Common wisdom among neuroscientists holds that cerebellar learning and synaptic plasticity are “somehow” different from their counterparts in other brain areas. This notion is largely based on the assumption that forms of cerebellar motor learning are mediated by long-term depression (LTD) of synaptic transmission at parallel fiber (PF)–Purkinje cell (PC) synapses, whereas in other brain areas, such as the hippocampus, long-term potentiation (LTP) is seen as the cellular learning correlate. But what distinguishes cerebellar synaptic plasticity mechanisms from those at other types of synapses, for example, at the well characterized hippocampal CA3–CA1 synapse? In a recent issue of PNAS, Kakegawa and Yuzaki (1) demonstrate that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor delivery into synapses is involved in a recently discovered form of cerebellar LTP, enabling comparison with similar processes at hippocampal CA3–CA1 synapses.
At these glutamatergic synapses, fast excitation relies on AMPA receptors, which are heteromeric complexes of the four homologous subunits GluR1 to GluR4 (also GluRA to GluRD). In hippocampal pyramidal cells, the majority of AMPA receptors consist of GluR1–GluR2 and GluR2–GluR3 heteromeric complexes. The current understanding is that hippocampal plasticity largely rests on modifications of the GluR1 subunit, resulting in altered GluR1 subunit trafficking and/or single-channel conductance changes. Kakegawa and Yuzaki (1) present evidence that plasticity at cerebellar PF–PC synapses differs substantially from hippocampal plasticity and relies on GluR2 subunit trafficking. Their data provide an example of a form of LTP that depends on the activity-dependent insertion of GluR2 subunits into synapses. These findings complement an emerging picture of remarkable differences between hippocampal and cerebellar plasticity, but also astonishing similarities.
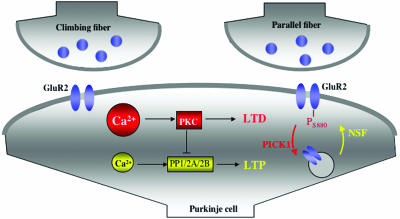
In several aspects, cerebellar plasticity provides a mirror image of hippocampal plasticity: hippocampal LTP induction requires large calcium transients that promote the activation of calcium/calmodulin-dependent kinase II (CaMKII) and protein kinase C (PKC), whereas hippocampal LTD relies on protein phosphatase (PP) activation after lower calcium transients (note that most PPs are not directly calcium-activated). In cerebellar plasticity, the kinase/phosphatase dependencies are inverse to the hippocampal ones (Fig. 1): LTD can be induced by paired PF and climbing fiber activity and requires a larger calcium transient than LTP induction (2), which can be observed after PF stimulation alone (3). PF-LTD is PKC-dependent (4), whereas the induction of LTP depends on phosphatases PP1, PP2A, and PP2B (5). These differences can also be seen at the molecular level: in hippocampal plasticity, GluR1 is phosphorylated at Ser-831 (a CaMKII/PKC phosphorylation site) during LTP, whereas for LTD induction, dephosphorylation occurs at Ser-845 (a PKA phosporylation site) (6). GluR1 endocytosis (and LTD) has been shown to be PP2B-dependent (7). In cerebellar PCs, GluR1 expression is weak (8), and the majority of AMPA receptors consist of GluR2–GluR3 heteromeric complexes. PF-LTD requires a PKC-dependent phosphorylation of GluR2 at Ser-880 (9) and is mediated by GluR2 endocytosis (10). As will be discussed below, GluR2 subunit trafficking occurs at hippocampal synapses as well, but GluR2 insertion is seen as a constitutive delivery mechanism that, in contrast to GluR1 subunit trafficking, is not driven by synaptic activity (11).
Fig. 1.
LTP and LTD induction mechanisms at cerebellar PF–PC synapses. For simplicity, climbing fiber (CF) and PF terminals are shown to contact the same postsynaptic compartment. The LTD induction cascade is shown in red: a large calcium transient (resulting from paired PF and CF activity) promotes PKC activation, which phosphorylates GluR2 at Ser-880. GluR2 endocytosis requires binding of GluR2 to protein interacting with C-kinase1 (PICK1). The LTP cascade is shown in yellow: lower calcium transients (resulting from PF activity) promote phosphatase activation (only PP2B is directly calcium-regulated). GluR2 insertion requires GluR2 binding to NSF.
In a recent issue of PNAS, Kakegawa and Yuzaki (1) present a different view on GluR2 subunit trafficking. They demonstrate that, during cerebellar LTP, GluR2 subunits are delivered to synapses and that this GluR2 insertion is an activity-dependent process that involves NO-mediated N-ethylmaleimide-sensitive factor (NSF) binding to GluR2. These findings provide evidence of activity-dependent GluR2 synapse delivery underlying LTP induction. In this study, LTP was monitored by using whole-cell patch-clamp recordings from PCs in mouse cerebellar slices. Previously, it has been described that LTP can be elicited by bath application of NO donors (3). Kakegawa and Yuzaki (1) use the NO donor diethylamine NO sodium salt, which leads to a postsynaptically expressed potentiation. Expression of PF-LTD involves a clathrin-mediated endocytosis of GluR2 subunits (10) after binding of GluR2 to protein interacting with C-kinase1 (PICK1) (12). To test whether GluR2 subunit insertion mediates PF-LTP in turn, botulinum toxin (BoTx; light chain) was added to the pipette saline, which interferes with soluble NSF attachment protein receptor (SNARE)-dependent exocytosis. BoTx application blocked LTP, whereas heat-inactivated BoTx did not. NSF–GluR2 binding is required for the membrane insertion and stabilization of GluR2 (13). It has been shown that NO can trigger this process by promoting S-nitrosylation of NSF (14). To examine whether this NSF–GluR2 interaction is required for PF-LTP, Kakegawa and Yuzaki (1) added pep-R845A to the pipette saline; this peptide interferes with the binding of NSF to GluR2. Interestingly, pep-R845A caused an excitatory postsynaptic current amplitude decrease and subsequently blocked LTP induction. These observations suggest that the NSF-dependent GluR2 insertion is not only required for the constitutive synapse delivery of GluR2 (11) but can be involved in LTP as well. Typically, the term “activity-dependent” relates to synaptic activity; thus a form of NO-triggered LTP is not strictly activity-dependent. However, this distinction is not valid here, as synaptically induced LTP and NO-evoked LTP have been shown to occlude each other (3) and it is known that the NO pathway can be synaptically triggered.
Previously, it was shown that PF-LTP induction requires lower calcium transients than PF-LTD induction (2) and that PF-LTP depends on phosphatase rather than kinase activity (5). In their article, Kakegawa and Yuzaki (1) confirm that PF-LTP is enhanced by low concentrations of the calcium chelator 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) (5 mM), but is blocked by higher BAPTA concentrations (30 mM). They also tested for the effects of previous application of the phorbol ester 12-O-tetradecanoyl-phorbol-13-acetate (TPA), which activates PKC and promotes synaptic depression in PCs. Previous TPA treatment leads to enhanced diethylamine NO sodium salt-evoked LTP.
This observation suggests that a larger potentiation can be achieved when the synapses were previously depressed, implying that LTP itself is not PKC-dependent. Finally, they tested for the involvement of CaMKII in LTP induction by applying the CaMKII inhibitor autocamtide-2 related inhibitory peptide (AIP). When AIP was added to the pipette saline, LTP was not affected, indicating that LTP is not CaMKII-dependent.
GluR2 subunit trafficking mediates bidirectional cerebellar plasticity.
It is obvious from the LTP data presented here (1) and from previous work on GluR2 endocytosis in LTD (10) that GluR2 subunit trafficking mediates bidirectional cerebellar plasticity. Remarkably, the molecular mechanisms underlying GluR2 subunit trafficking seem to be the same at hippocampal synapses: NSF–GluR2 binding promotes GluR2 membrane insertion at both hippocampal (15) and cerebellar (16) synapses. At both types of synapses, GluR2 subunits can be internalized by clathrin-mediated endocytosis (10, 17) after GluR2 phosphorylation at Ser-880, which subsequently results in a depression that occludes LTD (9, 18). Thus, whereas kinase activity in CA1 pyramidal cells phosphorylates GluR1 and promotes LTP, GluR2 phosphorylation in PCs and CA1 pyramidal cells causes endocytosis and can promote LTD. In GluR1–GluR2 heteromeres, GluR1 dominates the trafficking behavior (13). What role remains for hippocampal GluR2-dominated trafficking (e.g., GluR2–GluR3 heteromeres)? It has been suggested that NSF-dependent GluR2 insertion is crucial for the constitutive delivery of GluR2 to synapses (11). Recent studies suggest that GluR2 trafficking is not only about changing AMPA receptor densities, but might also alter the ratio of GluR2-containing and GluR2-lacking receptors with consequences for the calcium permeability (19). The effect of GluR2 subunit endocytosis on synaptic strength remains unclear. GluR2 endocytosis results in a depression that occludes LTD (18). However, a decrease in the GluR2 content of AMPA receptors can also cause a potentiation, because GluR2-containing receptors have a lower single-channel conductance than GluR2-lacking receptors (20). This observation suggests that, under some conditions, exocytosis of GluR1 subunits and endocytosis of GluR2 subunits, both of which are triggered by kinase activity, can act in concert to promote hippocampal LTP induction.
The article by Kakegawa and Yuzaki (1) suggests a less ambiguous key role of GluR2 subunit trafficking in cerebellar plasticity: GluR2 synapse delivery underlies LTP at PF–PC synapses. This form of GluR2 insertion is activity-dependent as it is triggered by NO-dependent activation of NSF, enabling binding of NSF to GluR2.
C.H. wrote the paper.
Conflict of interest statement: No conflicts declared.
See companion article on page 17846 in issue 49 of volume 102.
References
- 1.Kakegawa, W. & Yuzaki, M. (2005) Proc. Natl. Acad. Sci. USA 102, 17846–17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coesmans, M., Weber, J. T., De Zeeuw, C. I. & Hansel, C. (2004) Neuron 44, 691–700. [DOI] [PubMed] [Google Scholar]
- 3.Lev-Ram, V., Wong, S. T., Storm, D. R. & Tsien, R. Y. (2002) Proc. Natl. Acad. Sci. USA 99, 8389–8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansel, C., Linden, D. J. & D'Angelo, E. (2001) Nat. Neurosci. 4, 467–475. [DOI] [PubMed] [Google Scholar]
- 5.Belmeguenai, A. & Hansel, C. (2005) J. Neurosci. 25, 10768–10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, H.-K., Barbarosie, M., Kameyama, K., Bear, M. F. & Huganir, R. L. (2000) Nature 405, 955–959. [DOI] [PubMed] [Google Scholar]
- 7.Beattie, E. C., Carroll, R. C., Yu, X., Morishita, W., Yasuda, H., von Zastrow, M. & Malenka, R. C. (2000) Nat. Neurosci. 3, 1291–1300. [DOI] [PubMed] [Google Scholar]
- 8.Baude, A., Molnar, E., Latawiec, D., McIlhinney, R. A. J. & Somogyi, P. (1994) J. Neurosci. 14, 2830–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, H. J., Steinberg, J. P., Huganir, R. L. & Linden, D. J. (2003) Science 300, 1751–1755. [DOI] [PubMed] [Google Scholar]
- 10.Wang, Y. T. & Linden, D. J. (2000) Neuron 25, 635–647. [DOI] [PubMed] [Google Scholar]
- 11.Shi, S.-H., Hayashi, Y., Esteban, J. A. & Malinow, R. (2001) Cell 105, 331–343. [DOI] [PubMed] [Google Scholar]
- 12.Xia, J., Chung, H. J., Wihler, C., Huganir, R. L. & Linden, D. J. (2000) Neuron 28, 499–510. [DOI] [PubMed] [Google Scholar]
- 13.Song, I. & Huganir, R. L. (2002) Trends Neurosci. 25, 578–588. [DOI] [PubMed] [Google Scholar]
- 14.Huang, Y., Man, H.-Y., Sekine-Aizawa, Y., Han, Y., Juluri, K., Luo, H., Cheah, J., Lowenstein, C., Huganir, R. L. & Snyder, S. H. (2005) Neuron 46, 533–540. [DOI] [PubMed] [Google Scholar]
- 15.Lüscher, C., Xia, H., Beattie, E. C., Caroll, R. C., von Zastrow, M., Malenka, R. C. & Nicoll, R. A. (1999) Neuron 24, 649–658. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg, J. P., Huganir, R. L. & Linden, D. J. (2004) Proc. Natl. Acad. Sci. USA 101, 18212–18216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man, H.-Y., Lin, J. W., Ju, W. H., Ahmadian, G., Liu, L., Becker, L. E., Sheng, M. & Wang, Y. T. (2000) Neuron 25, 649–662. [DOI] [PubMed] [Google Scholar]
- 18.Seidenman, K. J., Steinberg, J. P., Huganir, R. L & Malinow, R. (2003) J. Neurosci. 23, 9220–9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner, S. M., Takamiya, K., Xia, J., Suh, J.-G., Johnson, R., Yu, S. & Huganir, R. L. (2005) Neuron 45, 903–915. [DOI] [PubMed] [Google Scholar]
- 20.Terashima, A., Cotton, L., Dev, K. K., Meyer, G., Zaman, S., Duprat, F., Henley, J. M., Collingridge, G. L. & Isaac, J. T. R. (2004) J. Neurosci. 24, 5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]