Summary
Genomics and proteomics may provide new ways of making the lives of bacteria more miserable
The 'finding' of some 'mislaid' anthrax by the Iraqi government in early March confirms that the spectre of biological warfare is still looming. But, if terrorists equipped with biological weapons seem to be the latest scourge of mankind, some even more cunning and deadlier enemies have been with us for millennia: bacteria that have craftily evolved to evade all attempts to outwit them, in a futile arms race that no side seems able to win. When Alexander Fleming discovered the antibacterial effect of penicillin in 1928, it seemed that science had finally hit upon the ultimate weapon. But we gave away our strategic advantage; by misusing antibiotics, we turned these seemingly God-sent cures into the very tools for training the enemy for even greater deviousness. The war seemed to be lost in the late 1990s, when multi-drug-resistant Staphylococcus aureus breached the last line of antibiotic defences, vancomycin.
But times are changing. One after the other, our bacterial foes are surrendering their secret arms to the microbial weapons inspectors: the sequencers and genomics experts who can lay bare their genomes in a matter of weeks, and inspect their armaments and biological weapons plants. The fruits of these grand microbial sequencing, genomics and proteomics efforts are having a varied effect on the war against bacterial infections. In some engagements, notably the development of new antibiotics, they are proving to be merely a useful addition. In vaccine research, however, they have already caused no less than a revolution.
A brief glimpse at the TIGR (The Institute for Genomic Research, Rockville, MA, USA) website (www.tigr.org) reveals the extent of our intelligence about the bacterial world: in addition to the 91 published genome sequences, another 90, many of them belonging to human pathogenic bacteria, are underway (Table 1). A tax-paying citizen would rightly ask, “So does this mean new treatments around the corner?” As far as antibiotics are concerned, the answer is probably “no”, because those best equipped to develop them are not driven by the needs of the taxpayer. “I'm very concerned that big pharma is dropping out of anti-infectives,” commented Julian Davies, Professor at the Department of Microbiology and Immunology, University of British Columbia, Canada, referring to a process of attrition that is driven by the need to produce ever larger cost:profit ratios. As the main players of pharmaceutical research and development expand and merge into ever larger colossi,
One after the other, our bacterial foes are surrendering their secret arms to the microbial weapons inspectors: the sequencers and genomics experts who can lay bare their genomes
most are trimming off this unprofitable branch of R&D. As Stewart Levy, President of the Association for the Prudent Use of Antibiotics, and Professor of Molecular Biology and Microbiology at Tufts University (Boston, MA, USA), remarked, “They would rather spend their millions on a drug that people will take for life.” Indeed, the blockbusters that are keeping big pharma going, such as blood pressure and cholesterol controlling drugs, provide returns in the region of a few billion US dollars a year; a new antibiotic, in contrast, may only make a few hundred million dollars.
Table 1.
Genomes of human pathogenic bacteria for which sequencing is in progress, but which are not yet published
| Genome | Size (Mb) | Institution | Funding | Anticipated completion |
|---|---|---|---|---|
| Actinobacillus actinomycetemcomitans | 2.2 | University of Oklahoma | NIDR | – |
| Actinomyces naeslundii | 3 | TIGR | NIH/NIDCR | 2003 |
| Anaplasma phagocytophila | 1.5 | TIGR | NIAID; Ohio State University | 2003 |
| Bacillus anthracis | 4.5 | TIGR | ONR; DOE; NIAID; DERA | – |
| Bacillus cereus | 5.2 | TIGR | NIH | – |
| Bacteroides forsythus | – | TIGR | – | – |
| Bacteroides fragilis | 5.3 | Sanger Centre | Beowulf Genomics | – |
| Bartonella henselae | 1.9 | University of Uppsala | SSF | Completed |
| Bartonella quintana | 1.6 | University of Uppsala | SSF | Completed |
| Bordetella bronchiseptica | 4.9 | Sanger Centre | Beowulf Genomics | – |
| Bordetella parapertussis | 3.9 | Sanger Centre | Beowulf Genomics | – |
| Bordetella pertussis | 3.88 | Sanger Centre | Beowulf Genomics | Completed |
| Brucella abortus | – | Lawrence Livermore National Laboratory | – | – |
| Burkholderia mallei | 6.0 | TIGR; USAMRIID | NIH; NIAID | 2003 |
| Burkholderia pseudomallei | 6.0 | Sanger Centre; DERA; Public Health Laboratory | Beowulf Genomics | – |
| Campylobacter jejuni | 1.7 | TIGR; USDA | USDA | 2003 |
| Chlamydia pneumoniae | 1.23 | Genset | – | Completed |
| Chlamydia pneumoniae | 1.2 | Gene Alliance | Byk Gulden | Completed |
| Chlamydia trachomatis | 1.038 | Genset | – | Completed |
| Clostridium difficile | 4.4 | Sanger Centre | Beowulf Genomics | – |
| Clostridium perfringens (strain ATCC 13124) | – | TIGR | – | Completed |
| Clostridium perfringens (strain SM101) | – | TIGR | – | – |
| Clostridium tetani | 4.4 | Göttingen Genomics Laboratory | Ministry of Lower Saxony for Science and Culture | 2003 |
| Corynebacterium diphtheriae | 3.1 | Sanger Centre; WHO; Public Health Laboratory | Beowulf Genomics | Completed |
| Coxiella burnetii | 2.1 | TIGR | NIAID; DARPA | 2003 |
| Escherichia coli (strain CFT073) | 5.23 | University of Wisconsin | NIAID | – |
| Escherichia coli (K1 strain RS218) | – | University of Wisconsin | NIAID | – |
| Enterococcus faecalis | 3.00 | TIGR | NIAID | Completed |
| Enterococcus faecium | 2.8 | Baylor College of Medicine; UTHHSC | NIH/NIAID | 2003 |
| Francisella tularensis (strain Schu 4) | 2.00 | European & North American Consortium | DARPA | 2003 |
| Francisella tularensis (strain LVS) | – | Lawrence Livermore National Laboratory | – | – |
| Fusobacterium nucleatum ssp. polymorphum | 2.4 | Baylor College of Medicine | NIH/NIDCR | 2003 |
| Haemophilus ducreyi | 1.76 | University of Washington; HTSC | NIAID | – |
| Klebsiella pneumoniae | – | Washington University Consortium | NIAID | – |
| Legionella pneumophila | 4.0 | Columbia Genome Center | NIAID | 2003 |
| Leptospira interrogans serovar icterohaemorrhagiae | 4.8 | Chinese National Human Genome Center, Shanghai | CNCBD; Science Technology Commission of Shanghai | Completed |
| Listeria monocytogenes | 2.9 | TIGR | USDA | – |
| Listeria ivanovii | – | Competence Center Pathogenomik; Instit Pasteur; University of Leon, Spain | – | 2003 |
| Mycobacterium avium | 4.70 | TIGR | NIAID | 2003 |
| Mycobacterium bovis | 4.4 | Sanger Centre; Institut Pasteur; VLA Weybridge | MAFF; Beowulf Genomics | – |
| Mycobacterium paratuberculosis | 5.00 | University of Minnesota | USDA/NRI; Minnesota Agricultural Experiment Station | 2003 |
| Mycobacterium smegmatis | 7.0 | TIGR | NIAID | 2003 |
| Mycoplasma hyopneumoniae* | 0.89 | University of Washington | – | Completed |
| Neisseria gonorrhoeae | 2.20 | University of Oklahoma | NIAID | – |
| Neisseria meningitidis | 2.2 | Sanger Centre | Beowulf Genomics | – |
| Porphyromonas gingivalis | 2.20 | TIGR; Forsyth Dental Center | NIDR | Completed |
| Prevotella intermedia | 3.8 | TIGR | NIH/NIDCR | 2003 |
| Rickettsia conorii | 1.2 | GENOSCOPE | – | – |
| Rickettsia typhi | 1.4 | Baylor College of Medicine; UTMB | NIH/NIAID | 2003 |
| Salmonella enterica (serovar Dublin) | – | University of Illinois | USDA | 2003 |
| Salmonella enterica (serovar Pulloram) | – | University of Illinois | USDA | – |
| Salmonella enterica (serovar Choleraesuis) | University of Illinois | USDA | – | |
| Salmonella enteritidis | 4.5 | University of Illinois | University of Illinois | – |
| Salmonella paratyphi A | 4.60 | Washington University Consortium | NIAID | – |
| Salmonella typhi | 5.08 | University of Wisconsin | NIAID | – |
| Salmonella typhimurium | 4.50 | Washington University Consortium | – | – |
| Shigella flexneri 2a | 4.65 | University of Wisconsin | NIAID | – |
| Staphylococcus aureus (strain COL) | 2.80 | TIGR | NIAID; MGRI | – |
| Staphylococcus aureus (strain 8325) | 2.80 | University of Oklahoma | NIAID; MGRI | – |
| Staphylococcus aureus (strain MRSA) | 2.8 | Sanger Centre; Trinity College, Dublin; WTCEID | Beowulf Genomics | – |
| Staphylococcus aureus (strain MSSA) | 2.8 | Sanger Centre; Trinity College, Dublin; WTCEID | Beowulf Genomics | – |
| Staphylococcus epidermidis (strain RP62A) | 2.4 | TIGR | NIH; NIAID | Completed |
| Staphylococcus epidermidis (strain ATCC 12228) | 2.4 | Chinese National Human Genome Center, Shanghai; Shanghai Medical University | – | 2003 |
| Streptococcus agalactiae | 2.1 | TIGR | NIAID | 2003 |
| Streptococcus gordonii | TIGR | – | – | |
| Streptococcus mitis | 2.2 | TIGR | NIH/NIDCR | 2003 |
| Streptococcus pneumoniae | 2.1 | TIGR | NIAID; University of Alabama | – |
| Streptococcus pyogenes | 1.98 | Sanger Centre; University of Newcastle | Beowulf Genomics | – |
| Streptococcus sobrinus | – | TIGR | – | – |
| Treponema denticola | 3.00 | Baylor College of Medicine; TIGR | NIH/NIDCR | 2003 |
| Yersinia pseudotuberculosis | – | Lawrence Livermore National Laboratory; Institut Pasteur | DOE–Chemical; Biological Non-proliferation Program; CEB; DGA | – |
*Belongs to the classification Mycoplasma, which can be considered to be between a virus and a bacterium. Source: The Institute for Genomic Research (TIGR) database of microbial genomes, March 2002. For full listings, including strain information, refer to http://www.tigr.org/tdb/mdb/mdbinprogress.html. For listings of published microbial genomes, refer to http://www.tigr.org/tdb/mdb/mdbcomplete.html. USAMRIID, US Army Medical Research Institute of Infectious Diseases; CEB, Center for Environmental Biotechnology, University of Tennesee, Knoxville, TA, USA; CNCBD, China National Center for Biotechnology Development; DARPA, US Defense Advanced Research Projects Agency; DERA, US Disaster Preparedness and Emergency Response Association; DGA, Delegation Generale pour l'Armement, Paris, France; DOE, US Department of Energy; MAFF, UK Ministry of Agriculture, Fisheries and Food, now part of the Department for the Environment, Food and Rural Affairs; Mb, megabases; MGRI, Merck Genome Research Institute; NIAID, US National Institute of Allergy and Infectious Diseases; NIDCR, US National Institute of Dental and Craniofacial Research; NIDR, US National Institute of Dental Research; NIH, US National Institutes of Health; NRI, US National Resources Institute; ONR, US Office of Naval Research; SSF, Stiftelsen för Strategisk Forskning (Swedish Foundation for Strategic Research); USDA, US Department of Agriculture; UTHHSC, Committee for the Protection of Human Subjects; UTMB, University of Texas Medical Branch; VLA, US Vetinary Laboratories Agency; WHO, World Health Organization; WTCEID, Wellcome Trust Centre for the Epidemiology of Infectious Disease.
This problem is not really helped by tipping a load of genomic data into the pharmaceutical laboratories because, as Levy pointed out, although we may be able to identify putative new targets, “we just don't have the molecules [to attack them].” Davies agrees that the real bottleneck today is synthetic chemistry. “One of the most important contributions will be pharmacogenomics,” according to him, but this is going to take a while to produce results. Exaggerating slightly, he asserted that “all the efforts of target-based drug discovery have failed in vitro because the target, even if it is an essential gene, is not necessarily a valid target in vivo.” Furthermore, the hardest part of producing a new antibiotic is not finding a target, but designing molecules to hit it that have acceptable profiles of toxicity and pharmacodynamics/kinetics. And so far, natural products have proved both more effective and less toxic than synthetic ones. But, as Miguel Vicente from the Centro Nacional de Biotecnologia in Madrid, Spain, observed, bacteria somewhere will probably have come in contact with these anti-bacterial compounds during their evolution, hence laying the foundations for the spread of resistance. With bacterial genome sequences at hand, science today has the opportunity to surprise the enemy with new approaches. Cationic peptides that are being developed for topical use and for coating in-dwelling medical devices (EMBO reports, 4, 114–117; 2003) are an example of such new developments, but they have had a bumpy ride with the regulatory authorities.
The retreat of big pharma from the battlefield of anti-infectives will, at least, give small companies, whose profit requirements are lower, the chance to fill this niche. However, they will have to get drug candidates at least as far as animal trials. “Large companies may enter later,” explained Levy, “but they won't be in the discovery phase.” A prerequisite for fast progress is the development of high-throughput structural biology techniques, but producing a broad-spectrum drug with a winning profile on all scores involves a large investment in synthetic chemistry and screening, and this is not something to which every small company can turn its hand.
And even if a new drug shows promise in clinical trials, it still has to overcome regulatory hurdles. Although the US Food and Drug Administration has introduced a fast-track approval procedure for new anti-infectives, it certainly has not become less stringent.
...public funding agencies [...] should certainly recognize untreatable bacterial infections as a socio-economic drain
Already, the decline in new drug approvals is raising concerns, as the Washington Post reported last November (November 18, 2002, page A1). Indeed, as Davies noted, penicillin, erythromycin, tetracycline and the aminoglycosides would probably not have passed the agency's scrutiny today. As a new miracle drug seems to be a vain hope at present, policy makers need to concentrate on public health measures and promote the prudent use of existing antibiotics. Compared with the USA, Europe—particularly Germany and Scandinavia—has achieved an encouraging decrease in the indiscriminate use of antibiotics; but, as Davies knows, antibiotic resistance never goes away. And the stabilization of resistance-creating mutations by compensatory mutations at other loci allows resistance to survive even in the absence of selective pressure. The most that one could hope for, using antibiotic rotation strategies, is a decrease in the incidence of resistance to 30%. But Levy stressed that for many problematic bacteria, it would be hard to find even two antibiotics to use in rotation. Add to this the fact that doctors do not like to be regulated, and it is clear that new research will have to provide a large part of the answer. For the US National Institutes of Health, the European Commission and other public funding agencies, who should urgently recognize untreatable bacterial infections as a socio-economic drain, this should signal a change in policy: a readjustment of expectations, and an increase in funding periods to a more realistic length.
But although genomics has not given antibiotic research the expected boost, it is overturning the field of vaccine research. Rino Rappuoli, Vice President of Vaccines Research at Chiron in Sienna, Italy, has been an instrumental figure in this revolution. “I believe that microbial genomics have shortened the time for vaccine development against microbes enormously,” he said. In 15 years of pre-genomic research on Meningococcus B, for example, almost no progress had been made. That has all changed: “Basically, in four years, we are in a phase I trial with antigens never seen before,” Rappuoli said. The principle of 'reverse vaccinology' (Fig. 1), pioneered by Chiron, is a classic case in which analysis of the sequences of all the proteins in a bacterium can greatly accelerate the search for an antigenic protein that is located on its surface. Hervé Tettelin, an Associate Investigator in Microbial Genomics at TIGR, which collaborates with Chiron, is in no doubt about the power of genomics approaches in identifying putative antigens for vaccine development. Using reverse vaccinology, TIGR and Chiron also identified around 600 Nisseria meningiti-dis outer-membrane-associated proteins. Twenty-five of these were shown to have bacteriocidal potential when used in combination with major histocompatibility complex protein and sera from mice that had been inoculated with purified bacterial protein isolates. Of these, seven are conserved across other Nisseria species, and, therefore, represent attractive candidates for broadspectrum vaccines.
Figure 1.
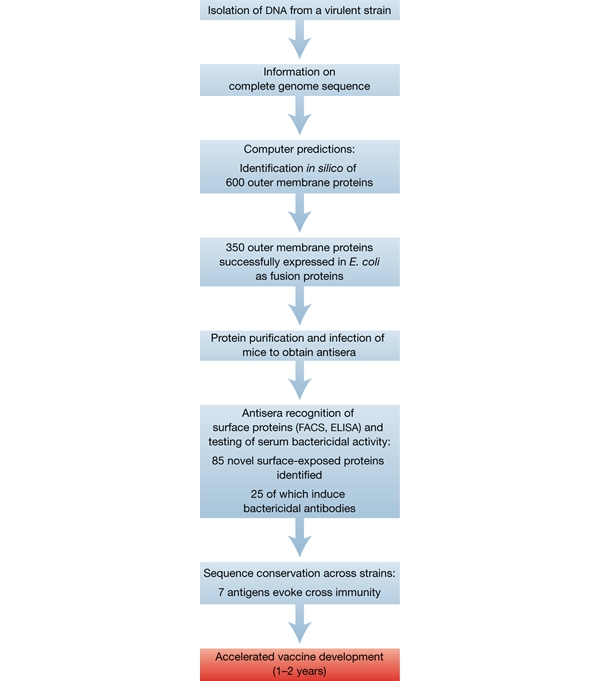
The principle of reverse vaccinology, using Neisseria meningitidis as an example.
This could solve the long-standing problem that current anti-Nisseria vaccines only protect against four out of the five main sera groups. “All proteins so far cloned, expressed and used for vaccine development had failed because they were not cross-protective,” explained Tettelin. In the case of group-B streptococci, too, there is no 'good' vaccine. But with sharper tools, provided by bacterial
The only certainty is that bacteria will continue to outwit us, and this problem demands engagement on all fronts
genomics, scientists are now able to rapidly find better and more numerous potential targets: “We did all this in 18 months,” said Tettelin, “compared with years of shooting in the dark. With genomics you're not missing anything; you have all the pieces in the puzzle.” Above all, the technology promises safer and more effective vaccines for a world that certainly has not escaped the possibility of another great epidemic. “I don't see a vaccine that can't be approached today,” Rappuoli added, “the problems are [now] socioeconomic barriers.”
Rappuoli's optimism also extends to the role of industry in developing anti-infectives. “I think [small biotech] will see a revolution that big pharma hasn't seen. Genomics has provided hundreds of new targets, but we haven't had enough time to exploit them yet.” Although big pharma—the likes of Aventis (Bridgewater, NJ, USA) and Merck Sharp & Dohme (Whitehouse Station, NJ, USA)—are drastically reducing their research spending on anti-infectives, one company at least claims not to have abandoned the field. GlaxoSmithKline (GSK), Brentford, UK, is “very committed to this area,” according to David Pompliano, Vice President of Biology in GSK's Microbial Musculoskeletal and Proliferative Diseases Drug Discovery unit. The challenge is to “find a molecule that will take out several proteins, or craft a molecule that's exquisitely specific [for non-mutable residues],” he explained. But he concedes that what industry misses is a “concentration on the 'developability' parameters around a compound,” adding “It's really a very difficult chemical problem.” Multidisciplinarity certainly helps, and GSK has invested heavily in this area. As part of their 'centres of excellence' for drug discovery, a large pharmacogenetics division and the involvement of early-stage clinicians supply the necessary expertise. A priority area of their research is pulmonary pneumonia, a market that seems likely to increase with the ageing population of western countries. Perhaps, in this area at least, “[we're] going to see in the next 4–5 years some very interesting molecules,” according to Pompliano. Vaccine research is another area of focus for this pharmaceutical giant, but they are not interested in the study of the interaction between host and pathogen genomes. This topic intrigues those that study tropical diseases, because susceptibility to, and severity of, infection depend greatly on host variability in human leucocyte antigens and MHCs, and can have a profound effect on vaccine development and administration strategies. But, sadly, this is not an area that the big guns can afford to be interested in.
Despite the promise of genomics, it would be unwise to rely solely on science or industry to come up with quick fixes for infectious diseases. Breakthroughs may well occur in certain fields, but the process of applying genomic and proteomic information to drug production will take a while yet, and will take time to gain momentum as it is heavily dependent on advances in combinatorial chemistry and on cheaper high-throughput technologies. The only certainty is that bacteria will continue to outwit us, and this problem demands engagement on all fronts, notably in public healthcare strategies and prescriptions. Policy making and funding must also increasingly take a longer view at an international level, as microbes respect no geopolitical boundaries. To win the forthcoming battles will require some considerable cunning and dedication. As Vicente remarked, “bacteria have been here longer than we have, and have an intelligence we don't yet understand.”


