Abstract
Background
Pathologists are often faced with the dilemma of whether to recommend continuation of methotrexate therapy for psoriasis within the context of an existing pro-fibrogenic risk factor, in this instance, patients with genetic hemochromatosis.
Case presentations
We describe our experience with two male psoriatic patients (A and B) on long term methotrexate therapy (cumulative dose A = 1.56 gms and B = 7.88 gms) with hetero- (A) and homozygous (B) genetic hemochromatosis. These patients liver function were monitored with routine biochemical profiling; apart from mild perivenular fibrosis in one patient (B), significant liver fibrosis was not identified in either patient with multiple interval percutaneous liver biopsies; in the latter instance this patient (B) had an additional risk factor of partiality to alcohol.
Conclusion
We conclude that methotrexate therapy is relatively safe in patients with genetic hemochromatosis, with no other risk factor, but caution that the risk of fibrosis be monitored, preferably by non-invasive techniques, or by liver biopsy.
Background
Liver fibrosis, increased liver collagen content, [1-3] damage to the canals of Hering, [4] and cirrhosis, have been reported in patients being treated with methotrexate for psoriasis [5] or rheumatoid arthritis [6] but not in the context of inflammatory bowel disease;[7,8] some studies suggest that this risk of liver fibrosis is overstated [9-11]. The duration of methotrexate therapy,[12] patient age, weekly oral dosage schedule,[13] cumulative methotrexate dose,[14] risk factors for non-alcoholic steatohepatitis (obesity, diabetes mellitus, glucose intolerance)[6,15-17] and concurrent alcohol ingestion [14,18] have been identified as significant risk factors in the evolution of liver fibrosis in these patients. By contrast long-term low-dose methotrexate therapy, in the absence of excess alcohol ingestion or other profibrogenic agents or diseases, have been reported as being relatively free of the risk of developing fibrosis [19,20]. It has been recommended that baseline pre-treatment liver biopsies are necessary to define the initial histologic status of the liver [21,22]. Although annual, or other suitable interval, liver biopsies are recommended with this treatment, to monitor development of liver fibrosis,[12,13,17,21] its necessity in the first five years of treatment[12] or the need at all for this invasive procedure [11] has been questioned; recent guidelines from the British Society of Gastroenterology do not include methotrexate as an indication for liver biopsy [23]. Ultrasound liver examination [24] and measurement of serum type III procollagen aminopeptide (PIIINP) [25-27] have been suggested as alternatives to liver biopsy; the former is useful only if the US appearances are normal as it is not discriminatory in identifying fibrosis [24]. We reflect on our experience with two patients receiving methotrexate for psoriasis, with genetic hemochromatosis (GH) as an "additional" risk factor for the development of liver fibrosis.
Case presentations
The clinical histories of two patients (patient A and patient B) with psoriasis, being treated with methotrexate, are summarised. During the course of their treatment, liver biopsies were performed as baseline to rule out and subsequently monitor the development of liver fibrosis as, at the present time PIIINP monitoring of liver fibrois is not available in our institution. The possibility of GH was queried on histological grounds and subsequently confirmed with genotyping.
Patient A
A 47 year-old male patient presented to the dermatologist October 1998 following an episode of severe flare up of psoriasis, which he had had for 15 years. His condition improved with coal tar, hydrocortisone ointment and UVB. However, after stopping UVB, his flare up recurred. Methotrexate was introduced to his treatment regime. After a satisfactory baseline blood test and a trial dose of 2.5 mg, he started methotrexate (10 mg/week) in April 1999. Two weeks later the dose was increased to 15 mg/week. In June 1999, the dose was increased to 17.5 mg/week and then back to 15 mg/week in July 1999 with improvement of his psoriasis. There was no history of excess alcohol ingestion.
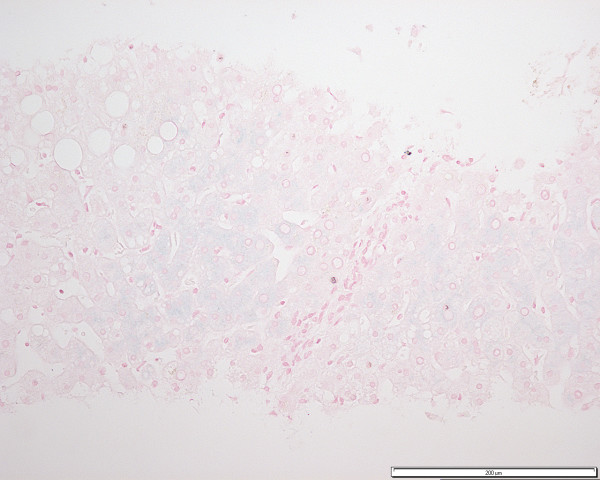
He had his first liver biopsy, six months later (October 1999) which showed moderate acinar zone 3 and 2 macrovesicular steatosis, with lesser amounts of microvesicular steatosis. Acinar zone 1, grade 1 parenchymal iron accumulation, with an acinar gradient, suggestive of heterozygous form of GH was seen with Perl's Prussian Blue (figure 1); iron was not detected in sinusoidal Kupffer cells or macrophages. There was no evidence of fibrosis or, accumulation of copper or alpha 1 anti-trypsin. Genotypic studies confirmed heterozygosity for the HFE gene mutation H63D (in the absence of C282Y).
Figure 1.
Perl's Prussian Blue stain for iron shows a typical pattern of iron accumulation seen in heterozygous GH (Patient A) (x20)
He had his second liver biopsy in November 2001; total cumulative methotrexate dose of 1.56 gm from his first liver biopsy. The biopsy showed moderate macrovesicular steatosis, mild steatohepatitis, grade 1 iron accumulation similar to his first biopsy but no fibrosis or contra-indication to the continued use of methotrexate.
Patient B
This 58 year-old patient was first seen by the dermatologist in October 1992 with psoriasis. He responded well with UVL and Alphosyl HC. In February 1993, his psoriasis got worse and methotrexate was started with a test dose of 5mg. Following satisfactory baseline blood tests, that was gradually increased to 30 mg/week. His skin condition improved significantly with treatment.
He stopped methotrexate before Christmas 1994 but subsequently he restarted it in March 1995. He had a liver biopsy in August 1995 (cumulative methotrexate dose of 2115 mg) which showed moderate steatosis, moderate deposition of hemosiderin within hepatocytes and no evidence of fibrosis. His liver function tests and full blood counts were normal at that time.
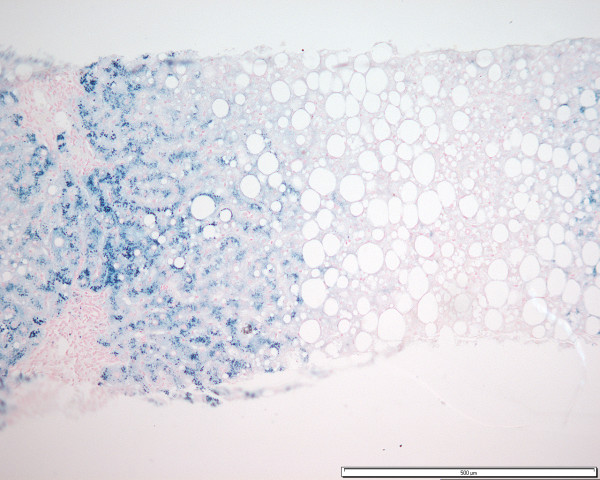
He was lost for follow up for 2 years between May 1996 to May 1998. When he was seen again in May 1998 he had slowly rising ALT and was still on methotrexate. Another liver biopsy (cumulative methotrexate dose of 5000 mg) was performed which showed moderate to severe macrovesicular steatosis with smaller amounts of microvesicular steatosis, predominantly in acinar zones 3 and 2. Grade 3 parenchymal iron accumulation, with an acinar gradient, was seen in acinar zone 1 (Figure 2) suggestive of homozygous GH. There was no evidence of fibrosis and thus no contra-indication for continued use of methotrexate. Genotypic studies confirmed homozygosity for HFE gene mutation C282Y, following which regular venesection was arranged. He continued on methotrexate until March 1999 when his ALT was raised at 96. However, as stopping methotrexate did not improve his liver function test result it was restarted together with UVL therapy in May 1999 for flare up.
Figure 2.
Grade 3 hepatocyte iron accumulation with an acinar distribution pattern consistent with homozygous GH (Patient B) (Perl's Prussian Blue: ×10).
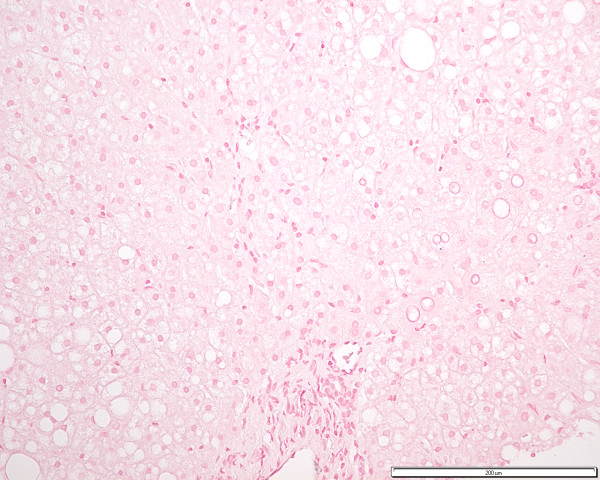
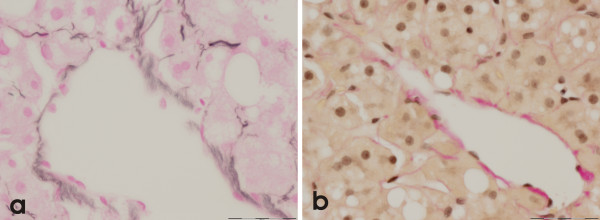
He continued with methotrexate despite failed attempt to reduce the dose. He had another liver biopsy in October 2001 (total cumulative methotrexate dose of 7880 mg) which showed moderate steatosis, moderate to severe steatohepatitis, marginal perivenular fibrosis but no residual iron (Figure 3); the former features were reminiscent of alcohol-induced liver damage (Figure 4). As there was no significant fibrosis, continued methotrexate administration was not contra-indicated although he was advised to curtail his alcohol ingestion.
Figure 3.
Following venesection (Patient B), there is no histological evidence of residual iron (×20).
Figure 4.
This figure shows a reticulin (a) and a Haematoxylin Van Gieson stain (b) of two terminal hepatic venules in which there is equivocal perivenular fibrosis (x40).
Discussion
Pathologists are occasionally faced with a decision of whether to recommend continuation of methotrexate therapy when a patient has an "additional" risk factor for the development of liver fibrosis and/or cirrhosis; in this instance with two patients on long-term methotrexate therapy who had GH. As far as we are aware, there have been no reports in the english literature that addresses this specific issue.
Patients with GH are at risk of developing liver fibrosis and, in the long term, liver cirrhosis; [28-30] there is a also, a risk of developing fibrosis with methotrexate therapy alone [31,32]. Cessation of methotrexate therapy is advised in the presence of significant liver fibrosis [13].
We have described our experience of two hemochromatotic patients on low dose on methotrexate therapy for relatively short periods of three and five years and relatively low cumulative doses. Although one of these patients with homozygous GH was also partial to alcohol, he did not show evidence of significant fibrosis apart from equivocal alcohol-associated perivenular fibrosis. In such instances, adequate counselling of the patient of the risk of excess alcohol in this setting is probably appropriate. There was no evidence of liver damage or fibrosis in our patient with heterozygous GH.
Although measurement of PIIINP can be useful in defining the development of liver fibrosis,[25] elevated or rising levels are not specific or sensitive for liver fibrosis; [25-27] however, liver biopsy has been recommended in patients whose PIINP levels increase from normal whilst they are being monitored for liver fibrosis [27]. Clearly PIIINP measurements will not distinguish fibrosis due to methotrexate or GH, in combination or alone or, fibrosis developing as a result of any other profibrogenic agent. We are unable to offer PIIINP measurements for evaluating liver fibrosis in our institution.
Conclusion
We conclude that methotrexate therapy is relatively safe in patients with GH but caution that the risk of fibrosis be monitored, preferably by non-invasive techniques, or by liver biopsy. We recommend that patients in this setting be advised of the potential for liver damage especially with "additonal" risk factors.
List of abbreviations
serum type III procollagen aminopeptide (PIIINP)
genetic hemochromatosis (GH)
ultraviolet B (UVB)
HFE: gene symbol
ultraviolet light (UVL)
alanine aminotransferase (ALT)
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
JM is the local liver pathologist who made the diagnosis of GH and together with MYL interrogated and prepared the manuscript. ADB provided expert review, advice and contributed to the preparation of the manuscript, as did NM who also initiated contact with these patients and secured consent. All authors have read and approved the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Joseph Mathew, Email: joe.mathew@rcht.cornwall.nhs.uk.
May Y Leong, Email: mayyingl@yahoo.com.
Nick Morley, Email: Nick.Morley@rcht.cornwall.nhs.uk.
Alastair D Burt, Email: A.D.Burt@newcastle.ac.uk.
References
- Bjorkman DJ, Hammond EH, Lee RG, Clegg DO, Tolman KG. Hepatic ultrastructure after methotrexate therapy for rheumatoid arthritis. Arthritis Rheum. 1988;31:1465–1472. doi: 10.1002/art.1780311202. [DOI] [PubMed] [Google Scholar]
- Nohlgard C, Rubio CA, Kock Y, Hammar H. Liver fibrosis quantified by image analysis in methotrexate-treated patients with psoriasis. J Am Acad Dermatol. 1993;28:40–45. doi: 10.1016/0190-9622(93)70006-f. [DOI] [PubMed] [Google Scholar]
- Jaskiewicz K, Voigt H, Blakolmer K. Increased matrix proteins, collagen and transforming growth factor are early markers of hepatotoxicity in patients on long-term methotrexate therapy. J Toxicol Clin Toxicol. 1996;34:301–305. doi: 10.3109/15563659609013794. [DOI] [PubMed] [Google Scholar]
- Hytiroglou P, Tobias H, Saxena R, Abramidou M, Papadimitriou CS, Theise ND. The canals of hering might represent a target of methotrexate hepatic toxicity. Am J Clin Pathol. 2004;121:324–329. doi: 10.1309/5HR9-0TNC-4Q4J-RXWX. [DOI] [PubMed] [Google Scholar]
- Nyfors A. Liver biopsies from psoriatics related to methotrexate therapy. 3. Findings in post-methotrexate liver biopsies from 160 psoriatics. Acta Pathol Microbiol Scand [A] 1977;85:511–518. doi: 10.1111/j.1699-0463.1977.tb03882.x. [DOI] [PubMed] [Google Scholar]
- Shergy WJ, Polisson RP, Caldwell DS, Rice JR, Pisetsky DS, Allen NB. Methotrexate-associated hepatotoxicity: retrospective analysis of 210 patients with rheumatoid arthritis. Am J Med. 1988;85:771–774. doi: 10.1016/s0002-9343(88)80019-6. [DOI] [PubMed] [Google Scholar]
- Lemann M, Zenjari T, Bouhnik Y, Cosnes J, Mesnard B, Rambaud JC, Modigliani R, Cortot A, Colombel JF. Methotrexate in Crohn's disease: long-term efficacy and toxicity. Am J Gastroenterol. 2000;95:1730–1734. doi: 10.1111/j.1572-0241.2000.02190.x. [DOI] [PubMed] [Google Scholar]
- Te HS, Schiano TD, Kuan SF, Hanauer SB, Conjeevaram HS, Baker AL. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2000;95:3150–3156. doi: 10.1111/j.1572-0241.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- Boffa MJ, Chalmers RJ, Haboubi NY, Shomaf M, Mitchell DM. Sequential liver biopsies during long-term methotrexate treatment for psoriasis: a reappraisal. Br J Dermatol. 1995;133:774–778. doi: 10.1111/j.1365-2133.1995.tb02754.x. [DOI] [PubMed] [Google Scholar]
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399–408. doi: 10.1111/j.1365-2230.1996.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Aithal GP, Haugk B, Das S, Card T, Burt AD, Record CO. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: are serial liver biopsies justified? Aliment Pharmacol Ther. 2004;19:391–399. doi: 10.1046/j.1365-2036.2004.01819.x. [DOI] [PubMed] [Google Scholar]
- Reynolds FS, Lee WM. Hepatotoxicity after long-term methotrexate therapy. South Med J. 1986;79:536–539. doi: 10.1097/00007611-198605000-00005. [DOI] [PubMed] [Google Scholar]
- Robinson JK, Baughman RD, Auerbach R, Cimis RJ. Methotrexate hepatotoxicity in psoriasis. Consideration of liver biopsies at regular intervals. Arch Dermatol. 1980;116:413–415. doi: 10.1001/archderm.116.4.413. [DOI] [PubMed] [Google Scholar]
- Nyfors A, Poulsen H. Morphogenesis of fibrosis and cirrhosis in methotrexate-treated patients with psoriasis. Am J Surg Pathol. 1977;1:235–243. doi: 10.1097/00000478-197709000-00005. [DOI] [PubMed] [Google Scholar]
- Langman G, Hall PM, Todd G. Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J Gastroenterol Hepatol. 2001;16:1395–1401. doi: 10.1046/j.1440-1746.2001.02644.x. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Koff R. A debate: should patients with rheumatoid arthritis on methotrexate undergo liver biopsies? Semin Arthritis Rheum. 1992;21:376–386. doi: 10.1016/0049-0172(92)90038-F. [DOI] [PubMed] [Google Scholar]
- Malatjalian DA, Ross JB, Williams CN, Colwell SJ, Eastwood BJ. Methotrexate hepatotoxicity in psoriatics: report of 104 patients from Nova Scotia, with analysis of risks from obesity, diabetes and alcohol consumption during long term follow-up. Can J Gastroenterol. 1996;10:369–375. doi: 10.1155/1996/213596. [DOI] [PubMed] [Google Scholar]
- Ashton RE, Millward-Sadler GH, White JE. Complications in methotrexate treatment of psoriasis with particular reference to liver fibrosis. J Invest Dermatol. 1982;79:229–232. doi: 10.1111/1523-1747.ep12500067. [DOI] [PubMed] [Google Scholar]
- Van Dooren-Greebe RJ, Kuijpers AL, Mulder J, De Boo T, Van de Kerkhof PC. Methotrexate revisited: effects of long-term treatment in psoriasis. Br J Dermatol. 1994;130:204–210. doi: 10.1111/j.1365-2133.1994.tb02901.x. [DOI] [PubMed] [Google Scholar]
- Lanse SB, Arnold GL, Gowans JD, Kaplan MM. Low incidence of hepatotoxicity associated with long-term, low-dose oral methotrexate in treatment of refractory psoriasis, psoriatic arthritis, and rheumatoid arthritis. An acceptable risk/benefit ratio. Dig Dis Sci. 1985;30:104–109. doi: 10.1007/BF01308193. [DOI] [PubMed] [Google Scholar]
- Kevat S, Ahern M, Hall P. Hepatotoxicity of methotrexate in rheumatic diseases. Med Toxicol Adverse Drug Exp. 1988;3:197–208. doi: 10.1007/BF03259882. [DOI] [PubMed] [Google Scholar]
- Dolan OM, Burrows D, Irvine A, Walsh M. The value of a baseline liver biopsy prior to methotrexate treatment. Br J Dermatol. 1994;131:891–894. doi: 10.1111/j.1365-2133.1994.tb08596.x. [DOI] [PubMed] [Google Scholar]
- British Society of Gastroenterology Guidelines on the use of liver biopsy in clinical practice. 2004.
- Coulson IH, McKenzie J, Neild VS, Joseph AE, Marsden RA. A comparison of liver ultrasound with liver biopsy histology in psoriatics receiving long-term methotrexate therapy. Br J Dermatol. 1987;116:491–495. doi: 10.1111/j.1365-2133.1987.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Zachariae H, Aslam HM, Bjerring P, Sogaard H, Zachariae E, Heickendorff L. Serum aminoterminal propeptide of type III procollagen in psoriasis and psoriatic arthritis: relation to liver fibrosis and arthritis. J Am Acad Dermatol. 1991;25:50–53. doi: 10.1016/0190-9622(91)70173-y. [DOI] [PubMed] [Google Scholar]
- Boffa MJ, Smith A, Chalmers RJ, Mitchell DM, Rowan B, Warnes TW, Shomaf M, Haboubi NY. Serum type III procollagen aminopeptide for assessing liver damage in methotrexate-treated psoriatic patients. Br J Dermatol. 1996;135:538–544. doi: 10.1046/j.1365-2133.1996.d01-1036.x. [DOI] [PubMed] [Google Scholar]
- Zachariae H, Heickendorff L, Sogaard H. The value of amino-terminal propeptide of type III procollagen in routine screening for methotrexate-induced liver fibrosis: a 10-year follow-up. Br J Dermatol. 2001;144:100–103. doi: 10.1046/j.1365-2133.2001.03959.x. [DOI] [PubMed] [Google Scholar]
- Mathew J. Burt AD . Cellular mechanisms of fibrogenesis in genetic haemochromatosis. In: Knook DL and Wisse E, editor. Cells of the hepatic sinusoid. Vol. 4 , The Kupffer Cell Foundation, Netherlands; 1993. pp. 274–276. [Google Scholar]
- Wojcik JP. Speechley MR. Kertesz AE. Chakrabarti S. PC A. Natural history of C282Y homozygotes for hemochromatosis. Can J Gastroenterol. 2002;16:297–302. doi: 10.1155/2002/161569. [DOI] [PubMed] [Google Scholar]
- Hubscher SG. Iron overload, inflammation and fibrosis in genetic haemochromatosis. J Hepatol. 2003;38:521–525. doi: 10.1016/S0168-8278(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Whiting-O'Keefe QE, Fye KH, Sack KD. Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am J Med. 1991;90:711–716. [PubMed] [Google Scholar]
- Zachariae H, Sogaard H, Heickendorff L. Methotrexate-induced liver cirrhosis. Clinical, histological and serological studies--a further 10-year follow-up. Dermatology. 1996;192:343–346. doi: 10.1159/000246408. [DOI] [PubMed] [Google Scholar]