Abstract
Cbl functions as an adaptor protein by interacting with other signalling molecules to form multimolecular complexes. Previous studies have proposed that Cbl is also a positive regulator of CrkL–C3G signalling, which leads to Rap1 activation. However, there is a lack of genetic evidence for a physiological function of Cbl in regulating this pathway. Here, we show that Cbl deficiency results in enhanced activation of Rap1. Cbl was shown to promote the ubiquitylation of CrkL without any apparent effect on its stability. Remarkably, the membrane translocation of C3G, its association with CrkL, and the guanine-nucleotide exchange activity of C3G were all increased in Cbl−/− thymocytes. Consistent with a function of Rap1 in integrin activation, enhanced integrin-mediated cell adhesion was also seen in Cbl−/− thymocytes. Thus, Cbl negatively regulates Rap1 activation, probably through a proteolysis-independent E3-ubiquitin-ligase activity of Cbl that modulates protein–protein interactions.
Introduction
Engagement of the T-cell antigen receptor (TCR) induces rapid activation of Ras, a small GTPase, which is an important molecule that triggers a cascade of protein kinases in the mitogen-activated protein kinase signalling pathway. Activated Ras is required for thymocyte selection and for the transcriptional regulation of genes encoding cytokines in peripheral T cells (Genot & Cantrell, 2000). There has, therefore, been great interest in understanding the downstream effectors and upstream regulators of Ras in T-cell signalling. Rap1, another small GTPase, is also activated after TCR ligation (Reedquist & Bos, 1998). Rap1 closely resembles Ras, and these proteins have almost identical effector domains. However, unlike Ras, Rap1 does not activate Raf1. Instead, Rap1 sequesters Raf1 from Ras, suggesting an antagonistic function for Rap1 in Ras signalling (Bos, 1998). All of the small GTPases, including Ras and Rap1, exist either in an inactive GDP-bound form or in an active GTP-bound form. Guanine-nucleotide exchange factors (GEFs) promote the formation of the active GTP-bound forms of GTPases, whereas GTPase-activating proteins stimulate their intrinsic GTPase activity and convert them to the GDP-bound form.
Cbl, a 120-kDa proto-oncoprotein, consists of an amino-terminal tyrosine-kinase-binding domain, a RING finger and carboxy-terminal proline-rich sequences containing several tyrosine phosphorylation sites (Thien & Langdon, 2001). Numerous studies during the past few years have shown that Cbl functions as an adaptor protein by interacting with protein tyrosine kinases (PTKs) and other crucial signalling molecules. More recently, Cbl was identified as an E3 ubiquitin (Ub) ligase, in which the RING finger recruits Ub-bound E2 and the variant src-homolgy 2 (SH2) domain binds to activated PTKs, enabling the transfer of Ub to them (Joazeiro et al., 1999; Levkowitz et al., 1999). These new studies suggest that Cbl might regulate T-cell function through its E3-Ub-ligase activity, resulting in the ubiquitylation of its binding partners. For example, Cbl promotes Ub conjugation to TCR-ζ through the adaptor function of Zap70 (Wang et al., 2001), which is consistent with the enhanced cellsurface expression of TCR/CD3 in Cbl−/− thymocytes (Murphy et al., 1998; Naramura et al., 1998).
Previous studies have also shown an activation-dependent interaction between Cbl and CrkL (reviewed in Thien & Langdon, 2001). CrkL is an adaptor protein that is composed of an N-terminal SH2 domain and two C-terminal SH3 domains. The SH2 domain has been shown to bind to Cbl and/or Cbl-b in an activation-dependent manner, and one of the CrkL C-terminal SH3 domains constitutively associates with C3G, a GEF that has been shown to be an exchange factor for Rap1 (Bos, 1998). Therefore, the Cbl–CrkL–C3G signalling pathway has been proposed to function in Rap1 activation and in the negative regulation of the Ras signalling pathway (Boussiotis et al., 1997).
Although Cbl is proposed to be a negative regulator of TCR signalling in T-cell lines and clones, the ablation of Cbl does not alter peripheral T-cell activation (Murphy et al., 1998; Naramura et al., 1998); Cbl deficiency only affects thymocyte development. In this study, we have examined the function of Cbl in CrkL–C3G signal transduction in thymocytes. Contrary to a study suggesting that Cbl positively regulates Rap1 activation in T-cell clones (Boussiotis et al., 1997), we provide genetic evidence that loss of Cbl results in enhanced Rap1 activation and increased levels of CrkL–C3G complex formation.
Results and Discussion
To determine whether Cbl is involved in the Rap1 signalling pathway, we analysed whether Cbl deficiency affects Rap1 activation. Rap1 exists in an inactive GDP-bound form and, when activated, is converted to a GTP-bound form. Techniques for the direct analysis of these two forms in cells have been ineffective due to the lack of a suitable antibody for the detection of the active form. However, it is known that only the GTP-bound active form of Rap1 is able to bind downstream effectors through their Rap1-binding domains (RBDs). One of these effectors, the Ral guanine-nucleotide dissociation stimulator (GDS), contains one of these domains, and its RBD binds the GTP-bound form of Rap1 with a higher affinity than it binds to other small GTPases, such as Ras (Bos, 1998). We took advantage of this system by performing a pull-down assay. The cell lysates of untreated or anti-CD3stimulated thymocytes were incubated with either glutathione-S-transferase (GST) alone or with a fusion of GST with the Ral RBD (GST–RBD), which were pre-coupled with glutathione-Sepharose beads. We found that GST–RBD pulled down Rap1 from thymocytes, and the association was further increased after stimulation with anti-CD3 (Fig. 1A).
Figure 1.
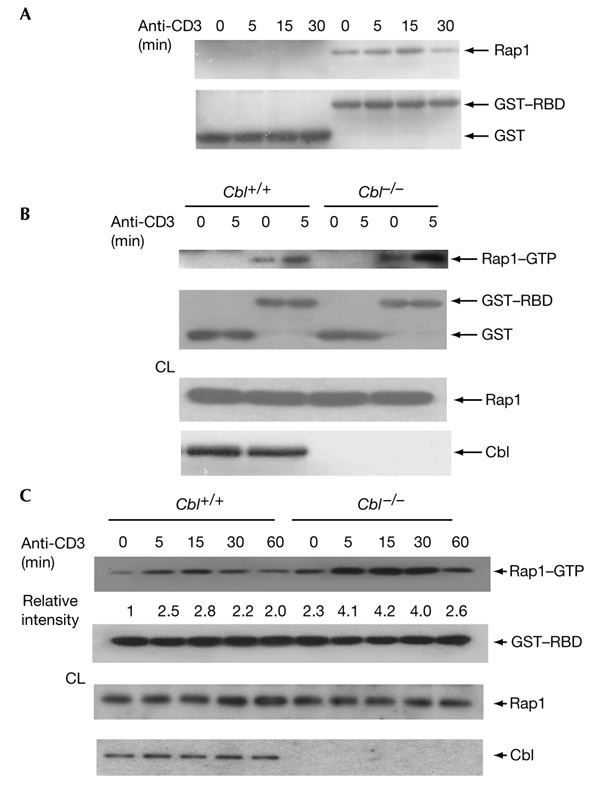
Upregulation of Rap1 activation in Cbl−/− thymocytes. (A) Pull-down assay for activated Rap1 in thymocytes. Cell lysates from unstimulated or anti-CD3-stimulated thymocytes were incubated with 4 μg of either glutathione-S-transferase (GST) alone or a fusion of GST to the Rap1-binding domain of the Ral guanine-nucleotide dissociation stimulator (GST–RBD). The precipitate was blotted with anti-Rap1 (top panel) and the membrane was reprobed with anti-GST (bottom panel). (B,C) Thymocytes from Cbl+/+ or Cbl−/− mice were stimulated with anti-CD3 for 5 min (B) or for varying durations (C), followed by pull-down assays with GST or GST–RBD. Aliquots of cell lysates (CL) were blotted with anti-Rap1 or anti-Cbl as indicated.
Cbl deficiency affects only thymocyte development, and does not affect peripheral T cells (Murphy et al., 1998; Naramura et al., 1998). We used the GST–RBD fusion protein to analyse Rap1 activation in Cbl+/+ and Cbl−/− thymocytes. A larger quantity of Rap1 was precipitated from unstimulated and anti-CD3stimulated Cbl−/− thymocytes than from Cbl+/+ cells (Fig. 1B), although the levels of Rap1 protein were the same in the Cbl+/+ and Cbl−/− populations. We examined further the kinetics of Rap1 activation by stimulating thymocytes for various periods of time. Rap1 activation in Cbl+/+ thymocytes peaked after stimulation for 15 min, then gradually declined (Fig. 1C). At any timepoint tested, we observed a stronger Rap1 activity in Cbl−/− thymocytes than in Cbl+/+ cells, even after stimulation for 6 h. Thus, in contrast to the previous observation that Cbl is a positive regulator of Rap1 (Boussiotis et al., 1997), our data suggest that Cbl deficiency results in increased Rap1 activation.
Cbl associates with CrkL in an activation-dependent manner, and this association has been implicated in the CrkL–C3G signalling that leads to Rap1 activation (Boussiotis et al., 1997; Schmitt & Stork, 2002). The recent identification of Cbl as an E3 Ub ligase prompted us to investigate whether Cbl can promote the conjugation of Ub to CrkL. For this purpose, we used a transient transfection system for the coexpression of Cbl, CrkL and haemagglutinin (HA)-tagged Ub (HA–Ub). Coexpression of Cbl with CrkL induced the formation of high-molecular-weight ladders, which were recognized by anti-HA. The slowly migrating smears indicated poly-ubiquitylation of CrkL itself, but not of CrkL-associated proteins, as we added SDS and N-ethyl-maleimide to the lysis buffer to disrupt protein–protein interactions. Stimulation with pervanadate increased ubiquitylation of CrkL (Fig. 2A). To confirm that a functional Cbl RING finger is required for CrkL ubiquitylation, we coexpressed CrkL with Cbl RING-finger mutants. Mutations of conserved Cys or Trp residues in the Cbl RING finger abolished the conjugation of Ub to CrkL (Fig. 2B). These data suggest that CrkL is a substrate for the Cbl E3 Ub ligase. To analyse further the effect of Cbl on CrkL ubiquitylation, we analysed endogenous CrkL ubiquitylation in Cbl+/+ and Cbl−/− thymocytes. Unstimulated Cbl+/+ thymocytes showed weak ubiquitylation of CrkL, which was increased after stimulation for 15 min with anti-CD3 (Fig. 2C). However, Cbl−/− thymocytes showed decreased ubiquitylation of CrkL in both unstimulated and anti-CD3stimulated cells, supporting the idea that CrkL is a physiological substrate for the Cbl E3 Ub ligase.
Figure 2.
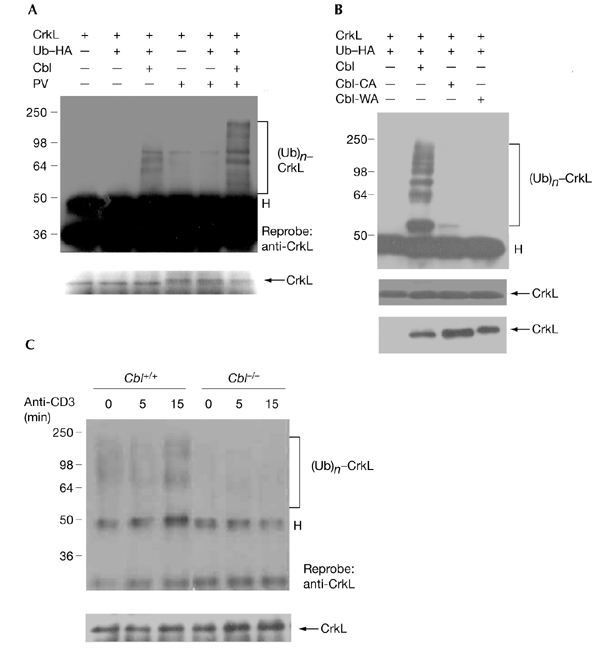
Cbl promotes ubiquitylation of CrkL. (A) 293T cells were transfected with plasmids as indicated, and the cells were left untreated or were treated with pervanadate (PV) for 30 min. The cell lysates were immunoprecipitated (IP) with anti-CrkL, blotting was carried out and detection was performed using anti-haemagglutinin (anti-HA). Poly-ubiquitylated CrkL ((Ub)n–CrkL) is indicated. The same membrane was reprobed with anti-CrkL. 'H' indicates the position of the IgG heavy chain. (B) Cells that coexpress wild-type Cbl or the Cbl RING-finger mutants Cbl-CA or Cbl-WA were analysed as in (A). (C) Thymocytes from Cbl+/+ and Cbl−/− mice were either unstimulated or were stimulated with anti-CD3 for the durations indicated, followed by immunoprecipitation with anti-CrkL, blotting, and detection using anti-ubiquitin (anti-Ub).
Ub conjugation to protein substrates can be a signal for their subsequent degradation by proteasomes (Hershko & Ciechanover, 1998). To understand the function of Cbl in the regulation of CrkL, we tested whether Cbl regulates CrkL stability by inducing its ubiquitylation. We compared the protein expression of CrkL and other signalling molecules in Cbl+/+ and Cbl−/− thymocytes before and after stimulation with anti-CD3 for various durations. It was found that CrkL is a stable protein, as there was no detectable change in its level even after stimulation of Cbl+/+ thymocytes for 6 h (Fig. 3A). Notably, Cbl deficiency did not affect the levels of CrkL before and after stimulation. Similarly, the levels of C3G and other Cbl-binding molecules, such as Zap70 and Grb2, were not different in Cbl+/+ and Cbl−/− thymocytes, either stimulated or unstimulated.
Figure 3.
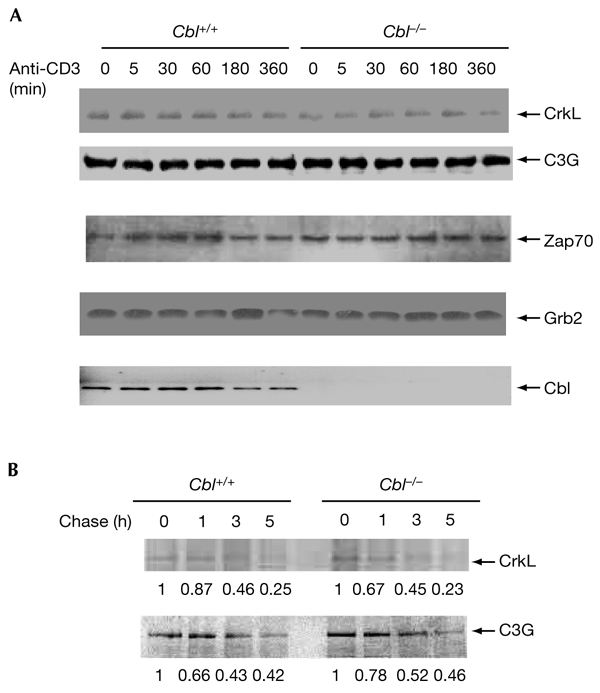
Cbl does not affect the stability of CrkL. (A) Thymocytes were stimulated for various durations and cell lysates were blotted with antibodies as indicated. (B) Thymocytes were pulse-labelled with [35S]methioine for 1 h and cells were chased for various durations. Cell lysates were immunoprecipitated with anti-CrkL or anti-C3G, samples were separated by SDS–polyacrylamide gel electrophoresis, and autoradiography was carried out. The data were quantified and normalized. The original intensity was considered to be the basal level (1.0) for each row. The relative intensity is labelled below each panel.
To confirm that Cbl does not affect the stability of CrkL, we performed pulse-chase experiments by radiolabelling endogenous CrkL in thymocytes. The levels of 35S-labelled CrkL in wild-type thymocytes were similar to those in Cbl−/− thymocytes over a period of 5 h (Fig. 3B). Similarly, the amounts of radiolabelled C3G were the same in Cbl+/+ and Cbl−/− thymocytes. Together, the results suggest that Cbl regulates CrkL in a proteolysis-independent manner.
The stable expression of CrkL in Cbl−/− thymocytes led us to investigate whether Cbl modulates the biological function of CrkL. CrkL interacts through its SH3 domain with the proline-rich region of C3G (Knudsen et al., 1994). We analysed whether Cbl deficiency affects the CrkL–C3G interaction. Immunoprecipitation with anti-CrkL was carried out on samples from thymocytes (either untreated or stimulated with anti-CD3), followed by immunoblotting with anti-C3G. Anti-CrkL immunoprecipitates from unstimulated thymocytes contained C3G (Fig. 4A). Stimulation with anti-CD3 for 30 min caused an increase in the association between CrkL and C3G. Thus, in contrast with our results from Jurkat T cells (Elly et al., 1999), CrkL–C3G binding was not constitutive in thymocytes. Instead, the interaction is dynamic and is regulated by thymocyte activation. Remarkably, the amount of C3G in anti-CrkL immunoprecipitates was increased in Cbl−/− thymocytes. In contrast to Cbl+/+ thymocytes, anti-CD3 treatment increased the level of CrkL–C3G association after stimulation for 5 min and 15 min, and these levels then declined to the basal level after further stimulation. These results suggest that Cbl functions in the regulation of the CrkL–C3G interaction.
Figure 4.
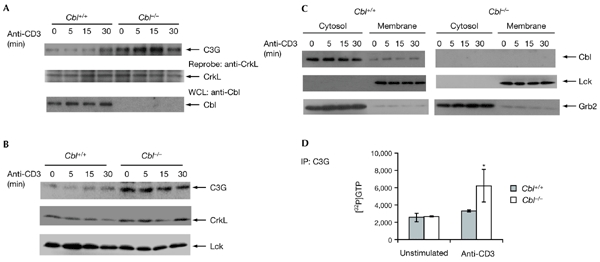
Cbl regulates C3G activation. (A) The CrkL–C3G interaction was increased in Cbl−/− thymocytes. Thymocytes from Cbl+/+ and Cbl−/− mice were either unstimulated or stimulated with anti-CD3 for the durations indicated, followed by immunoprecipitation (IP) with anti-CrkL, blotting, and detection with anti-C3G. The membrane was reprobed with anti-CrkL. (B) Membrane translocation of C3G was increased in Cbl−/− thymocytes. Cells treated as in (A) were subjected to subcellular fractionation and the membrane fraction was immunoblotted with anti-C3G, anti-CrkL or anti-Lck as indicated. (C) The membrane and cytosolic fractions from (B) were immunoblotted with anti-Cbl, anti-Lck or anti-Grb2. (D) Guanine-nucleotide exchange factor assay. Anti-C3G immunoprecipitates from thymocytes treated as in (A) were analysed for exchange activity using recombinant Rap1 as a substrate. The binding of [32P]GTP to Rap1 was measured using a liquid scintillation counter.
C3G has been shown to act as a GEF for Rap1 (Bos, 1998). The increased amount of GTP-bound Rap1 in Cbl−/− thymocytes and the enhanced interaction between CrkL and C3G suggests that the activation of C3G may be altered by Cbl deficiency. One of the mechanisms by which C3G activity is regulated is through its translocation. It has been shown that C3G is translocated to the membrane on stimulation by insulin in adipocytes, and this translocation is mediated by CrkII, a CrkL homologue (Chiang et al., 2001). We tested whether C3G translocation occurs in thymocytes. Thymocytes, either untreated or stimulated with anti-CD3 for various durations, were subjected to subcellular fractionation. The membrane fraction was probed with an anti-C3G antibody. C3G was detected in the membrane fraction in unstimulated thymocytes (Fig. 4B). Stimulation of thymocytes with anti-CD3 increased C3G translocation, particularly after stimulation for 15 min or 30 min. The same membrane was reprobed with anti-CrkL and with anti-Lck, and no apparent increase in membrane translocation of these proteins was observed after thymocyte stimulation. Notably, the amount of C3G in unstimulated Cbl−/− thymocytes was greater than that in Cbl+/+ thymocytes that had been stimulated for 30 min. The translocated C3G in unstimulated Cbl−/− thymocytes may represent a saturated state, as further stimulation of these thymocytes did not increase C3G translocation to the membrane fraction. In addition, the effect of Cbl deficiency on C3G translocation was specific, as the amounts of CrkL and Lck were similar in the membrane fractions of Cbl+/+ and Cbl−/− thymocytes. Membrane and cytosolic fractions were also immunoblotted with antibodies against Cbl, Lck and Grb2 to ensure the proper separation in the fractionation procedure. As shown in Fig. 4C, Cbl and Grb2 were detected mainly in the cytosolic pools, whereas Lck was mainly detected in the membrane fractions.
To demonstrate further a direct role of Cbl in C3G signalling that leads to Rap1 activation, we performed an in vitro GEF assay using immunopurified C3G from wild-type and Cbl−/− thymocytes, and recombinant Rap1 as a substrate. We found that the C3G guanine-nucleotide exchange activity for Rap1 was significantly increased in anti-CD3-stimulated thymocytes from Cbl−/− mice (Fig. 4D).
It was recently reported that Rap1 induces integrin activation (Sebzda et al., 2002). To determine whether Cbl is involved in similar pathways, we first examined whether Cbl affects thymocyte adhesion to BSA, fibronectin, Fc (antibody crystallizable fragment) and intracellular cell-adhesion molecule 1 (ICAM1)–Fc. More Cbl−/− than Cbl+/+ thymocytes attached to fibronectin-coated wells in both unstimulated and anti-CD3stimulated cells (Fig. 5A), whereas binding to BSA was almost the same in the two cell populations. Similarly, the percentage of adherent Cbl−/− thymocytes in ICAM1-coated wells was also increased when cells were stimulated with anti-CD3 (Fig. 5B). We also examined the binding of ICAM1 by leukocyte-function-associated antigen 1 (Lfa1) at the thymocyte cell surface by flow cytometry. Cbl−/− thymocytes showed stronger staining for ICAM1 than did wild-type thymocytes (Fig. 5C). As a control, the cellsurface expression of Lfa1 was also examined, and no difference was found between wild-type and Cbl−/− thymocytes (Fig. 5D). These data suggest that the increased binding to ICAM1–Fc in Cbl−/− thymocytes is not due to a change in cellsurface expression, but to an increased binding of Lfa1.
Figure 5.
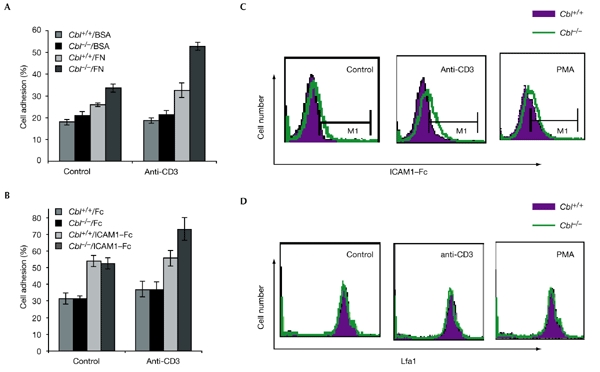
Thymocyte adhesion to fibronectin and intracellular cell-adhesion molecule 1. (A) Thymocytes were added to wells that had been pre-coated with fibronectin (FN) or BSA, and the cells attached to the wells were quantified and represented as a percentage of total cell input. (B) Thymocyte adhesion to wells coated with intracellular cell-adhesion molecule 1 (ICAM1) fused to Fc (antibody crystallizable fragment; ICAM1–Fc) or Fc only. (C) Thymocytes were either left untreated or were stimulated with anti-CD3 or phorbol myristate acetate (PMA) and were stained with Fc alone or ICAM1–Fc and examined by flow cytometry. Only the data from the specific staining for ICAM1–Fc is shown. (D) Cells treated as in (C) were stained with anti-leukocyte-function-associated antigen 1 (CD11a).
Here, we have shown that Cbl induces ubiquitylation of CrkL and negatively regulates the CrkL–C3G interaction, C3G translocation and its GEF activity. This is in contrast to the view that Cbl is a positive mediator of Rap1 activation in T cells (Boussiotis et al., 1997) and fibroblasts (Schmitt & Stork, 2002). We therefore propose a revised model in which Cbl acts as an E3 ligase to promote Ub conjugation to CrkL. However, CrkL ubiquitylation does not lead to its degradation. Ub conjugation to CrkL might cause a structural hinderance to its interaction with C3G. For example, Ub conjugation to the CrkL SH3 domain may prevent its association with the proline-rich sequences in C3G. This is supported by a recent finding that Ub conjugation to the p85 subunit of phosphatidylinositol-3-OH kinase affects its association with CD28/CD3 proteins in T cells (Fang & Liu, 2001). In addition, ubiquitylation has also been implicated in protein processing, and in the regulation of transcription and of protein kinases (Deng et al., 2000; Hoppe et al., 2000; Kaiser et al., 2000). It is clear that one of the mechanisms by which Cbl family proteins negatively regulate TCR signalling is through the ubiquitylation of their binding proteins, which affects the formation of multimolecular complexes.
Previous studies on the function of Rap1 in T-cell activation have used T-cell lines or clones (Boussiotis et al., 1997; Carey et al., 2000; Reedquist & Bos, 1998), and have suggested that Rap1 is antagonistic to Ras activation. To address a physiological role of Rap1 in T cells in vivo, Cantrell and co-workers have recently produced transgenic mice that express an active form of Rap1 specifically in the T-cell lineage (Sebzda et al., 2002). They found that Rap1 did not antagonize Ras signalling, as was previously suggested. On the contrary, Rap1 activation induced integrin activation and the positive selection of thymocytes. We observed increased thymocyte adhesion to fibronectin and enhanced binding to ICAM1–Fc in Cbl−/− thymocytes. Thus, our data provide further support for a role of Rap1 in integrin activation. Although the detailed mechanism by which Rap1 enhances cell adhesion remains to be elucidated, our results implicate Cbl as a negative regulator of integrin activation in T cells through the modulation of the CrkL–C3G–Rap1 signalling pathway.
Although Cbl and Cbl-b are highly homologous, mice with targeted alterations to these genes show markedly different phenotypes in T cells. Whereas Cbl−/− mice show only altered thymocyte development (Murphy et al., 1998; Naramura et al., 1998), Cbl-b−/− mice showed abnormalities only in peripheral T cells (Bachmaier et al., 2000; Chiang et al., 2001). We propose that this phenomenon is caused by differential targeting of substrates for ubiquitylation (Liu & Gu, 2002). However, a Cbl/Cbl-b conditional double-deficiency induced more severe autoimmunity and sustained peripheral T-cell activation (Naramura et al., 2002). Therefore, Cbl and Cbl-b may have overlapping functions in regulating TCR signalling. Indeed, we recently found that Cbl-b also targets CrkL for ubiquitylation and affects CrkL–C3G signalling in peripheral mature T cells (data not shown). Thus, CrkL may be a common target for Cbl-family E3 Ub ligases.
Methods
Antibodies and plasmids.
Polyclonal antibodies specific to Cbl, CrkL, C3G, Rap1, Zap70, Lck and Grb2, and monoclonal antibodies specific to c-Myc, HA and Ub were purchased from Santa Cruz Biotechnology. A monoclonal antibody specific to Rap1 was purchased from PharMingen. A phosphotyrosine-specific monoclonal antibody was purified from the culture supernatant of the 4G10 B-cell hybridoma line, and an anti-CD3-ε monoclonal antibody was purified from the culture supernatant of B-cell hybridoma line 145-2C11.
Cbl complementary DNAs that encode wild-type Cbl or Cbl RING-finger mutants (with Cys to Ala (Cbl-CA) or Trp to Ala (Cbl-WA) mutations), a Ub cDNA with an HA epitope tag, and the CrkL plasmid have been described previously (Elly et al., 1999; Wang et al., 2001). The bacterial expression plasmid that contains GST–RBD was provided by J. Bos. Production of the GST fusion protein was performed as described previously (Joazeiro et al., 1999).
Mice.
Cbl-deficient (Cbl−/−) mice on a C57BL/6 background were provided by M. Naramura and H. Gu. Thymocytes were collected from thymi of 6–8-week-old mice. Single-cell suspensions of thymocytes were prepared at 3 × 107 cells ml−1 in RPMI 1640 (Irvine Scientific) supplemented with 5% FCS.
Thymocyte activation.
Thymocytes were either left untreated, or were stimulated with 5 μg ml−1 anti-CD3-ε. Cells were incubated on ice for 10 min before stimulation at 37 °C for various durations as indicated.
Cell transfection.
293T cells were cultured in DMEM (Irvine Scientific) containing 10% FCS, 100 units of penicillin ml−1 and 100 units of streptomycin ml−1. Cells were transfected with plasmids containing Cbl, Cbl mutants, HA–Ub and CrkL (usually 1–5 μg total) using lipofectamine (Gibco-BRL). After 48 h, cells were collected and resuspended in 0.5 ml of DMEM. Cells were either untreated, or were treated with pervanadate for 30 min at 37 °C. Cells were then pelleted and resuspended in 1 × Nonidet P-40 lysis buffer containing 1% Nonidet P-40, 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 5 mM sodium pyrophosphate, 2 mM orthovanadate and 10 μg ml−1 of both aprotinin and leupeptin. Cells were lysed for 10 min at 4 °C, and insoluble materials were removed by centrifugation at 15,000g at 4 °C for 10 min. For the detection of ubiquitylated proteins, 0.1% SDS and 5 mM N-ethylmaleimide (Aldrich) were added to the lysis buffer to disrupt non-specific protein interactions.
Pull-down assays.
Thymocytes (3 × 107 cells), either unstimulated or stimulated with anti-CD3 for various durations, were washed twice with ice-cold PBS and lysed in a buffer containing 50 mM Tris-HCl, pH7.5, 200 mM NaCl, 10% glycerol, 1% Nonidet P-40, 5 mM MgCl2, 1 mM PMSF, 1 mM orthovanadate and 10 μg ml−1 of both aprotinin and leupeptin, at 4 °C for 30 min. Lysates were cleared by centrifugation at 15,000g at 4 °C for 15 min. Supernatants were incubated with 4 μg of GST or GST–RBD fusion protein, which were precoupled to glutathione–sepharose. After incubation for 1 h at 4 °C, beads were washed three times in lysis buffer and resuspended in Laemmli buffer. Samples were analysed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) using 12% gels, followed by transfer to polyvinylidene membranes. Affinity-purified activated Rap1 was detected by immunoblotting using an anti-Rap1 antibody.
Subcellular fractionation.
Thymocytes (2 × 107 cells) were resuspended in hypotonic buffer (containing 42 mM KCl, 10 mM HEPES (pH 7.4), 5 mM MgCl2 and 10 μg ml−1 of both aprotinin and leupeptin) at 4 °C, and incubated on ice for 15 min. Cells were transferred to a 1-ml syringe and sheared by passing them 5 times through a 30-gauge needle. The lysates were centrifuged at 200g for 10 min to remove nuclei and cell debris, and the supernatant was collected and centrifuged at 13,000g for 60 min at 4 °C. The supernatant containing the cytosolic fraction was collected, and the pellet was resuspended in lysis buffer (containing 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% NP-40, and 10 μg ml−1 of both aprotinin and leupeptin), vortexed for 5 min at 4 °C, and centrifuged again at 13,000g for 60 min at 4 °C. The supernatant containing the particulate membrane fraction was then diluted to a concentration equivalent to that of the cytosolic fraction and separated by SDS–PAGE.
Cell-adhesion assay.
96-well Maxisorp Immuno flat-bottomed plates (Nunc) were coated with 10 μg ml−1 BSA, fibronectin (Calbiochem), ICAM1–Fc or Fc only (R&D Systems). Thymocytes (1 × 106) in PBS containing 0.5% BSA were plated in triplicate and incubated with anti-CD3-ε (10 μg ml−1) or phorbol myristate acetate (PMA; 10 ng ml−1) for 30 min at 37 °C. Plates were washed three times with pre-warmed serum-free RPMI 1640. Adherent cells were quantified, and the results were expressed as a percentage of the total number of cells added.
Flow cytometry.
Single-cell suspensions of thymi were stained with fluorescein isothiocyanate (FITC)-conjugated anti-Lfa1 (CD11a, clone 2D7; PharMingen) for 1 h at 4 °C. Cells were then washed three times with PBS. For the analysis of thymocyte binding to soluble mouse ICAM1, 1 × 105 thymocytes were washed and resuspended in PBS with 0.1% BSA. Cells were either left untreated or were stimulated with anti-CD3-ε (10 μg ml−1) or PMA (10 ng ml−1) for 1 h at 37 °C. Soluble recombinant mouse ICAM1–Fc or Fc only (both at 10 μg ml−1) were added to the cells. After a 30-min incubation at 37 °C, the cells were washed twice in ice-cold PBS, 0.1% BSA and incubated with 10 μg ml−1 FITC-conjugated anti-Fc (Jackson Immunoresearch) for 1 h at 4 °C. Cells were washed twice in PBS with 0.1% BSA. Data acquisition and analysis were performed using a FACSCalibur machine (Becton Dickinson) with CellQuest software.
Pulse-chase assays.
Thymocytes (2 × 107) from Cbl+/+ or Cbl−/− mice were cultured in methionine-free DMEM containing 5% dialysed FCS for 1 h at 37 °C. After stimulation with anti-CD3-ε antibody, the cells were labelled for 1 h with 100 μCi [35S]methionine (ICN Biomedicals). Cells were then washed three times and cultured for various durations in complete DMEM medium containing 10% FCS. At each timepoint, cell lysates were immunoprecipitated with a specific antibody and resolved by SDS–PAGE using a 10% gel, and autoradiography was carried out. The radiolabelled protein bands were quantified using NIH Image 1.61 software.
C3G guanine-nucleotide exchange factor assay.
The purified His–Rap1 fusion protein was incubated with 1 mM GDP for 25 min at 20 °C in loading buffer (20 mM Tris-HCl, pH7.5, 50 mM NaCl, 3 mM MgCl2, 0.1 mM dithiothreitol (DTT), 0.1 mM EDTA). After purification using Microcon 10 columns (Amicon), the GDP-loaded His–Rap1 protein was added to exchange-assay buffer (20 mM Tris-HCl, pH 7.5, 80 mM NaCl, 5 mM MgCl2, 0.1 mM EDTA, 0.1 mM DTT, 0.5 mg ml−1 BSA and 100 mM GTP). Immunoprecipitated C3G from 2 × 107 thymocytes (30 μl) was added to the assay mixture containing 10 μCi of [α-32P]GTP and 5 μg of His–Rap1–GDP (the substrate) in a final volume of 40 μl. After incubation for 30 min at 30 °C, 10 μl of the sample was removed and added to 290 μl of wash buffer (20 mM Tris-HCl, pH 7.5, 80 mM NaCl, 10 mM MgCl2, 7 mM 2-mercaptoethanol) at 4 °C to stop the reaction. The diluted sample was poured onto a nitrocellulose membrane filter (pore size 0.45 μm; Schleicher & Schuell, Inc.). The membrane was washed twice with wash buffer. The radioactivity trapped on the filter was counted using a liquid scintillation counter. Each experiment was performed in triplicate.
Acknowledgments
We thank A. Altman and our colleagues at the Division of Cell Biology for support and discussion, M. Naramura and H. Gu for providing Cbl−/− mice, and J. Huang and J. Bos for reagents. This work was supported by the National Institutes of Health grant RO1DK56558 to Y.-C.L.
References
- Bachmaier K. et al. (2000) Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature, 403, 211–216. [DOI] [PubMed] [Google Scholar]
- Bos J.L. (1998) All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J., 17, 6776–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis V.A., Freeman G.J., Berezovskaya A., Barber D.L. & Nadler L.M. (1997) Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science, 278, 124–128. [DOI] [PubMed] [Google Scholar]
- Carey K.D. et al. (2000) CD28 and the tyrosine kinase Lck stimulate mitogen-activated protein kinase activity in T cells via inhibition of the small G protein Rap1. Mol. Cell. Biol., 20, 8409–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S.H. et al. (2001) Insulinstimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature, 410, 944–948. [DOI] [PubMed] [Google Scholar]
- Deng L. et al. (2000) Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell, 103, 351–361. [DOI] [PubMed] [Google Scholar]
- Elly C. et al. (1999) Tyrosine phosphorylation and complex formation of Cbl-b upon T cell receptor stimulation. Oncogene, 18, 1153–1162. [DOI] [PubMed] [Google Scholar]
- Fang D. & Liu Y.-C. (2001) Proteolysis-independent regulation of phosphatidylinositol 3-kinase by Cbl-b-mediated ubiquitylation in T cells. Nature Immunol., 2, 870–875. [DOI] [PubMed] [Google Scholar]
- Genot E. & Cantrell D.A. (2000) Ras regulation and function in lymphocytes. Curr. Opin. Immunol., 12, 289–294. [DOI] [PubMed] [Google Scholar]
- Hershko A. & Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem., 67, 425–479. [DOI] [PubMed] [Google Scholar]
- Hoppe T., Matuschewski K., Rape M., Schlenker S., Ulrich H.D. & Jentsch S. (2000) Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell, 102, 577–586. [DOI] [PubMed] [Google Scholar]
- Joazeiro C.A., Wing S.S., Huang H., Leverson J.D., Hunter T. & Liu Y.C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science, 286, 309–312. [DOI] [PubMed] [Google Scholar]
- Kaiser P., Flick K., Wittenberg C. & Reed S.I. (2000) Regulation of transcription by ubiquitylation without proteolysis: Cdc34/SCF (Met30)-mediated inactivation of the transcription factor Met4. Cell, 102, 303–314. [DOI] [PubMed] [Google Scholar]
- Knudsen B.S., Feller S.M. & Hanafusa H. (1994) Four proline-rich sequences of the guanine-nucleotide exchange factor C3G bind with unique specificity to the first Src homology 3 domain of Crk. J. Biol. Chem., 269, 32781–32787. [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Liu Y.-C. & Gu H. (2002) Cbl and Cbl-b in T-cell regulation. Trends Immunol., 23, 140–143. [DOI] [PubMed] [Google Scholar]
- Murphy M.A. et al. (1998) Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol. Cell. Biol., 18, 4872–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M., Kole H.K., Hu R.-J. & Gu H. (1998) Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl Acad. Sci. USA, 95, 15547–15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M., Jang I.K., Kole H., Huang F., Haines D. & Gu H. (2002) c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nature Immunol., 3, 1192–1199. [DOI] [PubMed] [Google Scholar]
- Reedquist K.A. & Bos J.L. (1998) Costimulation through CD28 suppresses T cell receptor-dependent activation of the Ras-like small GTPase Rap1 in human T lymphocytes. J. Biol. Chem., 273, 4944–4949. [DOI] [PubMed] [Google Scholar]
- Schmitt J.M. & Stork P.J. (2002) PKA phosphorylation of Src mediates cAMP's inhibition of cell growth via Rap1. Mol. Cell, 9, 85–94. [DOI] [PubMed] [Google Scholar]
- Sebzda E., Bracke M., Tugal T., Hogg N. & Cantrell D.A. (2002) Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signalling. Nature Immunol., 3, 251–258. [DOI] [PubMed] [Google Scholar]
- Thien C.B. & Langdon W.Y. (2001) Cbl: many adaptations to regulate protein tyrosine kinases. Nature Rev. Mol. Cell Biol., 2, 294–307. [DOI] [PubMed] [Google Scholar]
- Wang H.Y. et al. (2001) Cbl promotes ubiquitination of the T cell receptor-ζ through an adaptor function of Zap-70. J. Biol. Chem., 276, 26004–26011. [DOI] [PubMed] [Google Scholar]


