Abstract
A series of experiments, using cell culture models or in vitro assays, has shown that the RNA-binding protein HuR increases the half-life of some messenger RNAs that contain adenylate/uridylate-rich decay elements. However, its function in an integrated system has not yet been investigated. Here, using a mouse model, we report that misregulation of HuR, due to expression of an HuR transgene, prevents the production of fully functional gametes. This work provides the first evidence for a physiological function of HuR, and highlights its involvement in spermatogenesis.
Introduction
Regulation of the half-lives of certain messenger RNAs has important consequences for growth and development. In eukaryotes, cell proliferation and differentiation are mainly controlled by the expression of early response genes (ERGs), which encode proto-oncogene products (for example, c-Myc, c-Fos and c-Jun), cytokines and growth factors. Of the short-lived mRNAs transcribed from these genes, many contain cis-acting elements, which are known as adenylate- and uridylate-rich elements (AREs), in their 3′ untranslated regions. These AREs are partly responsible for the rapid degradation of the mRNAs that contain them (Chen & Shyu, 1995; Wilson & Brewer, 1999). The occurrence of AREs in mRNAs encoding a wide range of proteins (Bakheet et al., 2001) suggests that they have regulatory functions in a variety of biological processes. A large group of proteins, known as AU-binding proteins (AUBPs), regulate the cellular half-lives of many mRNAs by interacting directly with their AREs (Chen & Shyu, 1995; Wilson & Brewer, 1999). One of these proteins, HuR, is a ubiquitously expressed member of the embryonic-lethal abnormal vision (ELAV) family of RNA-binding proteins (Good, 1995), and is known to stabilize ARE-containing mRNAs (Fan & Steitz, 1998a; Peng et al., 1998; Wang et al., 2000a,b). Although it is mainly localized to the nucleus, HuR can be transported to the cytoplasm (Fan & Steitz, 1998a,b; Keene, 1999). The fact that HuR can shuttle between the nucleus and the cytoplasm suggests that, in addition to its function in the stabilization of ERG mRNAs, HuR might function as one of the main export adaptors for this important class of mRNAs (Gallouzi & Steitz, 2001). Moreover, this two-way traffic might be involved in regulating cell growth and differentiation (Wang et al., 2000a). These data show the importance of cellular localization for HuR function, and therefore suggest that this protein may be regulated differently in response to various developmental or environmental conditions, it might have several functions in mRNA metabolism.
To analyse the role of HuR in physiological situations, in which all the signals involved in modulating its activity are functioning, we produced β-actin–HuR transgenic mice, and showed that HuR overexpression during gametogenesis is correlated with a low rate of transgene transmission. Our results suggest that regulated HuR expression is required for proper spermatogenesis.
Results and Discussion
We designed a β-actin–HuR construct (BA–HuR), in which a sequence encoding a c-Myc-tagged HuR protein was placed under the control of the ubiquitously expressed β-actin promoter (see Fig. 1A, and Methods). We derived six founders, and analysed transgene expression by western blot analysis using a specific antibody against the c-Myc tag, 9E10 (see Methods). Unexpectedly, the transgene was not detected in any of the somatic tissues analysed, which were brain, gastrointestinal tract, heart, liver, kidney, lung, spleen, thymus and skin (Fig. 2A, and data not shown). However, it was expressed at high levels in germ cells (Fig. 2A; Gouble, 2001). These data differ from the results we obtained using the β-actin–Auf1 mice, which ubiquitously expressed the Auf1–hnRNPD transgene (Gouble et al., 2002). Because mice from all of the BA–HuR lines showed the same restricted pattern of HuR expression, we concluded that this was a result of the sequence of the BA–HuR transgene itself, and not of the sites of transgene integration into the mouse genome. In addition, Southern blot analysis showed that the CpG island in the β-actin promoter was methylated to a greater extent in the DNA from BA–HuR mice compared with that from β-actin–Auf1 mice, suggesting that this methylation contributes to gene silencing (Fig. 2B).
Figure 1.
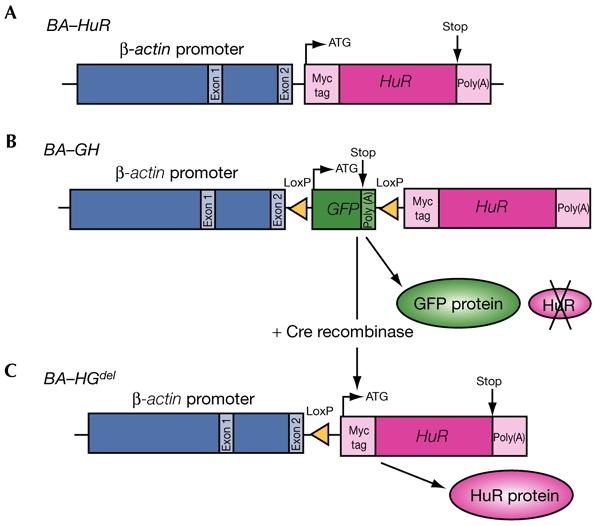
Transgene structure. Schematic representation of the β-actin–HuR (BA–HuR) (A), β-actin–loxP–GFP–loxP–HuR (BA–GH) (B) and BA–HGdel (C) transgenes. GFP, green fluorescent protein.
Figure 2.
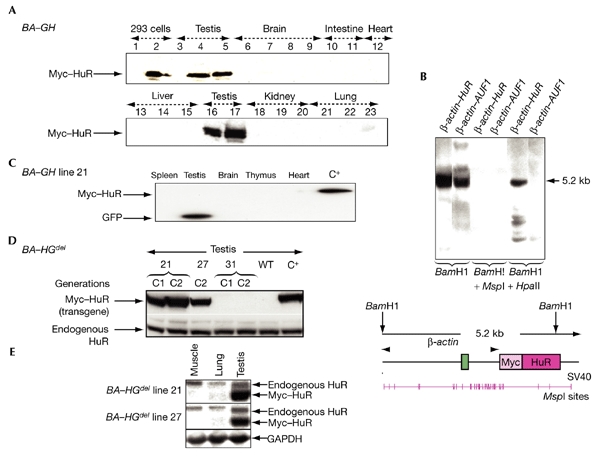
Analysis of transgene expression. (A) Western blot analysis of Myc–HuR expression using the 9E10 anti-Myc antibody on samples from 293 cells (either untransfected (lane 1), or transfected with the β-actin–HuR (BA–HuR) construct (lane 2), and from various organs, as indicated, from wild-type (lanes 3, 6, 10, 13, 18 and 21), BA–HuR line 18 (lanes 4, 5, 7, 8, 11, 15, 17, 20 and 23) or BA–HuR line 6 (lanes 9, 12, 14, 16, 19 and 22) mice. (B) Comparison of the methylation status of β-actin regulatory sequences in BA–HuR and β-actin–Auf1 mice. A Southern blot was carried out on DNA that was extracted from brain, digested with the indicated restriction enzymes, and hybridized with a β-actin probe. The structure of the β-actin–HuR transgene and the location of the MspI and HpaII sites are shown in the bottom panel. (C) Western blot analysis of transgene expression in BA–GH line 21 using anti-green-fluorescent-protein (anti-GFP) and anti-Myc antibodies to analyse samples from spleen, testis, brain, thymus and heart. C+ indicates a C1 mouse from the BA–HGdel line 21 different to that used for the first five lanes. (D). Myc–HuR (transgenic) and endogenous HuR expression, as detected by using the anti-Myc or anti-HuR antibodies on testes from BA–HGdel lines 21, 27 and 31 (from the C1 and C2 generations) and from wild-type mice. (E) Northern blot analysis was carried out on RNA extracted from muscle, lung and testis from BA–HGdel line 21 and line 27 mice, and the blot was hybridized with an HuR or GAPDH probe. BA–Auf1, β-actin–Auf1; SV40, simian virus 40; WT, wild type.
Surprisingly, although fertile, the founders did not transmit the transgene to their offspring, or only did so at a low rate (5–18%; Table 1). This could be explained by either 100% mosaicism, due to late transgene integration, leading to a low percentage of transgenic primordial germ cells, or by a reduction in the number of functional transgenic gametes, due to the presence, or expression, of the transgene during gametogenesis.
Table 1.
Transgene transmission to the descendents of β-actin–HuR founders
| Generation |
Transmission (%) |
|||||
|---|---|---|---|---|---|---|
| Founder 2 (F) | Founder 5 (F) | Founder 21 (M) | Founder 6 (M) | Founder 18 (M) | Founder 19 (M) | |
| F1 (F0 × WT) | 0 (n = 16) | 0 (n = 41) | 0 (n = 57) | 7 (n = 27) | 18 (n = 16) | 5 (n = 85) |
F, female; GFP, green fluorescent protein; M, male; n, total number of progeny; WT, wild type.
To test the latter possibility, we used the Cre–LoxP system to conditionally express HuR. We made a β-actin–LoxP–GFP–LoxP–HuR construct (BA–GH) (Fig. 1B), obtained three founders and made transgenic lines. As seen previously in BA–HuR mice, transgene expression, analysed by green fluorescent protein (GFP) detection, was restricted to the testes in mice from the three BA–GH lines. In addition, because of the presence of the upstream GFP cassette, transgenic HuR protein was not detected (Fig. 2C, and data not shown). However, the pattern of transmission of the transgene to subsequent generations was compatible with that of a Mendelian trait, except in the case of founder 27, which showed characteristics of germline mosaicism (Table 2). These results show that when the HuR transgene is silent, neither the expression of GFP, nor the presence of the transgene, alters transgene transmission in the various BA–GH lines.
Table 2.
Transgene transmission to the descendents of β-actin–loxP–GFP–loxP–HuR founders
| Generation |
Transmission (%) |
||
|---|---|---|---|
| Founder 21 (F) | Founder 27 (M) | Founder 31 (M) | |
| F1 (F0 × WT) | 58 (n = 17) | 16 (n = 56) | 31 (n = 42) |
| N2 (F1 × WT) | 39 (n = 28) | 31 (n = 36) | 34 (n = 58) |
| C1 (F1 × Cre) | 39 (n = 19) | 31 (n = 61) | 34 (n = 35) |
| C2TgP (WT × C1) | 13 (n = 52) | 17 (n = 70) | 31 (n = 23) |
| C2TgM (C1 × WT) | 7 (n = 28) | 8 (n = 86) | 41 (n = 49) |
F, female; GFP, green fluorescent protein; M, male; n, total number of progeny; TgM, maternal transgene; TgP, paternal transgene; WT, wild type.
To analyse the effects of HuR transgene expression, the GFP cassette was deleted in vivo by crossing F1 (or N2) BA–GH males with pgk–Cre transgenic females, which express Cre recombinase during oogenesis (Fig. 1C). In this case, recombination should take place as early as the pronuclear fusion step (Lallemand et al., 1998). After the cross was carried out, the expected number of transgenic mice (known as BA–HGdel mice) were obtained in the resulting C1 generation; this number was similar to that obtained in the N2 generation for the three BA–GH lines 21, 27 and 31 (Table 2). However, when the BA–HGdel C1 mice were crossed with wild-type mice to produce the C2 generation, we observed a large decrease in the number of transgenic offspring, although the sizes of the litters were normal (eight or nine mice). This was seen for BA–HGdel lines 21 and 27, but not for line 31, regardless of whether the transgene was of paternal (C2TgP) or maternal (C2TgM) origin (Table 2).
To understand why transgene transmission was different in line 31 from that in lines 21 and 27, Southern blotting was carried out, followed by sequential hybridization with HuR, β-actin and GFP probes (Fig. 3A). Our interpretation of the results (Fig. 3B) is as follows: in mice from BA–HGdel line 21, after Cre recombination, a single complete copy of the BA–HGdel transgene remained, which hybridized with the β-actin and HuR probes, but not with the GFP probe. In mice from BA–HGdel line 27, two copies of the transgene, in opposite orientations, one of which contained truncated β-actin sequences, were identified. These did not hybridize with the GFP probe. Finally, in mice from BA–HGdel line 31, truncations of the most 5′ and the most 3′ copies of the concatamer resulted in an incomplete deletion of the GFP sequences in the remaining copy. Hence, whereas the HuR transgene should be expressed in mice from BA–HGdel lines 21 and 27, no such expression is expected in mice from BA–HGdel line 31.
Figure 3.
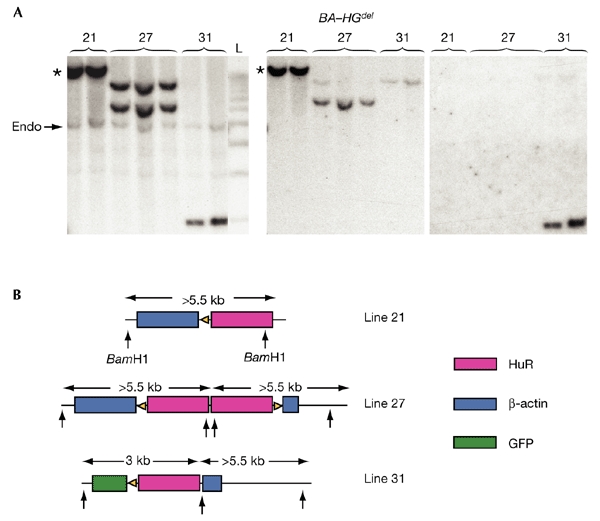
Analysis of transgene configuration in BA–HGdel transgenic lines. (A) Southern blot analysis was carried out on DNA extracted from BA–HGdel mice after Cre excision in vivo and digestion with BamHI. When several copies of the BA–GH transgene are located in a concatameric array in the mouse genome, Cre-mediated recombination between LoxP sites with the same orientation removes the intervening DNA, leaving one copy of the BA–HGdel transgene at the original chromosomal location (indicated by asterisks). (B) Interpretation of the number of copies of the transgene and their orientation in each BA–HGdel line. The arrows indicate the location of the BamHI restriction sites; the numbers (in kilobases) indicate the sizes of the fragments recognized by the different probes. L, 1-kb ladder; Endo, endogenous HuR.
To verify this, we carried out western blotting using proteins extracted from the testes of mice from BA–HGdel lines 21, 27 and 31. Myc–HuR protein was expressed in the testes of mice from BA–HGdel lines 21 and 27, in both the C1 and C2 generations, but not in the testes of wild-type or BA–HGdel line 31 mice (Fig. 2D, and data not shown). These data show a strong correlation between Myc–HuR expression in testis and a low rate of transgene transmission, as only mice expressing the HuR transgene showed a large reduction in the number of their transgenic offspring.
To determine the nature of this defect in spermatogenesis, we carried out a detailed analysis of transgene expression in BA–HuR and BA–HGdel testes. As shown by immunohistochemistry, carried out on tissues from BA–HuR mice, none of the spermatogonia, located at the base of the seminiferous epithelium, expressed the transgene, whereas a low level of labelling was seen in the primary spermatocytes, in both the nucleus and the cytoplasm. By contrast, transgene expression was high in the round spermatids, mostly in the nuclei, whereas it was only just detectable in the elongated spermatids (Fig. 4A,B). This was not seen in β-actin–Auf1 testes, which, although showing the same pattern of expression in the spermatocytes and the round spermatids, showed high expression of the Myc-tagged Auf1 transgene in the elongated spermatids (data not shown). This difference could be explained either by a lack of HuR transgene expression during late steps of differentiation, or by a loss of elongated spermatids expressing the HuR transgene, but not the Auf1 transgene. This, in turn, could lead to a marked reduction in the number of transgenic spermatozoa, explaining the low rate of HuR transgene transmission. This was confirmed by a detailed analysis of Myc–HuR expression in the spermatids at stages VIII–XII. Although there was a high level of expression of the transgene in approximately 70% of the spermatids (stages VIII and IX), a progressive loss of expression was seen in the later stages: only 20% of the elongated spermatids (stage X) expressed the transgene, and no labelling was detected in stages XI and XII. These data were reproducible in all of the three BA–HuR lines tested (Fig. 4A,B, and data not shown).
Figure 4.
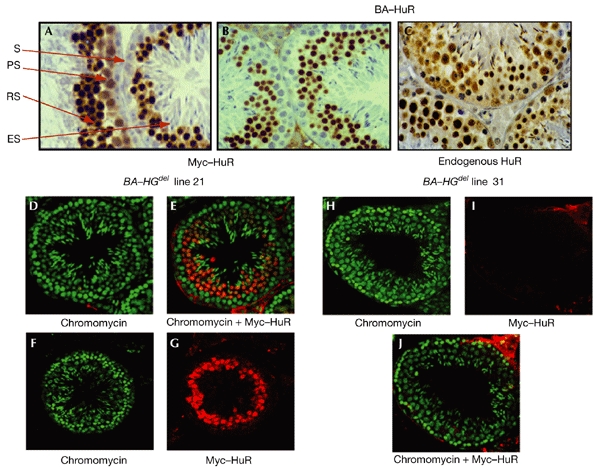
Analysis of endogenous and transgenic HuR expression at the cellular level. (A–C) Immunohistochemical analysis; testis sections (3–5 μm) from β-actin–HuR (BA–HuR) lines 19 (A) and 18 ((B) and (C)) were incubated with the 9E10 antibody to detect the HuR transgene ((A) and (B)), or with the 19F12 antibody to detect endogenous HuR (C). (D–J) Confocal analysis. Testis sections (∼60 μm) from BA–HGdel lines 21 (D–G) and 31 (H–J) were incubated with chromomycin (green), which specifically stains nuclei (D, F, H), or with 9E10 (red), which detects transgenic HuR (G, I). Double labelling gave an orange staining, which was mainly restricted to the round spermatid population; this was only visible in BA–HGdel line 21 sections (E), and not in BA–HGdel line 31 sections (J). ES, elongated spermatids; PS, primary spermatocytes; RS, round spermatids; S, spermatogonia.
The dynamic pattern of transgene expression observed in BA–HuR testes was also seen in BA–HGdel testes, as analysed by immunofluorescence. Analysis of chromomycin-labelled sections of testes showed strong orange (Fig. 4E) or red (Fig. 4G) staining, corresponding to HuR transgene expression in the round spermatids of mice from BA–HGdel line 21, but not in those from line 31 (Fig. 4I,J). Again, weak orange/red staining in the elongating spermatids of BA–HGdel mice from lines 21 and 27 was detected, confirming the results of the immunohistochemical analysis of testes from the various BA–HuR lines (Fig. 4A,B). This supports the hypothesis that a loss of elongated spermatids occurs in the transgenic mice.
The results obtained from the immunohistochemical and immunofluorescence analyses of Myc–HuR expression led us to speculate that the low rates of transmission in BA–HuR mice and mice from BA–HGdel lines 21 and 27 were due either to ectopic expression of HuR protein, or to overexpression of this protein at a specific stage of spermatogenesis. To investigate this, we analysed the expression of endogenous HuR protein in the testis, which had not been done previously. We showed, by western blot analysis, that endogenous HuR is present in wild-type and transgenic testes, and that its level of expression is not modified by transgene expression (Fig. 2D). The difference in the levels of expression of Myc–HuR and endogenous HuR was assessed further by northern blot analysis. mRNA expression from the HuR transgene was shown to be seven and five times higher than endogenous HuR mRNA in the testes of mice from BA–HGdel lines 21 and 27, respectively (Fig. 2E). We examined endogenous HuR expression at the cellular level, during spermatogenesis, by immunohistochemical analysis of seminiferous tubule sections of transgenic or wild-type testes (Fig. 4C, and data not shown). In both cases, HuR protein was expressed throughout spermatogenesis. These results show that HuR is overexpressed in the round spermatids of BA–HuR and BA–HGdel mice, which suggests that the problem in transgene transmission is due to HuR overexpression at this stage of gametogenesis.
The cell population in which HuR is mainly overexpressed during spermatogenesis is characterized by a massive wave of transcriptional activity, leading to the activation of many post-meiotic genes (Sassone-Corsi, 2002). HuR might, therefore, be required during spermatogenesis to maintain the expression of some of these genes by stabilizing their mRNAs. In transgenic testes, HuR overexpression might inappropriately control mRNA destiny (its nucleocytoplasmic transport, stability or translation), thus altering the differentiation and maturation of the spermatids, in which the expression of HuR target mRNAs is misregulated. The recent identification of numerous transcripts expressed during spermatogenesis might be helpful in identifying the mRNA targets of HuR (Fujii et al., 2002). However, these targets might either be specific to a particular stage of spermatogenesis, or be expressed in other organs. As the low rate of transgene transmission was not only observed in the male, but also in the female germline (Tables 1 and 2), HuR overexpression might deregulate a process common to ovogenesis and spermatogenesis due to impaired mRNA metabolism.
Whatever the mRNA targets of HuR might be, our data clearly show that the effect of HuR on them is different from that of Auf1. Indeed, we have made four β-actin–Auf1 lines that express a high level of transgenic Auf1 in spermatids, and in which there is no defect in transgene transmission (Gouble et al., 2002). These results suggest that these two AUBPs, which both recognize AUUUA repeats that are found in numerous ARE-containing mRNAs, do not regulate the same set of mRNAs during spermatogenesis.
In summary, we have shown that misregulation of HuR, due to HuR transgene expression, impairs transgene transmission. Our data provide the first demonstration that this RNA-binding protein has a crucial function in a physiological context, and highlights the importance of HuR regulation for proper germ-cell differentiation. The β-actin–Auf1 and β-actin–HuR transgenic mice that we generated, which express increased levels of either Auf1 or HuR, are potentially important tools for identifying the set of mRNAs that are differentially regulated by these two AUBPs during gametogenesis.
Methods
Production of HuR transgenic mice.
For BA–HuR lines, the human HuR complementary DNA (GenBank accession number U38175; Ma et al., 1996) was cloned in-frame with a Myc tag, and was inserted into blunt-ended pBAP (Gunning et al., 1987). The resulting plasmid was named BA–HuR. A 5.5-kb fragment, consisting of β-actin and Myc–HuR sequences, was microinjected into fertilized (CBA × C57Bl/6) × (CBA × C57Bl/6) oocytes to obtain transgenic founders (Brinster & Palmiter, 1986), which were then crossed with (CBA × C57Bl/6) wild-type mice, followed by intercrossing or backcrossing with wild-type mice to create transgenic lines.
For BA–GH lines, an EGFP cassette, containing enhanced GFP (EGFP) sequences flanked by two LoxP sites (a gift from S. Tajbakhsh), was inserted between the β-actin and Myc–HuR sequences in the BA–HuR construct to produce the BA–GH construct. A 7.1-kb fragment, consisting of the BA–GH sequences, was microinjected into fertilized (CBA × C57Bl/6) × (CBA × C57Bl/6) oocytes to obtain transgenic founders, which were crossed with wild-type (CBA × C57Bl/6) mice to produce F1 transgenic mice. These mice were then backcrossed to wild-type mice to produce N2 transgenic mice.
For BA–HGdel lines, in vivo excision of the GFP cassette was carried out by crossing F1 or N2 BA–GH males to pgk–Cre transgenic females (Lallemand et al., 1998). The resulting BA–HGdel C1 mice (male or female) were backcrossed to wild-type mice to produce BA–HGdel C2 transgenic mice.
For expression analysis, the animals used were of the same age (3–6 months), the same weight, and did not show any morphological or anatomical abnormalities.
Protein extraction and quantitative western blot analysis.
Protein extraction and quantitative western blot analyses were carried out as described previously (Gouble et al., 2002). The following antibodies were used: monoclonal anti-human-c-Myc antibody, 9E10; monoclonal anti-GFP, JL-8 (Clontech) and monoclonal anti-HuR, 19F12 (a gift from H. Furneaux; Rodriguez-Pascual et al., 2000), which reacts specifically with the endogenous HuR protein, and not with the Myc-tagged HuR protein.
Immunohistochemical and immunofluorescence analyses.
Immunohistochemistry was carried out as described by Gouble et al. (2002). For immunofluorescence, sections of testes were blocked and permeabilized in 10% goat serum, 1% BSA, 1% Triton X-100 in PBS, incubated with primary antibody (9E10 or 19F12) for 1 h at 20 °C, and washed in PBS. Immunostaining was detected using a rhodamine-conjugated goat anti-mouse antibody (Alexa 546, Molecular Probes). For nuclear staining, samples were incubated in freshly made chromomycin A3 solution (Sigma) in PBS and 150 mM MgCl2, and were washed in PBS. Sections were analysed using a Leica SP2 confocal microscope equipped with helium–neon lasers and appropriate filter combinations.
Genotyping and Southern blot analysis.
Southern blot and PCR analyses were carried out using genomic DNA, which was extracted from the tails of wild-type or transgenic mice.
Northern blot analysis.
Total RNA was extracted using Trizol (Invitrogen), and the RNA species were separated by electrophoresis through a denaturing gel (2.2 M formaldehyde, 1.2% agarose) and transferred to a nylon membrane. DNA probes were labelled with [32>P]dCTP using a random-priming kit (Amersham Pharmacia Biotech).
Acknowledgments
We are grateful to G. Delsol, M. March, S. Grazide and T. Dimeglio for their contributions to this work. We also thank M. Poiret, R. Feil, J. Smith and C. Babinet for helpful discussions. The mice were produced in the Service de Transgenèse and analysed in the Plateforme d'Histopathologie Expérimentale and d'Imagerie Cellulaire (IFR 109). This work was supported by Association pour la Recherche contre le Cancer contract 9842, ARECA network Pôle Protéomique et Cancer. M.L.-M. and A.G. are supported by ARC grants.
References
- Bakheet T., Frevel M., Williams B., Greer W. & Khabar K. (2001) ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res., 29, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. & Palmiter R. (1986) Introduction of genes into the germ lines of animals. Harvey Lect., 80, 1–38. [PMC free article] [PubMed] [Google Scholar]
- Chen C.-Y.A. & Shyu A.B. (1995) AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci., 20, 465–470. [DOI] [PubMed] [Google Scholar]
- Fan X. & Steitz J. (1998a) Overexpression of HuR, a nuclear–cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J., 17, 3448–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. & Steitz J. (1998b) HNS, a nuclear–cytoplasmic shuttling sequence in HuR. Proc. Natl Acad. Sci. USA, 95, 15293–15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Tamura K., Masai K., Tanaka H., Nishimune Y. & Nojima H. (2002) Use of stepwise subtraction to comprehensively isolate mouse genes whose transcription is up-regulated during spermiogenesis. EMBO J., 3, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi I.-E. & Steitz J.A. (2001) Delineation of mRNA export pathways by the use of cell-permeable peptides. Science, 294, 1895–1901. [DOI] [PubMed] [Google Scholar]
- Good P.J. (1995) A conserved family of elav-like genes in vertebrates. Proc. Natl Acad. Sci. USA, 92, 4557–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouble A. (2001) Etude de la Fonction des Proteines AUF1 et HuR in vivo dans des souris transformiques. Thesis, Université Paul Sabatier, Toulouse, France.
- Gouble A., Grazide S., Meggetto F., Mercier P., Delsol G. & Morello D. (2002) A new player in oncogenesis: AUF1/hnRNPD overexpression leads to tumorigenesis in transgenic mice. Cancer Res., 62, 1489–1495. [PubMed] [Google Scholar]
- Gunning P., Hardeman E., Wade R., Ponte P., Bains W., Blau H. & Kedes L. (1987) Differential patterns of transcript accumulation during human myogenesis. Mol. Cell. Biol., 7, 4100–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. (1999) Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc. Natl Acad. Sci. USA, 96, 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand Y., Luria V., Haffner-Krausz R. & Lonai P. (1998) Maternally expressed PGK–Cre transgene as a tool for early and uniform activation of the Cre sitespecific recombinase. Transgenic Res., 7, 105–112. [DOI] [PubMed] [Google Scholar]
- Ma W.J., Cheng S., Campbell C. & Furneaux H.M. (1996) Cloning and characterization of HuR, a ubiquitously expressed ELACV-like protein. J. Biol. Chem., 271, 8144–8151. [DOI] [PubMed] [Google Scholar]
- Peng S.S.Y., Chen C.-Y.A., Xu N. & Shyu A.B. (1998) RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J., 15, 3461–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pascual F., Hausding M., Ihrig-Biedert I., Furneaux H., Levy A.P., Forstermann U. & Kleinert H. (2000) Complex contribution of the 3′-UTR region to the expressional regulation of the human inducible nitric oxide synthase gene: involvement of the RNA-binding protein HuR. J. Biol. Chem., 275, 26040–26049. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P. (2002) Unique chromatin remodelling and transcriptional regulation in spermatogenesis. Science, 296, 2176–2178. [DOI] [PubMed] [Google Scholar]
- Wang W., Caldwell M.C., Lin S., Furneaux H. & Gorospe M. (2000a) HuR regulated cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J., 19, 2340–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Furneaux H., Cheng H., Caldwell M.C., Hutter D., Liu Y., Holrook N. & Gorospe M. (2000b. b) HuR regulates p21 mRNA stabilization by UV light. Mol. Cell. Biol., 20, 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. & Brewer G. (1999) The search for trans-acting factors controlling messenger RNA decay. Prog. Nucleic Acid Res. Mol. Biol., 62, 257–291. [DOI] [PubMed] [Google Scholar]


