Abstract
Activating the immune system to trigger a specific response is a major challenge in vaccine development. In particular, activating sufficient cytotoxic T lymphocyte-mediated cellular immunity, which is crucial for the treatment of many diseases including cancer and AIDS, has proven to be especially challenging. In this study, antigens were encapsulated in acid-degradable polymeric particle carriers to cascade cytotoxic T lymphocyte activation. To target dendritic cells, the most potent antigen-presenting cells, the particle carriers, were further conjugated with monoclonal antibodies. A series of ex vivo and in vivo studies have shown increased receptor-mediated uptake of antibody-conjugated particles by dendritic cells as well as migration of particle-carrying dendritic cells to lymph nodes and stimulation of naïve T cells leading to enhanced cellular immune response as confirmed by specific cell lysis and IFN-γ secretion.
Keywords: acid-degradable particle, drug delivery, targeted vaccine
Vaccination has been regarded as one of the most cost-efficient strategies to prevent and treat many diseases. Unlike conventional medicines directly working on specific targets, vaccines initiate a cascade of immune response against a disease; therefore, successful delivery of small amounts of antigens can lead to effective and highly specific protection from a disease. Immune responses are categorized as cellular and humoral immunities. Cytotoxic T lymphocyte (CTL)-mediated cellular immunity removes target cells infected by intracellular virus and bacteria, whereas antibody (Ab)-mediated humoral immunity inactivates extracellular pathogens. The type of immune response is determined by the intracellular fate of antigens in antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs). Peptides derived from antigens, which are degraded in the cytosolic space, are presented by major histocompatibility complex (MHC) I molecules and initiate cellular immunity, whereas peptides from extracellularly endocytosed antigens, which are degraded in the lysosome, are presented by MHC II molecules followed by activation of Ab-secreting B cells and assisting in CTL activation (1). Therefore, one of the challenges in vaccine development is to activate CTL-mediated responses in addition to humoral protection through the delivery of antigens by conventional means such as injection or nasal spray (i.e., extracellular delivery) (2). For example, Ab-mediated protection is not as efficient as cellular immunity against cancer (3, 4), and, in the treatment of AIDS, both the eradication of HIV-infected cells and the inactivation of viral particles are crucial (5, 6). It has been shown that the MHC I-directed presentation of antigens was significantly higher for antigens delivered through acid-degradable particle carriers than observed with the delivery of free proteins (7). Recently, particle carriers obtained using both degradable monomers and degradable cross-linkers were shown to dramatically increase MHC I-directed antigen presentation while also conserving MHC II-directed antigen presentation (8).
Another challenge in the design of successful vaccines is the targeting of APCs because free antigens can be endocytosed easily by most types of cells; therefore, it is critical to design a delivery vehicle capable of preferential targeting of APCs. Professional APCs such as DCs constitute one of the most interesting classes of APCs to target because DCs are the most potent APCs for the stimulation of naïve T cells. Numerous studies have shown that DCs orchestrate immune response and migrate into secondary lymphoid organs such as lymph nodes (LNs) and spleen where they stimulate naïve T cells (9, 10). In normal tissues, other APCs such as macrophages need to encounter a previously activated T cell with the antigen-specific T cell receptor to stimulate it.
However, targeting DCs in vivo is very difficult because, in normal tissues, they exist in significantly lower numbers than other APCs such as macrophages. To overcome this problem, acid-degradable particles encapsulating a model antigen, ovalbumin (OVA), were conjugated with anti-DEC-205 (anti-CD205) monoclonal Abs (mAbs). DEC-205 was chosen because it is an endocytosis receptor that is only expressed by lymphoid, interstitial, epidermal Langerhans DCs, and thymic endothelial cells, unlike mannose and Fc receptors, which are expressed by both macrophages and DCs (11, 12).
Materials and Methods
Production of Anti-DEC-205 mAbs. Rat IgG2a anti-DEC-205 mAb producing hybridoma cell line (NLDC-145) was purchased from American Type Culture Collection and cultured in a complete medium that was RPMI medium 1640 supplemented with 10% (vol/vol) FBS (HyClone), 2 mM l-glutamine, 1.5 g/liter sodium bicarbonate, 4.5 g/liter glucose, 10 mM Hepes, and 1.0 mM sodium pyruvate (all from GIBCO/BRL). For proliferation, cell densities were maintained between 105 and 106 viable cells per ml as suggested by American Type Culture Collection. For the production of Abs, 5 × 106 cells were inoculated in 100 ml of fresh medium and cultured for a further 7 days. The harvested supernatant was filtered, then centrifuged at 1,200 × g for 10 min to remove cells and debris. The purified Abs were obtained by running the filtered supernatant through a protein G column (Pierce). Concentrations of rat IgG2a Abs were determined by an ELISA kit (Bethyl Laboratories, Montgomery, TX).
Bone Marrow-Derived DCs (BMDCs) and Bone Marrow-Derived Macrophages (BMMφs). Femurs and tibias of 2- to 4-month-old female C57BL/6 mice (The Jackson Laboratory) were extracted after they were killed, and the bone marrow was flushed out by using a 25-gauge needle with 5 ml of RPMI 1640 medium supplemented with 10% (vol/vol) FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, and 55 μM 2-mercaptoethanol (all from GIBCO/BRL). After the red blood cells were removed by treating with RBC lysis buffer (Gentra Systems), the remaining cells were cultured with RPMI 1640 medium and further supplemented with 5% (vol/vol) granulocyte–macrophage colony-stimulating factor-containing supernatant (20 ng/ml) for DC differentiation or L-cell conditioned medium for macrophage differentiation (13). After 7 days of cultivation in a 6-well plate, the suspended dendritic and adhered macrophage cells were inoculated onto a 96-well plate at 5 × 104 cells per well.
Preparation of Anti-DEC-205 mAb-Conjugated Particles. Particle carriers encapsulating OVA were prepared (14) by using an acid-degradable cross-linker and an acid-degradable primary amine monomer as described in ref. 8. Dried particles (10 mg) were resuspended in 1 ml of PBS (pH 8.0) and mixed with 1 mg of Bis(sulfosuccinimidyl)suberate (BS3) (Pierce) followed by stirring at room temperature for 30 min. After centrifugation and washing twice, the particles in 0.5 ml of PBS (pH 8.0) were mixed with a solution (0.5 ml) containing 0.1 mg of anti-DEC-205 mAb. As a control, keyhole limpet hemocyanin (KLH)-specific rat IgG2a mAbs were purchased from eBioscience (San Diego, CA) and used to prepare isotype Ab-conjugated particles. Ab-containing solutions were run through a buffer-exchanging column to equilibrate the buffer to PBS (pH 8.0) before conjugation. After stirring 30 min at room temperature, the particles were centrifuged and washed twice with PBS. Conjugation efficiency was determined by hydrolyzing a weighed amount of particles in a acetic acid buffer (pH 5.0) for 10 min, then quantifying Ab concentration in the aliquot with ELISA. The amount of encapsulated OVA was quantified as 100 μg/mg particles by using the method described in ref. 8.
The size of the particle carriers in PBS (pH 7.4) was measured by using a dynamic light scattering (DLS) particle sizer (Malvern Instruments, Southborough, MA). Hydrolysis of the particles was confirmed by incubating 1 mg of anti-DEC-205-conjugated particles in 1 ml of acetic acid buffer (pH 5.0) for 10 min at room temperature followed by size measurement of the hydrolyzed aliquot using DLS. To visualize the conjugation of Anti-DEC-205 mAbs with particle carriers, R-phycoethrin-conjugated-anti-rat IgG Abs were mixed with anti-DEC-205-conjugated particles in PBS (pH 7.4) with 5% FBS. After washing twice, the particles were observed by using a LS450 laser-scanning confocal microscope (Zeiss).
Analysis of Particle Endocytosis. Anti-DEC-205 mAb- or isotype mAb-conjugated particles (1 mg per well of six-well plate) encapsulating Alexa Fluor 488 (Molecular Probes)-conjugated OVA were incubated with BMDCs or BMMφs(1 × 106 cells per well). After various times of incubation, the cells were washed, trypsinized, and analyzed by a Beckman Coulter EPICS XL flow cytometer. The relative numbers of internalized particles were quantified and compared by mean channel values of 1 × 105 cells.
In Vivo DC Targeting. Anti-DEC-205 mAb- or isotype mAb-conjugated particles encapsulating Alexa Fluor 488-conjugated OVA (1 mg) were s.c. injected into the base of the tail of female C57BL/6 mice (three mice per group). To prevent hydrolysis of the particles, nondegradable methylene bisacrylamide (Bio-Rad) was used to prepare the particle carriers. In a control experiment, 0.1 mg of Alexa Fluor 488-conjugated OVA was injected. After 18 h, popliteal LNs (PLNs), inguinal LNs (ILNs), and mesenteric LNs (MLNs) were isolated from the mice injected with particles or free proteins, and lymphocytes were harvested. The cells were further stained with anti-DEC-205, CD11c, CD11b, and CD8α mAbs. The types of cells carrying the fluorescent-labeled particles were determined by flow cytometry. Note that a single mouse has two ILNs and PLNs, and multiple MLNs. Therefore, each data point represents the mean of at least six measurements.
Intracellular Cytokine Staining. Spleen cells from C57BL/6 mice vaccinated with anti-DEC-205 mAb-conjugated particles, isotype mAb-conjugated particles encapsulating OVA (1 mg), or 0.1 mg of free OVA (five mice per group) were removed after 10 days. The splenocytes were cultured for 4 h in the presence of brefeldin A and 100 nM SIINFEKL peptide. They were stained for CD8, fixed and permeabilized, and stained for IFN-γ. All reagents were from the Cytofix/Cytoperm kit (Pharmingen) and were used according to the manufacturer's instructions.
CTL Activity Assay. Female C57BL/6 mice were vaccinated twice, 14 days apart, by s.c. injection into the base of the tail. Three sets of vaccinations were performed as follows: (i) with anti-DEC-205 mAb-conjugated particles, (ii) with isotype mAb-conjugated particles encapsulating OVA (1 mg), or (iii) with 0.1 mg of free OVA (five mice per group). A week after the second vaccination, spleens were isolated, and the activity of CTLs was measured. EL4 cells (targets) were stained with PKH26 (Sigma) according to the manufacturer's instructions, incubated with spleen cells (responders) from vaccinated mice at 37°C for 2 h at various effector-to-target (E:T) ratios, stained for 30 min on ice with 1 mM YoPro1 (Molecular Probes), and analyzed by FACS. Specific lysis of the PKH+ target cells was calculated as [%YoPro1+ve – %YoPro1+ve at E:T ratio = 0]/[100 – %YoPro1+ve at E:T ratio = 0] × 100. As negative controls we included unpulsed EL4 cells incubated with spleen cells from vaccinated mice.
Statistical Data Analysis. Multiple experimental data were obtained and are presented as means ± standard deviations. Statistical comparisons were done at a significance level of P < 0.05 using Student's t test.
Results and Discussion
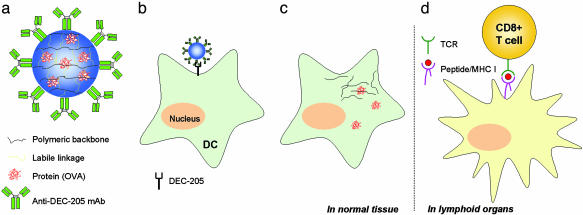
To target DCs in vivo and to initiate a directed CTL-mediated immune response, anti-DEC-205 mAbs were conjugated to the acid-degradable particle carriers, which in earlier work (8) were shown to induce remarkable MHC I-directed antigen presentation while also conserving MHC II presentation (Fig. 1a). The new carrier particles conjugated with anti-DEC-205 mAbs were designed to encounter and be taken up efficiently by DCs through receptor-mediated endocytosis in vivo (Fig. 1b). A beneficial feature of the use of anti-DEC-205 mAb-conjugated particles is that mature DCs (mDCs) as well as immature DCs are targeted. These mDCs express high levels of DEC-205 on their cell surface and are able to stimulate naïve T cells with high efficiency (12). Because of their great lability to acid, the particles are hydrolyzed in the acidic lysosome of the DCs and release the encapsulated antigens into the cytoplasm (Fig. 1c) (15). The released antigens then are degraded as intracellular pathogens by the proteasome. After migrating to lymphoid organs, DCs present antigen-derived peptides on MHC I molecules to CD8+ T cells, which differentiate into CTLs upon successful activation through their T cell receptor (Fig. 1d).
Fig. 1.
Schematic diagram of enhanced CTL activation by DC-targeting acid-degradable particles. (a) OVA was encapsulated in acid-degradable polymeric particles further conjugated with anti-DEC-205 mAbs. (b) The particles are taken up by DEC-205 expressing DCs in vivo. (c) After hydrolysis in the acidic lysosome of DCs, encapsulated OVA is released into the cytoplasm. (d) Intracellularly processed OVA-derived peptides are presented as a complex with MHC I and recognized by CD8+ T cells with the corresponding T cell receptors in secondary lymphoid organs (e.g., LNs and spleen). After successful ligation, CD8+ cells differentiate into CTLs.
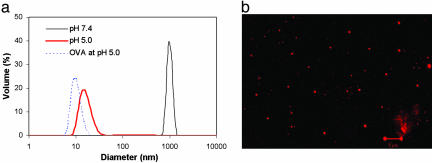
Hydrolysis of the anti-DEC-205 mAb-conjugated acid-degradable particles was verified by using particle-size analysis by means of dynamic light scattering (Fig. 2a). Under the lysosomal condition (i.e., pH 5.0), most of the particles are hydrolyzed rapidly. Although two peaks representing free proteins and hydrolyzed linear polymers could be expected to be observed by dynamic light scattering, only one (red bold line) was actually observed. Because this peak corresponds to a size larger than that of free OVA (dotted line in Fig. 2a), it suggests that the OVA may form aggregates with the hydrolyzed polymer carrier. Laser-scanning confocal micrography showed a high density of anti-DEC-205 mAbs at on all of the observed acid-degradable particles that were labeled with fluorescent anti-rat IgG2a Abs (Fig. 2b). Quantification of anti-DEC205 mAbs in the hydrolyzed particle solutions by ELISA revealed the efficiency to be 2.5 ng of mAbs per mg of particle. This low level of conjugation is expected because the mAbs are covalently immobilized only on the surface of the particles.
Fig. 2.
Hydrolysis and confocal micrograph of DEC-205 mAb-conjugated acid-degradable microparticles. (a) Acid-degradable particles (size ≈ 1 μm; solid black line) hydrolyzed completely after 10-min incubation in vitro at pH 5.0 (solid red line). The size of the hydrolyzed particles was larger than free OVA (dotted blue line) implying the presence of aggregates of OVA and hydrolyzed polymer. (b) Conjugation of anti-DEC-205 mAbs on the degradable particles was confirmed by laser-scanning confocal microscopy after incubation with fluorescence-labeled anti-rat IgG2a Abs. (Scale bar: 5 μm.)
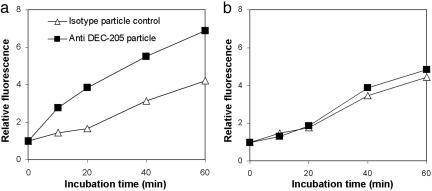
DCs are known to uptake antigens by means of macropinocytosis, whereas macrophages uptake by means of phagocytosis. In other words, DCs can endocytose relatively small particles compared with macrophages (16). Therefore, to enhance DC-specific uptake, it is important to target DCs by using their endocytosis receptor. Because DEC-205 is an endocytosis receptor, acid-degradable particles conjugated with anti-DEC-205 mAb are expected to be taken up by DCs more efficiently than control particles conjugated to isotype rat IgG2a mAb. To verify this hypothesis of DC-specific endocytosis, particles conjugated with anti-DEC-205 or isotype mAbs were prepared. To facilitate identification, OVA was conjugated with the pH-insensitive and FITC-like fluorescent dye Alexa Fluor 488, and the labeled protein also was encapsulated in the particles. Ex vivo incubation of BMDCs and BMMφs showed that more particles conjugated with anti-DEC-205 mAb were taken up by BMDCs than particles conjugated with isotype mAb (Fig. 3a). In contrast, no difference in endocytosis efficiency was observed for uptake of both types of particles by BMMφs (Fig. 3b). These results clearly confirm that enhanced DC-specific endocytosis of particles can be achieved through their conjugation with anti-DEC-205 mAbs.
Fig. 3.
Enhanced endocytosis of anti-DEC-205 mAb-conjugated particles only by DCs. (a) Anti-DEC-205 mAb-conjugated and anti-KLH rat IgG2a particles encapsulating fluorescence-labeled OVA were incubated with BMDCs. The relative amount of internalized particles was compared with the relative fluorescence intensities quantified as mean channel values. A significant enhancement of particle endocytosis by DCs was observed with anti-DEC-205 mAb-conjugated particles. (b) Incubation of particles with BMMφs with the same particles used for BMDCs showed no difference in endocytosis.
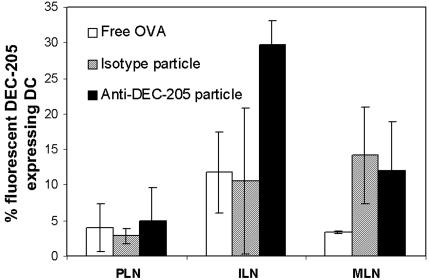
Having demonstrated the increased uptake of OVA-encapsulated particles by DCs compared with BMMφs ex vivo, we wished to investigate whether immunization of mice with the particles would also lead to increased uptake by DCs. To this end mice were injected s.c. with free OVA or with particles conjugated with either anti-DEC-205 or isotype mAbs, and their LNs were harvested 18 h later. OVA was labeled with Alexa Fluor 488 and further used either as the free protein or encapsulated in the particles. Mice injected with anti-DEC-205-conjugated particles showed a large amount of fluorescent DEC-205+ DCs (≈30%) in the draining inguinal LN, indicating that efficient uptake of the particles by DCs had taken place (Fig. 4). A significantly lower amount of DCs carrying particles were observed in mice injected with either free OVA or isotype mAb-conjugated particles compared with those injected with anti-DEC-205-conjugated particles. Analysis of DEC-205+ DCs from MLN showed both isotype and anti-DEC-205-conjugated particles had been taken up (≈10%), whereas free OVA had not (Fig. 4). Previous studies have shown that DCs migrate to draining LNs such as inguinal LN, but not to nondraining LNs (e.g., MLN), within the first 18 h after s.c. injection of OVA (17). However, incubation of DCs with acid-degradable particles has been shown to induce maturation (15), which would increase the homing efficiency of the DCs to the LNs by up-regulating adhesion molecules. This finding is possibly why more fluorescent DEC-205+ DCs were observed in the MLNs of mice injected with either isotype or anti-DEC-205-conjugated mAb particles when compared with those injected with free OVA (Fig. 4). No difference was seen in popliteal LNs between the three injection groups. This finding is expected because this study used an injection into the base of the tail, and only DCs injected into the footpad migrate to the popliteal LNs (18). Overall, these results clearly confirmed that DEC-205+ DCs migrated efficiently to the LNs after capture of anti-DEC-205 mAb-conjugated particles.
Fig. 4.
In vivo DC targeting using anti-DEC-205 mAb-conjugated particles. Fluorescence-labeled anti-DEC-205 mAb- or anti-KLH rat IgG2a mAb-conjugated particles were injected s.c. into the tail of mice. After 18 h, popliteal LNs, inguinal LNs, and MLNs were isolated, and lymphocytes were analyzed by flow cytometry. More fluorescent DEC-205 expressing DCs harvested from the mice injected with anti-DEC-205 mAb-conjugated particles were identified than from the mice injected with anti-KLH rat IgG2a mAb-conjugated particles and fluorescent OVA.
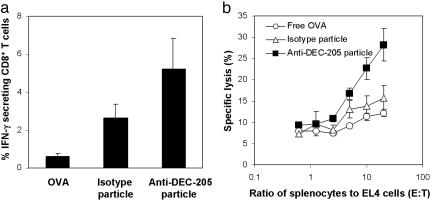
CTL activation upon recognition of the MHC I molecule loaded with the antigenic peptide leads to secretion of IFN-γ to assist in the efficient activation of the immune system. The ability of the conjugated particles to initiate an antigen-specific CTL response in vivo through cytosolic release of the encapsulated OVA was investigated. Mice were vaccinated with acid-degradable particles conjugated with anti-DEC-205 mAb or isotype mAbs or with free OVA. Spleen cells were isolated 10 days later, and the number of IFN-γ secreting antigen-specific CD8+ T cells was quantified. Responding to antigen-specific stimulation, mice vaccinated with anti-DEC-205 mAb-conjugated degradable particles had twice as many IFN-γ producing CD8+ T cells compared with those vaccinated with isotype mAb-conjugated particles (5% vs. 2.5%) and >5 times as many as those vaccinated with free OVA (5% vs. 0.6%) (Fig. 5a). Specific interactions between the MHC/peptide of target cells and the T cell receptor of the CTL induce binding of LFA-1 integrin receptors on the CTL surface to intercellular adhesion molecules on the target cells. This polarized interaction triggers release of perforin and granzymes, thus killing the target cell. The ability of vaccination with DEC-205 or isotype mAb-conjugated particles to induce protective immunity by CTLs was measured by specific lysis of cells presenting OVA-derived peptides on their surface. Splenocytes isolated from mice vaccinated with anti-DEC-205 mAb-conjugated particles, isotype mAb-conjugated particles, or free OVA were incubated with OVA-derived SIINFEKL (SL8) peptide pulsed target EL4 cells and assayed for their CTL activity (Fig. 5b). CTL activity of spleen cells from mice vaccinated with anti-DEC-205 mAb-conjugated particles against MHC I/SL8-expressing cells was greater than that observed for mice vaccinated with isotype mAb-conjugated particles or free OVA.
Fig. 5.
Efficient in vivo CTL activation by vaccination with anti-DEC-205 mAb-conjugated particles. Mice were injected with anti-DEC-205 mAb or anti-KLH IgG2a mAb-conjugated particles or free OVA. After 10 days spleen cells were removed and assayed for SL8-specific responses by IFN-γ (a) or CTL activity (b). (a) More SL8 specific IFN-γ secreting cells were identified from spleen cells isolated from the mice vaccinated with anti-DEC-205 mAb-conjugated particles than those vaccinated with isotype mAb-conjugated particles or free OVA. (b) Enhanced CTL activity of spleen cells isolated from the mice vaccinated with anti-DEC-205 mAb-conjugated particles was observed compared to mice vaccinated with anti-KLH mAb-conjugated particles or free OVA.
One of the unique characteristics of in vivo DCs is cross-presentation, which is the presentation of extracellular antigens by MHC I. It was shown that cross-presentation of antigens internalized in the particulate carrier is more efficient than that achieved with soluble extracellular antigens because of distinctive antigen processing pathways (19). In other words, it is not only the degradability of the particles but also the particle carrier itself that can help increase CTL activation by MHC I-directed antigen presentation. However, it was shown that nondegradable particles were much less effective at cross-presenting antigens than degradable particles (8). This result indicates that cross-presentation by plain particle carriers is not efficient enough to stimulate a sufficient number of CD8+ T cells to differentiate into active CTLs. This finding emphasizes the benefit derived from the use of the acid-degradable particle carriers to confer efficient CTL-mediated immunity. It also has been reported that antigen conjugated with anti-DEC-205 mAb improved MHC I-directed antigen presentation (12, 20). Therefore, anti-DEC-205-conjugated acid-degradable particles encapsulating antigen increase the efficiency of CD8+ T cell activation, receptor-mediated endocytosis, and in vivo DC targeting.
Some subsets of DCs including plasmacytoid DCs (pDCs), which are known to play an important role in antiviral responses by bridging the innate and adaptive immunities, do not express DEC-205 (21, 22). The use of particle carriers conjugated with anti-DEC-205 mAb is therefore not suitable for pDC targeting. Therefore, to further enhance CTL activation to a multitude of antigens, the use of a particle carrier conjugated with a universal DC targeting molecule would be more beneficial. However, it is not known at this time which subsets of DCs contribute most to in vivo CTL activation, although enhanced CTL activation was observed with targeting DCs other than pDCs in this study.
Acknowledgments
This work was supported by National Institutes of Health Grant R01-EB005824-01 and in part by U.S. Department of Energy Grant DE-ACO3-76SF00098 for the development of functionalized stimuli-responsive nanoparticles.
Author contributions: Y.J.K. and J.M.J.F designed research; Y.J.K. and E.J. performed research; Y.J.K. and E.J. contributed new reagents/analytical tools; Y.J.K, E.J., and N.S. analyzed data; and Y.J.K., E.J., N.S. and J.M.J.F. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: APC, antigen-presenting cell; DC, dendritic cell; BMDC, bone marrow-derived DC; BMMφ, bone marrow-derived macrophage; CTL, cytotoxic T lymphocyte; KLH, keyhole limpet hemocyanin; LN, lymph node; MLN, mesenteric LN; OVA, ovalbumin.
References
- 1.Germain, R. N. (1994) Cell 76, 287–299. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky, J. A., Ahlers, J. D. & Belyakov, I. M. (2001) Nat. Rev. Immunol. 1, 209–219. [DOI] [PubMed] [Google Scholar]
- 3.Huang, A. Y. C., Golumbek, P., Ahmadzadeh, M., Jaffee, E., Pardoll, D. & Levitsky, H. (1994) Science 264, 961–965. [DOI] [PubMed] [Google Scholar]
- 4.Qin, Z., Richter, G., Schüler, T., Ibe, S., Cao, X. & Blankenstein, T. (1998) Nat. Med. 5, 627–630. [DOI] [PubMed] [Google Scholar]
- 5.Letvin, N. L. & Walker, B. D. (2003) Nat. Med. 9, 861–866. [DOI] [PubMed] [Google Scholar]
- 6.Emini, E. A. & Koff, W. C. (2004) Science 304, 1913–1914. [DOI] [PubMed] [Google Scholar]
- 7.Murthy, N., Xu, M., Schuck, S., Kunisawa, J., Shastri, N. & Fréchet, J. M. J. (2003) Proc. Natl. Acad. Sci. USA 100, 4995–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon, Y. J., Standley, S. M., Goodwin, A. P., Gillies, E. R. & Fréchet, J. M. J. (2005) Mol. Pharm. 2, 83–91. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 10.Lanzavecchia, A. & Salluto, F. (2001) Cell 106, 263–266. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, W., Swiggard, W. J., Heufler, C., Peng, M., Mirza, A., Steinman, R. M. & Nussenzweig, M. C. (1995) Nature 375, 151–155. [DOI] [PubMed] [Google Scholar]
- 12.Bonifaz, L. C., Bonnyay, D. P., Charalambous, A., Darguste, D. I., Fujii, S.-I., Soares, H., Brimnes, M. K., Moltedo, B., Moran, T. M. & Steinman, R. M. (2004) J. Exp. Med. 199, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stier, E. M., Mandal, M. & Lee, K.-D. (2005) Mol. Pharm. 2, 74–82. [DOI] [PubMed] [Google Scholar]
- 14.Murthy, N., Thng, Y. X., Schuck, S., Xu, M. C. & Fréchet, J. M. J. (2002) J. Am. Chem. Soc. 124, 12398–12399. [DOI] [PubMed] [Google Scholar]
- 15.Kwon, Y. J., Standley, S. M., Goh, S. L. & Fréchet, J. M. J. (2005) J. Controlled Release 105, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiele, L., Rothen-Rutishauser, B., Jilek, S., Wunderli-Allenspach, H., Merkle, H. P. & Walter, E. (2001) J. Controlled Release 76, 59–71. [DOI] [PubMed] [Google Scholar]
- 17.Ingulli, E., Ulman, D. R., Lucido, M. M. & Jenkins, M. K. (2002) J. Immunol. 169, 2247–2252. [DOI] [PubMed] [Google Scholar]
- 18.Kupiec-Weglinski, J. W., Austyn, J. M. & Morris, P. J. (1988) J. Exp. Med. 167, 632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerman, A. L., Kyritsis, C., Tampe, R. & Cresswell, P. (2005) Nat. Immunol. 6, 107–113. [DOI] [PubMed] [Google Scholar]
- 20.Bonifaz, L., Bonnyay, D., Mahnke, K., Rivera, M., Nussenzweig, M. C. & Steinman, R. M. (2002) J. Exp. Med. 196, 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colonna, M., Trinchieri, G. & Liu, Y.-J. (2004) Nat. Immunol. 5, 1219–1226. [DOI] [PubMed] [Google Scholar]
- 22.McKenna, K., Beignon, A.-S. & Bhardwaj, N. (2005) J. Virol. 79, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]