa cell line (constitutive proteasome) were coincubated with 30-mer insulin B chain peptides at 37°C for 20 h. Digestion mixtures were analyzed by MS as described in Materials and Methods. The intensity is expressed as the percentage of total summed mass-peak intensities of digested 30-mer. Predicted epitope used for CTL induction is in bold.
Detection of Insulin B10-18-Specific CD8+ T Cells. The insulin B10-18 peptide was shown to be by far the best binder to HLA-A*0201 (see Table 1), and this peptide is processed by both immuno and constitutive proteasomes. PBMCs from a healthy donor were primed with this peptide in vitro and tested for the presence of insulin B10-18-specific CD8 T cells. To facilitate the detection and quantification of insulin B10-18-specific HLA-A*0201-restricted CD8+ T cells, we constructed an HLA-A2insB10-18 tetramer. Blood cell samples were stained with the HLA-A2insB10-18 tetramer and then analyzed by flow cytometry. After several rounds of in vitro stimulation with insulin B10-18, >2% of the CD8+ T cells were positive for the HLA-A2insB10-18 tetramer. Specificity was confirmed by using a HLA-A2 tetramer containing an irrelevant (HA-1) peptide serving as negative control.
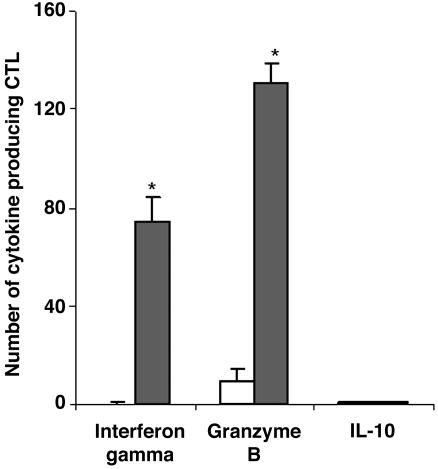
IFN-γ and Granzyme B Production. Activated CD8+ T cells are known to produce high levels of the cytokine IFN-γ and the cytotoxic enzyme granzyme B. We determined the number of IFN-γ-, granzyme B-, and IL-10-producing CD8+ T cells by ELISpot analysis (Fig. 2). CD8+ T cells produced high levels of IFN-γ and granzyme B upon stimulation by the insulin B10-18 peptide (P < 0.0005). IL-10 was not produced by peptide-specific CD8+ T cells. These results indicate that the CD8+ T cells show potential cytolytic activity upon stimulation by the insulin B10-18 peptide.
Fig. 2.
Cytotoxic cytokine release by insulin B10-18-specific CD8+ T cells after stimulation with insulin B10-18. A total of 50,000 CD8+ T cells, either untreated (open bars) or treated (filled bars) with insulin B10-18, were cultured on anti-INF-γ-coated, anti-granzyme B-coated, or IL-10 antibody-coated culture plates. INF-γ, anti-granzyme B, and IL-10 release was measured by ELISpot analysis. The bars show the number of cells producing IFN-γ, granzyme B, or IL-10. Data are expressed as means ± SD. The cut-off line represents the mean plus 3 × SD of specific CTLs frequencies in HLA-A2-positive nondiabetic subjects. *, P < 0.0005 compared with the untreated CTLs.
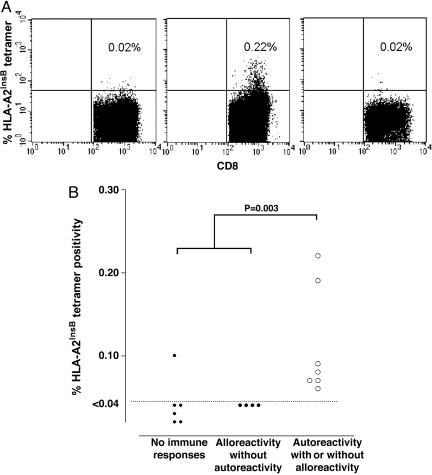
Detection of Insulin B Chain10-18-Specific CD8+ T Cells in Type 1 Diabetic Patients. We could not readily detect CD8 T cells binding the HLA-A2insB10-18 tetramer in PBMC of long-standing diabetes patients (data not shown). Therefore, we monitored the presence of insulin B10-18-specific CD8+ T cells in type 1 diabetic patients receiving islet allografts. These patients expressed different patterns of β cell function and alloreactivity and autoreactivity to islet determinants as described (26, 27). We determined the presence in vivo of insulin B10-18-specific CD8+ T cells in recurrent autoimmunity after islet transplantation. We screened nine HLA-A*0201-positive islet recipients for levels of insulin B10-18-specific CD8+ T cells in peripheral blood by using ex vivo tetramer staining. Representative stainings are depicted in Fig. 3A. We were able to demonstrate their presence in the circulation of type 1 diabetic islet transplant recipients with recurrent autoimmunity and islet allograft loss (with or without concurrent alloreactivity against the allograft), but not in patients with persistent β cell function or patients who lost islet function in combination with induction of allograft rejection without evidence of autoimmunity. HLA-A2insB10-18 tetramer staining revealed a positive correlation between detection of autoreactive CD8+ T cells and development of recurrent autoimmunity after islet transplantation and subsequent graft failure in type 1 diabetic patients (P = 0.003) (Fig. 3B).
Fig. 3.
Insulin B10-18-specific CD8 frequencies in peripheral blood samples of islet allograft recipients who have persistent islet graft function versus recurrent autoimmunity within 1 year after islet transplantation. (A) Representative examples of tetramer stainings of HLA-A2-negative subjects (Left), patients with recurrent type 1 diabetes after islet transplantation (Center), and patients with successfully restored β cell function without islet autoimmunity (Right). (B) Insulin B10-18-specific CD8 frequencies in the circulation of type 1 diabetic islet transplant recipients with recurrent autoimmunity and islet allograft loss (with or without concurrent alloreactivity against the allograft), patients with persistent β cell function, and patients who lost islet function in combination with induction of allograft rejection without evidence of autoimmunity. HLA-A2insB10-18 tetramer staining revealed a positive correlation between detection of autoreactive CD8+ T cells and development of recurrent autoimmunity after islet transplantation and subsequent graft failure in type 1 diabetic patients.
Discussion
β cell destruction is mediated by T cells in both human and NOD murine autoimmune diabetes (1, 31). Although epitopes recognized by CD4+ T cells and autoantibodies have been described in type 1 diabetes (32), the nature of the HLA class I epitopes has remained unknown. Our study provides evidence for CD8+ T cell autoreactivity to insulin in type 1 diabetes. Two HLA-A*0201-restricted epitopes, derived from glutamic acid decarboxylase and islet amyloid polypeptide, have previously been described (11, 12), but a relation with β cell destruction and type 1 diabetes could not be established. In this study, we demonstrate that a significant proportion of CD8+ T cells from islet transplant recipients with recurrent autoimmunity and loss of insulin production recognized the insulin B chain 10-18.
Although insulin is produced and secreted through secretory granules, this molecule is frequently mistargeted to the cytosol, particularly under stressed conditions. This phenomenon could be partly related to the structure of insulin, containing three cysteine disulfide bridges. Consequently, the insulin B chain may become the target of proteolytic digestion by cytosolic proteasomes and transportation to the endoplasmic reticulum (33), where it can bind to MHC class I molecules and become available to CD8+ T cells on the surface of β cells. To test this possibility, we first determined the potential of insulin B chain-derived peptides to bind to HLA-A*0201. The selected peptides consist of anchor residues at positions 2 and/or 9 (28, 29). The peptide insulin B chain 10-18 HLVEALYLV fitted perfectly to HLA-A*0201 and was subsequently confirmed with an HLA binding assay. Despite the fact that a potential peptide displays an appropriate MHC class I binding motif, it needs to be efficiently processed by the cellular machinery. Because proteasomes are the major proteases involved in the generation of MHC class I-bound peptides, we investigated whether the HLVEALYLV peptide could be generated by immuno and constitutive proteasomes. Proteasomes tend to generate the exact C-terminal ends of HLA class I ligands, but do not necessarily generate the exact N terminus required for HLA class I binding, thus frequently generating N-terminally extended precursors (34). We showed that both immuno and constitutive proteasomes generate the exact C terminus of the insulin B10-18 peptide. Four natural HLA-A*0201 ligands, derived from the insulin B chain itself, were directly generated by the 20S proteasomes in vitro. The 9-, 10-, and 11-mer peptides were translocated to the endoplasmic reticulum by the peptide transporter TAP1 (data not shown), and distinct aminopeptidase(s) in the cytosol or endoplasmic reticulum will conceivably trim the N termini of these presented peptides to their appropriate size to allow binding to HLA-A2.
The autoreactive T cell line against the insulin B chain 10-18 was generated from PBMCs of a healthy blood donor by in vitro priming with dendritic cells. Three similar attempts to isolate autoreactive CD8+ T cells from long-standing type 1 diabetic patients failed. Although this observation could have resulted from practical insufficiencies and low precursor frequencies, we favor the possibility that peripheral CD8+ T cell autoreactivity is suppressed when the vast majority of β cells have been destroyed. In healthy donors, however, the immune system appears ignorant rather than tolerant (35), and this ignorance can be lost upon vigorous priming with epitope-presenting dendritic cells, as demonstrated here.
Islet transplantation provides a unique opportunity for monitoring islet destruction in a relatively short time window. Our study demonstrates that elevated insulin B10-18-specific CD8+ T cell precursor frequency in the peripheral blood of islet recipients precedes recurrent autoimmunity as measured by T cell proliferation of CD4+ T cells, islet autoantibodies, and loss of β cell function (26, 27). This result indicates that HLA-A2insB10-18 tetramer staining is strongly correlated with detection of autoreactive CD8+ T cells and recurrent autoimmunity and graft failure after islet transplantation in type 1 diabetic patients, although this staining does not prove a causal relationship between these T cells and the destruction process, because loss of β cell function was often seen in combination with alloreactivity against the islet graft. These results suggest that elevated levels of insulin B10-18-specific CD8+ T cells in peripheral blood might be used as an indicator of early islet allograft loss, thereby facilitating efforts to preserve islet mass. In addition, the use of HLA-A2insB10-18 tetramers may facilitate the prediction of islet allograft loss before the onset of clinical symptoms. The frequency of insulin B10-18-specific CD8+ T cells may be perceived as low. However, we believe that the precursor frequencies detected in type 1 diabetes patients eliciting recurrent autoimmunity and loss of islet graft function is in fact relatively high, as compared with other reports on T cell autoreactivity enumerating precursor frequencies by either limiting dilution (36, 37), HLA class II tetramers (38), or ELISpot. With all of these methods, the precursor frequencies of circulating autoreactive T cells are between 0 and 10 million. We reason that the relatively high percentages of tetramer-staining CD8 that we measured in type 1 diabetes patients losing their islet allograft resulted from a massive T cell (re)activity that was evidently detectable in peripheral blood. Intriguingly, similar to findings by Reijonen et al. (38) on HLA class II stainings with autoantigenic epitopes, the intensity of the staining was relatively low compared with that against viral epitopes, suggestive of low avidity T cell autoreactivity that may bear relevance to lack of thymic deletion. Our report demonstrates detection of autoreactive T cells with tetramers without the need of an in vitro round of restimulation.
A number of approaches might be applied to promoting antigen-specific tolerance for the prevention of type 1 diabetes. Several studies have shown that prevention of diabetes can be achieved in animal models by the down-regulation of autoreactive T cells after administration of immunodominant peptides derived from the major β cell antigens. Prevention of diabetes in animal models has been achieved through oral, intranasal, i.v., or s.c. administration of B9 peptide from the insulin B chain (39), peptide B24-C36 from proinsulin (7, 40), peptides from glutamic acid decarboxylase 65 (41–43), or peptides from the heat-shock protein hsp60 (44, 45). However, the short half-life of peptides in the circulation and the need for repeated administrations are some of the limitations of peptide therapy. Our study demonstrates that ignorance or tolerance to islet autoantigen can be lost upon in vitro priming with dendritic cells loaded with the autoantigenic peptide.
In conclusion, we provide strong evidence that peptide 10-18 of the insulin B chain is associated with recurrent autoimmunity and loss of β cell function in type 1 diabetic islet transplant recipients. Thus, tetramers containing this peptide might be tools for diagnosis of disease onset.
Acknowledgments
We thank Prof. Sjaak Neefjes for his contribution to the TAP studies and Peter de Koning and Arnoud de Ru for excellent technical assistance. This study was supported by Juvenile Diabetes Research Foundation Grant 4-2001-434, Dutch Diabetes Research Foundation Grant 2001.06.001, a Centre for Medical Systems Biology grant, and a Centre of Excellence grant approved by the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research. This study was conducted within the framework of the Juvenile Diabetes Research Foundation Center for β Cell Therapy in Europe with the collaboration of the Eurotransplant Foundation (Leiden, The Netherlands).
Author contributions: F.O., J.W.D., and B.O.R. designed research; G.G.M.P. and C.A.M.B. performed research; O.H.M.T., M.G.D.K., P.A.v.V., B.K., and D.P. contributed new reagents/analytic tools; G.G.M.P., F.O., J.W.D., and B.O.R. analyzed data; and G.G.M.P., J.W.D., and B.O.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: NOD, nonobese diabetic; CTL, cytotoxic T lymphocyte; TAP, transporter associated with antigen presentation; PBMC, peripheral blood mononuclear cell.
References
- 1.Roep, B. O. (2003) Diabetologia 46, 305–321. [DOI] [PubMed] [Google Scholar]
- 2.Roep, B. O., Arden, S. D., De Vries, R. R. & Hutton, J. C. (1990) Nature 345, 632–634. [DOI] [PubMed] [Google Scholar]
- 3.Arif, S., Tree, T. I., Astill, T. P., Tremble, J. M., Bishop, A. J., Dayan, C. M., Roep, B. O. & Peakman, M. (2004) J. Clin. Invest. 113, 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serreze, D. V., Leiter, E. H., Christianson, G. J., Greiner, D. & Roopenian, D. C. (1994) Diabetes 43, 505–509. [DOI] [PubMed] [Google Scholar]
- 5.Wicker, L. S., Leiter, E. H., Todd, J. A., Renjilian, R. J., Peterson, E., Fischer, P. A., Podolin, P. L., Zijlstra, M., Jaenisch, R. & Peterson, L. B. (1994) Diabetes 43, 500–504. [DOI] [PubMed] [Google Scholar]
- 6.Wong, F. S., Visintin, I., Wen, L., Flavell, R. A. & Janeway, C. A., Jr. (1996) J. Exp. Med. 183, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiLorenzo, T. P., Graser, R. T., Ono, T., Christianson, G. J., Chapman, H. D., Roopenian, D. C., Nathenson, S. G. & Serreze, D. V. (1998) Proc. Natl. Acad. Sci. USA 95, 12538–12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marron, M. P., Graser, R. T., Chapman, H. D. & Serreze, D. V. (2002) Proc. Natl. Acad. Sci. USA 99, 13753–13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fennessy, M., Metcalfe, K., Hitman, G. A., Niven, M., Biro, P. A., Tuomilehto, J. & Tuomilehto-Wolf, E. (1994) Diabetologia 37, 937–944. [DOI] [PubMed] [Google Scholar]
- 10.Robles, D. T., Eisenbarth, G. S., Wang, T., Erlich, H. A., Bugawan, T. L., Babu, S. R., Barriga, K., Norris, J. M., Hoffman, M., Klingensmith, G., et al. (2002) Clin. Immunol. 102, 217–224. [DOI] [PubMed] [Google Scholar]
- 11.Panagiotopoulos, C., Qin, H., Tan, R. & Verchere, C. B. (2003) Diabetes 52, 2647–2651. [DOI] [PubMed] [Google Scholar]
- 12.Panina-Bordignon, P., Lang, R., van Endert, P. M., Benazzi, E., Felix, A. M., Pastore, R. M., Spinas, G. A. & Sinigaglia, F. (1995) J. Exp. Med. 181, 1923–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wegmann, D. R., Norbury-Glaser, M. & Daniel, D. (1994) Eur. J. Immunol. 24, 1853–1857. [DOI] [PubMed] [Google Scholar]
- 14.Daniel, D., Gill, R. G., Schloot, N. & Wegmann, D. (1995) Eur. J. Immunol. 25, 1056–1062. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama, M., Abiru, N., Moriyama, H., Babaya, N., Liu, E., Miao, D., Yu, L., Wegmann, D. R., Hutton, J. C., Elliott, J. F., et al. (2005) Nature 435, 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schloot, N. C., Roep, B. O., Wegmann, D., Yu, L., Chase, H. P., Wang, T. & Eisenbarth, G. S. (1997) Diabetologia 40, 564–572. [DOI] [PubMed] [Google Scholar]
- 17.Durinovic-Bello, I. I., Schlosser, M., Riedl, M., Maisel, N., Rosinger, S., Kalbacher, H., Deeg, M., Ziegler, M., Elliott, J., Roep, B. O., et al. (2004) Diabetologia 47, 439–450. [DOI] [PubMed] [Google Scholar]
- 18.Kent, S. C., Chen, Y., Bregoli, L., Clemmings, S. M., Kenyon, N. S., Ricordi, C., Hering, B. J. & Hafler, D. A. (2005) Nature 435, 224–228. [DOI] [PubMed] [Google Scholar]
- 19.Drijfhout, J. W., Brandt, R. M., D'Amaro, J., Kast, W. M. & Melief, C. J. (1995) Hum. Immunol. 43, 1–12. [DOI] [PubMed] [Google Scholar]
- 20.Kessler, J. H., Beekman, N. J., Bres-Vloemans, S. A., Verdijk, P., van Veelen, P. A., Kloosterman-Joosten, A. M., Vissers, D. C., ten Bosch, G. J., Kester, M. G., Sijts, A., et al. (2001) J. Exp. Med. 193, 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottenhoff, T. H., Geluk, A., Toebes, M., Benckhuijsen, W. E., van Meijgaarden, K. E. & Drijfhout, J. W. (1997) J. Immunol. Methods 200, 89–97. [DOI] [PubMed] [Google Scholar]
- 22.Neisig, A., Roelse, J., Sijts, A. J., Ossendorp, F., Feltkamp, M. C., Kast, W. M., Melief, C. J. & Neefjes, J. J. (1995) J. Immunol. 154, 1273–1279. [PubMed] [Google Scholar]
- 23.Altman, J. D., Moss, P. A., Goulder, P. J., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274, 94–96. [DOI] [PubMed] [Google Scholar]
- 24.Beekman, N. J., van Veelen, P. A., van Hall, T., Neisig, A., Sijts, A., Camps, M., Kloetzel, P. M., Neefjes, J. J., Melief, C. J. & Ossendorp, F. (2000) J. Immunol. 164, 1898–1905. [DOI] [PubMed] [Google Scholar]
- 25.Eggers, M., Boes-Fabian, B., Ruppert, T., Kloetzel, P. M. & Koszinowski, U. H. (1995) J. Exp. Med. 182, 1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keymeulen, B., Ling, Z., Gorus, F. K., Delvaux, G., Bouwens, L., Grupping, A., Hendrieckx, C., Pipeleers-Marichal, M., Van Schravendijk, C., Salmela, K., et al. (1998) Diabetologia 41, 452–459. [DOI] [PubMed] [Google Scholar]
- 27.Roep, B. O., Stobbe, I., Duinkerken, G., van Rood, J. J., Lernmark, A., Keymeulen, B., Pipeleers, D., Claas, F. H. & De Vries, R. R. (1999) Diabetes 48, 484–490. [DOI] [PubMed] [Google Scholar]
- 28.Hunt, D. F., Henderson, R. A., Shabanowitz, J., Sakaguchi, K., Michel, H., Sevilir, N., Cox, A. L., Appella, E. & Engelhard, V. H. (1992) Science 255, 1261–1263. [DOI] [PubMed] [Google Scholar]
- 29.Ruppert, J., Sidney, J., Celis, E., Kubo, R. T., Grey, H. M. & Sette, A. (1993) Cell 74, 929–937. [DOI] [PubMed] [Google Scholar]
- 30.van der Burg, S. H., Ras, E., Drijfhout, J. W., Benckhuijsen, W. E., Bremers, A. J., Melief, C. J. & Kast, W. M. (1995) Hum. Immunol. 44, 189–198. [DOI] [PubMed] [Google Scholar]
- 31.Liblau, R. S., Wong, F. S., Mars, L. T. & Santamaria, P. (2002) Immunity 17, 1–6. [DOI] [PubMed] [Google Scholar]
- 32.Verge, C. F., Stenger, D., Bonifacio, E., Colman, P. G., Pilcher, C., Bingley, P. J. & Eisenbarth, G. S. (1998) Diabetes 47, 1857–1866. [DOI] [PubMed] [Google Scholar]
- 33.Harding, H. P. & Ron, D. (2002) Diabetes 51, Suppl. 3, S455–S461. [DOI] [PubMed] [Google Scholar]
- 34.Mo, X. Y., Cascio, P., Lemerise, K., Goldberg, A. L. & Rock, K. (1999) J. Immunol. 163, 5851–5859. [PubMed] [Google Scholar]
- 35.Ohashi, P. S., Oehen, S., Buerki, K., Pircher, H., Ohashi, C. T., Odermatt, B., Malissen, B., Zinkernagel, R. M. & Hengartner, H. (1991) Cell 65, 305–317. [DOI] [PubMed] [Google Scholar]
- 36.Naik, R. G., Beckers, C., Wentwoord, R., Frenken, A., Duinkerken, G., Brooks-Worrell, B., Schloot, N. C., Palmer, J. P. & Roep, B. O. (2004) J. Autoimmun. 23, 55–61. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, J., Markovic-Plese, S., Lacet, B., Raus, J., Weiner, H. L. & Hafler, D. A. (1994) J. Exp. Med. 179, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reijonen, H., Novak, E. J., Kochik, S., Heninger, A., Liu, A. W., Kwok, W. W. & Nepom, G. T. (2002) Diabetes 51, 1375–1382. [DOI] [PubMed] [Google Scholar]
- 39.Daniel, D. & Wegmann, D. R. (1996) Proc. Natl. Acad. Sci. USA 93, 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez, N. R., Augstein, P., Moustakas, A. K., Papadopoulos, G. K., Gregori, S., Adorini, L., Jackson, D. C. & Harrison, L. C. (2003) J. Clin. Invest. 111, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian, J., Clare-Salzler, M., Herschenfeld, A., Middleton, B., Newman, D., Mueller, R., Arita, S., Evans, C., Atkinson, M. A., Mullen, Y., et al. (1996) Nat. Med. 2, 1348–1353. [DOI] [PubMed] [Google Scholar]
- 42.Tisch, R., Wang, B. & Serreze, D. V. (1999) J. Immunol. 163, 1178–1187. [PubMed] [Google Scholar]
- 43.Tian, J., Atkinson, M. A., Clare-Salzler, M., Herschenfeld, A., Forsthuber, T., Lehmann, P. V. & Kaufman, D. L. (1996) J. Exp. Med. 183, 1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias, D., Reshef, T., Birk, O. S., van der, Z. R., Walker, M. D. & Cohen, I. R. (1991) Proc. Natl. Acad. Sci. USA 88, 3088–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bockova, J., Elias, D. & Cohen, I. R. (1997) J. Autoimmun. 10, 323–329. [DOI] [PubMed] [Google Scholar]