Abstract
PDZD2 (PDZ-domain-containing 2; also known as PAPIN, AIPC and PIN1) is a ubiquitously expressed multi-PDZ-domain protein. We have shown that PDZD2, which shows extensive homology to pro-interleukin-16 (pro-IL-16), is localized mainly to the endoplasmic reticulum (ER). Pro-IL-16 is cleaved in a caspase-3-dependent mechanism to generate the secreted cytokine IL-16. The abundant expression of PDZD2 in the ER, and its sequence similarity to pro-IL-16, suggests that similar post-translational processing of PDZD2 may occur. Indeed, western blotting and mass spectrometry analysis of conditioned medium from cells transfected with epitope-tagged PDZD2 show that there is secretion of a PDZD2 peptide of approximately 37 kDa (sPDZD2, for secreted PDZD2) that contains two PDZ domains. Expression of PDZD2 was detected in several tissues. Furthermore, sPDZD2 secretion is suppressed by the mutation of a sequence that shows similarity to caspase recognition motifs or by treatment with a caspase inhibitor. In summary, PDZD2 is the first reported multi-PDZ protein that is processed by proteolytic cleavage to generate a secreted peptide containing two PDZ domains.
Introduction
PDZ (for PSD95, Discs-large and ZO-1) domains are conserved protein–protein interaction modules comprised of ∼80 to 100 amino acids (reviewed recently in Harris & Lim, 2001; Sheng & Sala, 2001). They recognize specific carboxy-terminal sequences and usually exist in proteins as tandem repeats. PDZ domains are thought to function by acting as molecular scaffolds to facilitate the assembly of macromolecular complexes.
PDZ domains are known best for their functions in the clustering of plasma-membrane-associated proteins and in intracellular signalling. However, the discovery of interleukin-16 (IL-16) suggests that PDZ-containing proteins can also mediate extracellular signalling. IL-16 was first identified as a T-lymphocyte chemotactic activity in mitogen-stimulated peripheral blood mononuclear cells (reviewed in Cruikshank et al., 2000). Secreted IL-16 is produced in several cell types from its multi-PDZ precursor, pro-IL-16, by caspase-3-mediated proteolysis (Zhang et al., 1998; Sciaky et al., 2000; Sharma et al., 2000).
PIN1/PAPIN/AIPC is a ubiquitously expressed multi-PDZ protein of unknown function. We first identified PIN1 (for PDZ domain protein 1 isolated from the rat insulinoma cell line INS-1; Thomas et al., 1999) as a protein that interacts with the basic helix–loop–helix transcription factor E12. Independently, full-length rat and human PIN1 complementary DNAs were cloned and named PAPIN (for plakophilin-related Armadillo-repeat-protein interacting PDZ protein; Deguchi et al., 2000) and AIPC (for activated in prostate cancer; Chaib et al., 2001), respectively, on the basis of the protein's binding and expression properties. Most recently, the gene encoding the human homologue of this protein was mapped to 5p13.2 and named PDZD2 by the Human Genome Nomenclature Committee (http://www.gene.ucl.ac.uk/nomenclature). PDZD2 is used as the name for PIN1/PAPIN/AIPC in this article to avoid confusion. In this report, we used V5- and green fluorescent protein (GFP)-tagged constructs to show that PDZD2 is localized to the endoplasmic reticulum (ER) and the nucleus. Importantly, we demonstrated by western blot analysis, mass spectrometry and site-directed mutagensis that PDZD2, like pro-IL-16, is proteolytically cleaved at its C terminus to generate a secreted peptide, sPDZD2 (for secreted PDZD2), that contains two PDZ domains. These findings give a new insight into the cellular role of PDZD2.
RESULTS
Analysis of the subcellular localization of PDZD2
Previous immunohistochemical analysis of PDZD2 expression gave conflicting results. PDZD2 was detected at cell–cell contacts in lung sections and in normal rat kidney (NRK) cells (Deguchi et al., 2000), whereas PDZD2 staining was reported to be mainly cytoplasmic in prostate sections (Chaib et al., 2001). To examine the subcellular localization of PDZD2 in more detail, we tagged the full-length rat protein at the C-terminus with the viral V5 epitope (the PDZD2C–V5 construct, Fig. 1A). After transient transfection into COS7 cells, the subcellular distribution of V5-tagged PDZD2 was analysed by immunostaining. V5 immunoreactivity was detected at the perinuclear region in most of the transfected cells (Fig. 1Ba). This perinuclear region was identified as the ER by counterstaining with fluorescent concanavalin A (conA; a lectin) conjugates that bind to glycoproteins in the ER (Fig. 1Bb,c). In addition, a smaller proportion of transfected cells (∼8%) showed immunoreactivity that co-localized with nuclear DNA labelled by propidium iodide (Fig. 1Bd–f). Analysis using a GFP-tagged construct, PDZD2N–GFP (Fig. 1A), revealed a similar pattern of expression, with a similar ratio of perinuclear versus nuclear stained cells (Fig. 1Bg,h), strongly suggesting that the expression pattern observed is independent of tagging.
Figure 1.
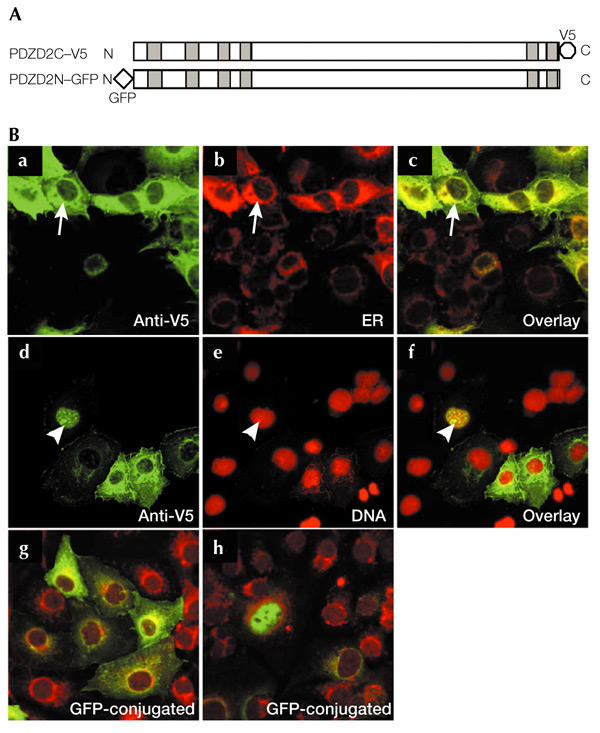
Subcellular localization of PDZD2. (A) The PDZD2C–V5 and PDZD2N–GFP constructs, containing a carboxy-terminal V5 tag and an amino-terminal green fluorescent protein (GFP) tag, respectively. Shaded boxes indicate the positions of the PDZ domains. (B) Localization of PDZD2 in COS7 cells. Cells transfected with PDZD2C–V5 (a–f) were immunostained with an anti-V5 antibody and counterstained with concanavalin A (conA) to stain the endoplasmic reticulum (ER) (b) or propidium iodide to detect nuclear DNA (e). Superimposed images are shown in c and f. Arrows indicate the co-localization of V5-tagged PDZD2 with the ER marker, and arrowheads indicate nuclear localization of PDZD2 (seen in ∼8% of the transfected cells). Fluorescent images of PDZD2N–GFP-transfected cells that display ER and nuclear staining, respectively, are shown in g and h; these cells were counterstained with conA.
Homology between PDZD2 and pro-interleukin-16
In GenBank searches for proteins homologous to PDZD2, pro-IL-16 shows the strongest match for the PDZD2 C terminus. Over a region of 215 amino acids (Fig. 2, in red brackets), there is 39.5% identity and 58.1% similarity. Of particular interest is the conservation of the tryptophan residue (indicated by a red arrowhead in Fig. 2) that was predicted to confer a unique binding property to the secreted PDZ domain in IL-16 (Muhlhahn et al., 1998). Of 312 human PDZ domains that we analysed from the SMART database (Schultz et al., 1998), only the final PDZ domains of pro-IL-16 and PDZD2 contain tryptophan at this position. This homology, together with the fact that PDZD2 is ER-localized, suggests that PDZD2 is subject to post-translational processing similar to that of pro-IL-16.
Figure 2.
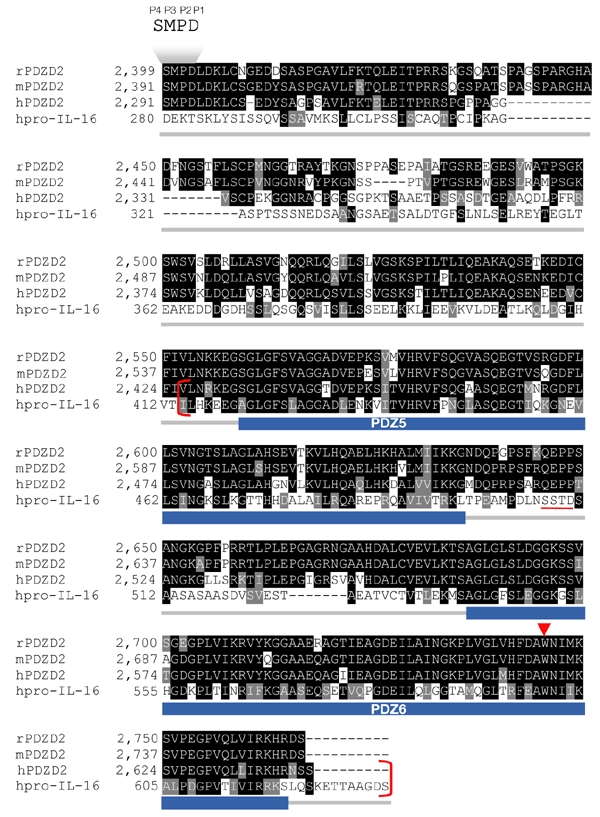
Sequence similarity between PDZD2 and pro-interleukin-16 at the carboxyl terminus. The prefix letter for each sequence name denotes the sequence source: h, human; m, mouse; r, rat. The GenBank accession numbers of rPDZD2, hPDZD2 and human pro-interleukin-16 (hpro-IL-16) are NP_075229, AAK07661 and AAB58261, respectively. The mouse PDZD2 sequence was obtained by translation of the Ensembl-predicted transcript ENSMUST00000037924. The positions of the two PDZ domains in the secreted form of PDZD2 (sPDZD2), PDZ5 and PDZ6, predicted according to the SMART database, are indicated by the blue bars below the aligned sequences. The 215-amino-acid region that is highly conserved (39.5% identity, 58.1% similarity) between human PDZD2 and pro-IL-16 is shown in red brackets. Of particular interest is the tryptophan residue within the PDZ binding cleft (indicated by the red arrowhead) that is conserved among all three PDZD2 homologues and pro-IL-16. The pro-IL-16 caspase recognition sequence, SSTD (Zhang et al., 1998), is underlined in red. The position of a similar sequence, SMPD (named P4 to P1, according to Earnshaw et al., 1999), which has been identified as being important for PDZD2 secretion, is indicated above the sequence.
Secretion of the PDZD2 carboxy-terminal peptide
To investigate the possible secretion of the PDZD2 C terminus, PDZD2C–V5-transfected COS7 cells were treated with serum-free medium, and the conditioned medium was collected for western blot analysis to detect V5-tagged peptides (Fig. 3A). Lysates prepared from the nuclear and cytoplasmic fractions of the remaining cells were analysed in parallel. It is interesting to note that two peptides of ∼38 kDa and ∼46 kDa were detected both in the conditioned medium and in the cytoplasmic fraction, but not in lysates from the nuclear fraction or from the empty vector control (Fig. 3A). These peptides seem to be synthesized in the cytoplasm (presumably in the ER), as they are absent from the nuclear fraction. However, detection of larger V5-tagged bands in both fractions of the cell lysate suggests that extensive proteolytic processing of the PDZD2 protein occurs, which cannot be suppressed by the protease inhibitors present. Under our experimental conditions, the full-length protein could only be visualized after long exposures (data not shown).
Figure 3.

Secretion of PDZD2. (A) Western blot. Conditioned medium (M), and subcellular fractions (nuclear (N) and cytoplasmic (C)), from COS7 cells transfected with PDZD2C–V5, or vector only, were analysed by immunoblotting using the anti-V5 antibody. (B) Mass spectrometry analysis. Peptides from the conditioned medium from cells transfected with PDZD2C–V5 or vector only were analysed by mass spectrometry. Arrows indicate the V5-tagged secreted PDZD2 peptide (∼38 kDa).
We also carried out mass spectrometric analysis of the conditioned medium to estimate more accurately the size of the PDZD2 peptides (Fig. 3B). When compared with the vector-only control, a prominent peak was observed at 38.7227 kDa, corresponding to the ∼38-kDa band detected by western blot analysis. The lack of a discernible peak at ∼46 kDa suggests that the larger band seen from western blots may correspond to a conformational variant of the ∼38-kDa peptide, which is only observed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) analysis. The ∼38-kDa (or ∼37-kDa when the V5 tag is excluded) peptide was named sPDZD2 to indicate its secreted nature. Secretion of sPDZD2 was also shown in transiently and stably transfected Chinese hamster ovary (CHO) cells, indicating that PDZD2 processing and sPDZD2 secretion are not specific to COS cells (data not shown).
Endogenous expression of secreted PDZD2
If proteolytic cleavage and secretion of PDZD2 is of physiological relevance, sPDZD2 should be detectable in tissue extracts. To analyse endogenous sPDZD2 expression by immunoblotting, we made a polyclonal antiserum against the peptide encoding amino acids 2,519–2,641 of the C terminus (Fig. 4A). This antiserum was shown to be specific by western blot and immunocytochemical analyses using extracts from PDZD2-transfected COS7 and CHO cells, and by using a recombinant IL-16 protein as a negative control (Fig. 4A,B). Low levels of endogenous PDZD2 expression, predominantly in the ER, were also detected in COS7 cells using this antiserum (Fig. 4Bg). Protein extracts from various mouse tissues were analysed for sPDZD2 expression using this specific antiserum (Fig. 4C). Importantly, the ∼37-kDa sPDZD2 peptide was detected in most of the tissue samples, albeit at varying levels. The full-length PDZD2 protein of ∼300 kDa was also detected after longer exposure times. We believe that the other bands of intermediate size correspond to intermediary processing products. This analysis indicates that endogenous synthesis of the sPDZD2 peptide occurs in several tissues.
Figure 4.
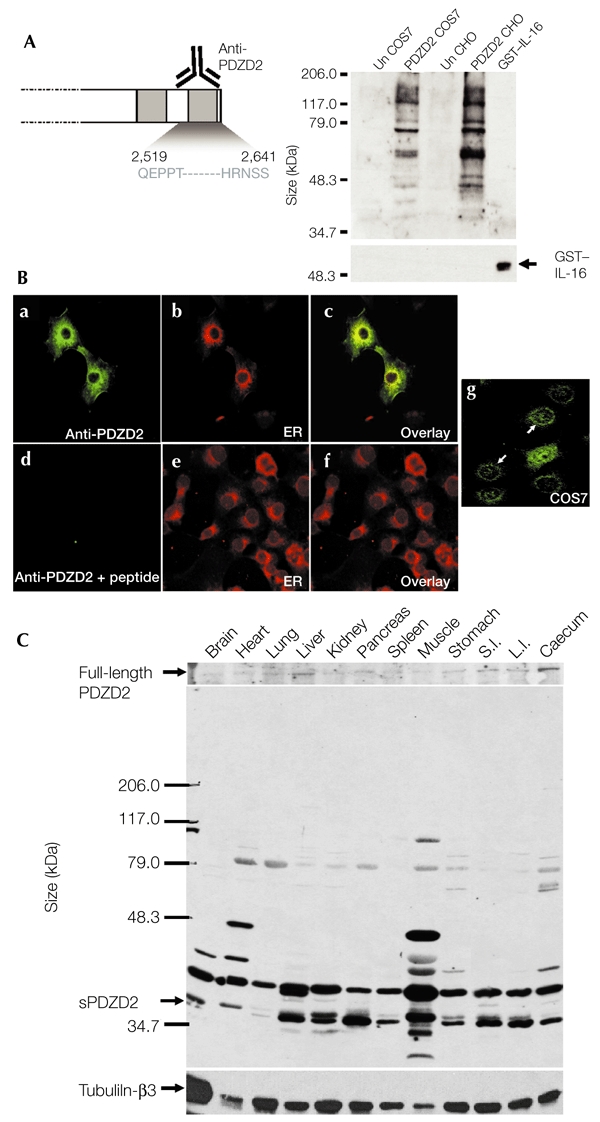
Expression of the secreted form of PDZD2 in several tissues. (A) Test of antiserum specificity by western blot analysis. Rabbit polyclonal anti-PDZD2 antiserum raised against the last PDZ domain (the 122 amino acids at the carboxyl terminus) was partially purified and tested for specificity by immunoblot analysis using cell lysates (20 μg) prepared from PDZD2-transfected and untransfected (Un) COS7 and Chinese hamster ovary (CHO) cells. Untransfected cells express low endogenous levels of PDZD2. A recombinant glutathione-S-transferase (GST)–interleukin-16 (IL-16) fusion protein was also made and tested in parallel. The anti-PDZD2 antiserum could not detect 20 ng of this fusion protein, which showed strong immunoreactivity with an anti-GST antibody. (B) Test of specificity by immunostaining. The specificity of the antiserum is also supported by the endoplasmic reticulum (ER) staining of PDZD2-transfected COS7 cells. Pre-incubation with the PDZD2 peptide (10 μg ml−1) abolished the immunoreactivity. The endoplasmic reticulum (ER) was detected by counterstaining with concanavalin A (ConA). c and f are superimposed images. g, endogenous PDZD2 expression in COS7 cells; arrows indicate the ER localization of PDZD2. (C) Western blot analysis of expression in different tissues. Protein extracts (20 μg) prepared from different tissues were analysed for PDZD2 expression using the partially purified anti-PDZD2 antiserum. The secreted PDZD2 (sPDZD2) peptide (∼37 kDa) was present in most of the tissues analysed. The upper panel shows expression of full-length PDZD2 (∼300 kDa), which was detectable after longer exposure of the blot. The blot was stripped and reprobed with anti-tubulin-β3 to control for possible loading differences. L.I., large intestine; S.I., small intestine.
Defining the cleavage site required for sPDZD2 secretion
The mass of sPDZD2 determined from the mass spectrometry analysis is consistent with proteoloytic cleavage C terminal to the Asp residue at position 2,402. The SMPD sequence (amino acids 2,399–2,402) resembles known caspase cleavage sites (Earnshaw et al., 1999), suggesting that PDZD2 cleavage may also be caspase-dependent. To test this, a ten-amino-acid region (residues 2,402–2,411), which contains three aspartate residues, was replaced with a single isoleucine residue (mutant 1, Fig. 5A). This PDZD2 deletion construct, when tested in CHO cells, had lost the ability to direct sPDZD2 secretion (Fig. 5B). Western blots were probed with our anti-PDZD2 antiserum, and loss of sPDZD2 expression was evident from both the conditioned medium and the cell lysate. To define more precisely the cleavage site, two more mutants (mutants 2 and 3, Fig. 5A) were generated with the aspartate residues at positions 2,402 and 2,404, respectively, substituted with alanine. The abolition of sPDZD2 secretion from cells transfected with mutant 2, but not from cells transfected with mutant 3, strongly supports the proposal that cleavage occurs at amino acid 2,402 (Fig. 5B).
Figure 5.
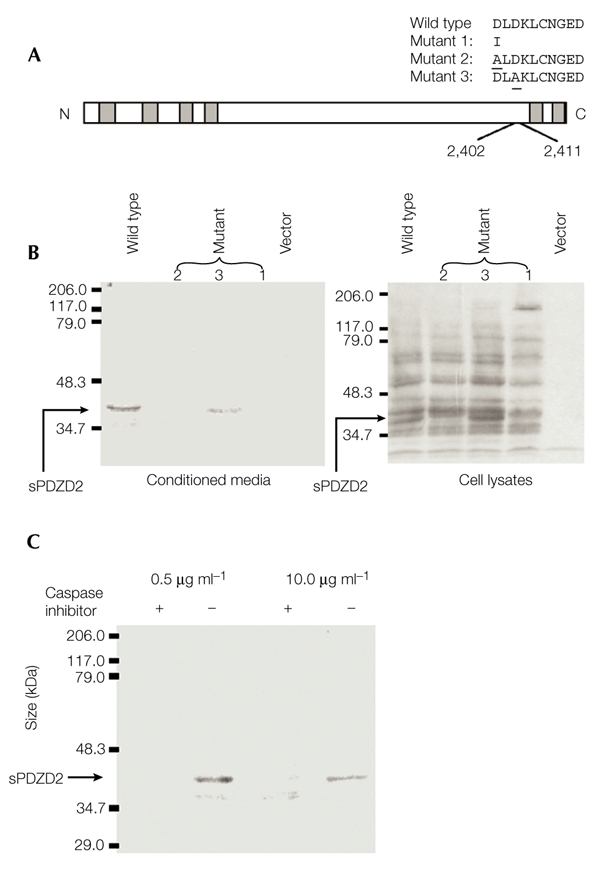
Characterization of the secretion of a processed form of PDZD2. (A) Schematic representation of mutant constructs (mutants 1–3). The underlined residues indicate the positions of aspartate residues in the wild-type PDZD2 protein that have been substituted for alanine in mutants 2 and 3. (B) Analysis of secretion of the secreted form of PDZD2 (sPDZD2). Conditioned media and whole-cell lysates were harvested from Chinese hamster ovary cells that were transfected with the different constructs. The media and lysates were then analysed by immunoblotting using the anti-PDZD2 antiserum. sPDZD2 secretion is lost with mutants 1 and 2 but not with mutant 3. (C) Effect of caspase inhibitor. The inhibitor B-D-FMK (+) or its inactive structural homologue Z-FA-FMK (−) were added to the serum-free medium during sPDZD2 collection at 0.5 μg ml−1 or 10 μg ml−1. B-D-FMK but not Z-FA-FMK inhibits sPDZD2 secretion.
B-D-FMK (Boc-Asp(OMe)-fluoromethyl ketone) is a general, competitive inhibitor of caspases that binds strongly to the caspase active-site, whereas Z-FA-FMK (Z-Phe-Ala-fluoromethyl ketone) is a structural homologue of B-D-FMK that shows no inhibitory activity. As expected, B-D-FMK, but not Z-FA-FMK, inhibits sPDZD2 secretion (Fig. 5C). Taken together, these data provide strong evidence that there is caspase-dependent cleavage at the SMPD site, which releases the last two PDZ domains of PDZD2 in the form of the sPDZD2 protein.
DISCUSSION
The subcellular localization of a protein usually provides clues to its cellular function. Using V5- and GFP-tagged constructs, we showed that PDZD2 is localized predominantly to the ER. This ER localization is not an artefact of overexpression, as a similar ER-localized expression of endogenous PDZD2 was seen in COS7 cells. The high levels of PDZD2 expression in the ER are consistent with the strong cytoplasmic staining of PDZD2 that has been detected previously in prostate tumours (Chaib et al., 2001), and is reminiscent of the perinuclear localization of pro-IL-16 in transfected COS cells (Y. Zhang et al., 2001). Western blot and mass spectrometry analyses indicate that cytoplasmic PDZD2 is proteolytically processed to generate the secreted PDZ-containing peptide sPDZD2. Similarly to that of IL-16, sPDZD2 secretion is caspase-dependent. Thus, sPDZD2 is only the second PDZ-containing protein that is known to be secreted.
The ER localization of PDZD2 detected in our study is not consistent with the claim made by Deguchi et al. (2000) that PDZD2 is localized to cell–cell contacts in NRK and lung epithelial cells. This discrepancy may simply reflect a cell-type difference, but intracellular staining was also evident in the immunocytochemical analysis of NRK cells (Deguchi et al., 2000). To solve this apparent dilemma, an extensive analysis of endogenous PDZD2 expression in diverse tissue and cell types using multiple antibodies is required.
Predictions based on the mass of sPDZD2 determined by mass spectrometry analysis, followed by mutagenesis, allowed us to pinpoint the site of PDZD2 cleavage to the peptide bond after the SMPD sequence. Cleavage at this site generates the sPDZD2 peptide, containing two PDZ domains, which is much larger than IL-16. Although the penultimate pro-IL-16 PDZ domain is not secreted, there is extensive similarity between this domain and the fifth PDZ domain of PDZD2 (Fig. 2). Recent analyses of the multi-PDZ proteins syntenin and GRIP show a co-operative effect between neighbouring PDZ domains (Grootjans et al., 2000; Q. Zhang et al., 2001). It will be interesting to know how the extra PDZ domain in sPDZD2 affects peptide structure and function. The presence of two PDZ domains also suggests the interesting possibility that sPDZD2 may interact with several proteins and facilitate formation of protein complexes extracellularly.
Structural nuclear magnetic resonance spectrometry analysis of IL-16 reveals a smaller cleft consisting of the amino-acid sequence GLGF, with a bulky tryptophan side-chain in the centre, that is predicted to alter the binding of PDZ ligands (Muhlhahn et al., 1998). sPDZD2 is the only other protein in the database that contains a tryptophan in the ligand-binding pocket. This suggests the intriguing possibility that the tryptophan side-chain might be important for the function of the secreted peptides, and that secreted peptides such as these are rare. IL-16, an atypical cytokine, was shown to function as a growth and differentiation factor in various cell types (Cruikshank et al., 1987; Parada et al., 1998; Szabo et al., 1998). We speculate that sPDZD2 may have a similar growth and/or differentiation function.
Interestingly, nuclear expression was also detected in transfected cells by immunocytochemical and western blot analyses, suggesting that PDZD2 might have a nuclear function. It is worth noting that our N- and C-terminal-tagged constructs show similar ratios of ER versus nuclear staining in transfected cells, showing that proteolytic cleavage is not associated with the differential targeting of the different termini of the PDZD2 protein. This is in contrast with pro-IL-16 processing in COS cells, in which the proteolytic cleavage that generates IL-16 is accompanied by nuclear translocation of the prodomain, but not of the full-length protein (Y. Zhang et al., 2001).
METHODS
Plasmid constructs.
The full-length rat PDZD2 cDNA (AF169411) in pCI–neo was a gift from Y. Takai and Y. Hata (Deguchi et al., 2000). The viral V5 epitope was added downstream of the PDZD2 coding sequence by a PCR-based strategy to generate PDZD2C–V5. To introduce GFP at the N terminus of PDZD2, the full-length coding sequence was subcloned into pEGFP–C2 (Clontech) to produce the construct PDZD2N–GFP. Mutagenesis was carried out using the QuikChangeTM site-directed mutagenesis kit from Stratagene. The nucleotide sequences of the different constructs were confirmed by DNA sequencing.
Transfections and immunocytochemistry.
FUGENE 6 (Roche) and Lipofectamine 2000 (Invitrogen) were used as transfection reagents for COS7 and CHO cells, respectively. After transfection (48 h), cells were fixed in ice-cold 50% acetone/50% methanol for 10 min, followed by blocking with 3% BSA/10% normal goat serum in PBS. The primary antibodies anti-V5 (1:500 dilution; Invitrogen) or anti-PDZD2 (1:1,000 dilution) were then applied, followed by a fluorescein isothiocyanate-conjugated secondary antibody (Alexa Fluor 488 rabbit anti-mouse IgG; 1: 250 dilution; Molecular Probes). Nuclei and ER were counterstained by incubation with propidium iodide (used at 25 μg ml−1 in PBS, 0.1% Triton X-100, for 10 s) and with fluorescent conA conjugates (Molecular Probes) at 20 μg ml−1 in PBS for 30 min, respectively. Images were acquired using a confocal microscope (Biorad MRC 1024).
Collection of conditioned media, subcellular fractionation and mass spectrometry.
To test for sPDZD2 secretion, COS or CHO cells, in 10-cm plates, were transfected with the PDZD2C–V5 construct. The next day, media were replaced with fresh, serum-free media. The media were collected 48 h later. Cells remaining on the plates were harvested, lysed and fractionated into cytoplasmic and nuclear fractions as previously described (Y. Zhang et al., 2001). The Voyager-DE STR Biospectrometry Workstation system (Perkin Elmer) was used for the analysis of peptides in the conditioned media. To verify that sPDZD2 secretion is caspase-dependent, the general caspase inhibitor B-D-FMK (Enzyme System Products), or its analogue, Z-FA-FMK, used as a control, were added to the serum-free media at 0.5 μg ml−1 or 10 μg ml−1.
Immunoblot analysis.
Protein samples were separated by SDS–PAGE in 12% Laemmli gels and electro-blotted onto Hybond C Super nitrocellulose membranes (Amersham Life Sciences). Blots were incubated with mouse anti-V5 antiserum (1:5,000 dilution; Invitrogen), mouse anti-tubulin-β3 antiserum (Ab-3; 1:100 dilution; Neomarkers), mouse anti-glutathione-S-transferase (GST) antiserum (1:500 dilution; Santa Cruz) or rabbit anti-PDZD2 antiserum (1:10,000 dilution). Antigen–antibody complexes were detected using secondary antibodies conjugated to horseradish peroxidase (1:7,500 dilution; Transduction Laboratories) and visualized using the SuperSignal ECL detection system (Pierce). Blots for reprobing were stripped in 0.1 M glycine buffer (pH 2.9).
Generation of the anti-PDZD2 antibody.
To raise rabbit polyclonal antiserum against the C terminus of PDZD2, a human recombinant intein–PDZD2 fusion protein (containing amino acids 2,519–2,641, which include the last PDZ domain) was synthesized using the IMPACT-CN system (New England Biolabs) in accordance with the manufacturer's instructions. Approximately 4 mg of peptide was generated, and this was lyophilized and sent for injection into rabbits by United States Biological. Antisera were partially purified by ammonium sulphate precipitation and by affinity purification using a Protein A/G column (Pharmacia).
Note added in proof: Recently, the official name of PDZD2 has been changed to PDZ domain containing 3 (PDZK3), according to the nomenclature of the Human Genome Nomenclature Committee (http://www.gene.ucl.ac.uk/nomenclature).
Acknowledgments
We are grateful to M. K. Thomas, K. Kramer, C.-C. Hui, N.-S. Wong, B. Wong and other lab members for their critical review of this manuscript. We thank Y. Takai and Y. Hata for providing the PAPIN cDNA, and V. Lam, P.-T. Cheung and J. D. Huang for sharing plasmids and reagents with us. This work was supported by a grant from the Research Grants Council of the Hong Kong Special Administration Region, China (HKU 7234/99M), awarded to K.-M. Y.
References
- Chaib H., Rubin M.A., Mucci N.R., Li L., Taylor J.M.G., Day M.L., Rhim J.S. & Macoska J.A. (2001) Activated in prostate cancer: a PDZ domain-containing protein highly expressed in human primary prostate tumors. Cancer Res., 61, 2390–2394. [PubMed] [Google Scholar]
- Cruikshank W.W., Berman J.S., Theodore A.C., Bernardo J. & Center D.M. (1987) Lymphokine activation of T4+ T lymphocytes and monocytes. J. Immunol., 138, 3817–3823. [PubMed] [Google Scholar]
- Cruikshank W.W., Kornfeld H. & Center D.M. (2000) Interleukin-16. J. Leukoc. Biol., 67, 757–766. [DOI] [PubMed] [Google Scholar]
- Deguchi M., Iizuka T., Hata Y., Nishimura W., Hirao K., Yao I., Kawabe H. & Takai Y. (2000) PAPIN. A novel multiple PSD-95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related armadillo repeat protein/δ-catenin and p0071. J. Biol. Chem., 275, 29875–29880. [DOI] [PubMed] [Google Scholar]
- Earnshaw W.C., Martins L.M. & Kaufmann S.H. (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem., 68, 383–424. [DOI] [PubMed] [Google Scholar]
- Grootjans J.J., Reekmans G., Ceulemans H. & David G. (2000) Syntenin–syndecan binding requires syndecan synteny and the co-operation of both PDZ domains of syntenin. J. Biol. Chem., 275, 19933–19941. [DOI] [PubMed] [Google Scholar]
- Harris B.Z. & Lim W.A. (2001) Mechanism and role of PDZ domains in signalling complex assembly. J. Cell Sci., 114, 3219–3231. [DOI] [PubMed] [Google Scholar]
- Muhlhahn P. et al. (1998) Structure of interleukin-16 resembles a PDZ domain with an occluded peptide binding site. Nature Struct. Biol., 5, 682–686. [DOI] [PubMed] [Google Scholar]
- Parada N.A., Center D.M., Kornfeld H., Rodriguez W.L., Cook J., Vallen M. & Cruikshank W.W. (1998) Synergistic activation of CD4+ T cells by IL-16 and IL-2. J. Immunol., 160, 2115–2120. [PubMed] [Google Scholar]
- Schultz J., Milpetz F., Bork P. & Ponting C.P. (1998) SMART, a simple modular architecture research tool: identification of signalling domains. Proc. Natl Acad. Sci. USA, 95, 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Brazer W., Center D.M., Cruikshank W.W. & Smith T.J. (2000) Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J. Immunol., 164, 3806–3814. [DOI] [PubMed] [Google Scholar]
- Sharma V., Sparks J.L. & Vail J.D. (2000) Human B-cell lines constitutively express and secrete interleukin-16. Immunology, 99, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M. & Sala C. (2001) PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci., 24, 1–29. [DOI] [PubMed] [Google Scholar]
- Szabo P., Zhao K., Kirman I., Le Maoult J., Dyall R., Cruikshank W. & Weksler M.E. (1998) Maturation of B cell precursors is impaired in thymic-deprived nude and old mice. J. Immunol., 161, 2248–2253. [PubMed] [Google Scholar]
- Thomas M.K., Yao K.M., Tenser M.S., Wong G.G. & Habener J.F. (1999) Bridge-1, a novel PDZ-domain coactivator of E2A-mediated regulation of insulin gene transcription. Mol. Cell. Biol., 19, 8492–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Fan J.S. & Zhang M. (2001) Interdomain chaperoning between PSD-95, Dlg, and Zo-1 (PDZ) domains of glutamate receptor-interacting proteins. J. Biol. Chem., 276, 43216–43220. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Center D.M., Wu D.M., Cruikshank W.W., Yuan J., Andrews D.W. & Kornfeld H. (1998) Processing and activation of pro-interleukin-16 by caspase-3. J. Biol. Chem., 273, 1144–1149. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kornfeld H., Cruikshank W.W., Kim S., Reardon C.C. & Center D.M. (2001) Nuclear translocation of the N-terminal prodomain of interleukin-16. J. Biol. Chem., 276, 1299–1303. [DOI] [PubMed] [Google Scholar]


