Abstract
During translation, a string of non-overlapping triplet codons in messenger RNA is decoded into protein. The ability of a ribosome to decode mRNA without shifting between reading frames is a strict requirement for accurate protein biosynthesis. Despite enormous progress in understanding the mechanism of transfer RNA selection, the mechanism by which the correct reading frame is maintained remains unclear. In this report, evidence is presented that supports the idea that the translational frame is controlled mainly by the stability of codon–anticodon interactions at the P site. The relative instability of such interactions may lead to dissociation of the P-site tRNA from its codon, and formation of a complex with an overlapping codon, the process known as P-site tRNA slippage. We propose that this process is central to all known cases of +1 ribosomal frameshifting, including that required for the decoding of the yeast transposable element Ty3. An earlier model for the decoding of this element proposed 'out-of-frame' binding of A-site tRNA without preceding P-site tRNA slippage.
Introduction
A crucial rule of protein synthesis is the triplet character of genetic decoding. How the translational machinery maintains the proper reading frame is a question of primary importance. A small number of genes, the translation of which requires a switch between open reading frames through efficient ribosomal frameshifting (Farabaugh & Björk, 1999; Brierley & Pennell, 2001; Baranov et al., 2002a, 2003), offer a unique tool for the investigation of this question. An understanding of the mechanism of frameshifting could provide an answer to how triplet decoding is controlled by the ribosome.
The first bacterial chromosomal gene that was found to require +1 frameshifting for its expression was the Escherichia coli gene prfB, which encodes release factor 2 (RF2; Craigen et al., 1985; Craigen & Caskey, 1986). The mechanism suggested for this involves slippage of the Psite tRNALeu from the codon CUU to the overlapping codon, UUU, in the frameshift site CUU UGA C (Craigen et al., 1985; Weiss et al., 1987), followed by binding of a tRNA to the +1-frame Asite codon. Further studies, using artificial constructs based on the RF2 frameshifting cassette, have supported this idea (Curran & Yarus, 1988; Curran, 1993). Efficient frameshifting was seen in those cases where the Psite tRNA was able to form good base pairing with the overlapping +1 codon, for example, UUU or CCC.
The site of the frameshifting required for decoding both the yeast transposable element Ty1 (Belcourt & Farabaugh, 1990) and the actin-filament-binding protein ABP140 (Asakura et al., 1998) is CUU AGG C. The mechanism originally suggested for this (Belcourt & Farabaugh, 1990) was similar to that suggested for prfB: Psite tRNA slippage followed by binding of a tRNA to the +1-frame A-site codon. The frameshifting required for Ty3 expression occurs at the sequence GCG AGU U (Farabaugh et al., 1993). Its Psite tRNA, decoding GCG, cannot form good base pairs with the +1 codon, CGA, leading to the hypothesis that efficient frameshifting occurs without P-site tRNA slippage, but with out-of-frame binding of tRNA at the A site (Farabaugh et al., 1993; Pande et al., 1995; Stahl et al., 2001). Recently, the same group raised the possibility that out-of-frame Asite tRNA binding is responsible for all cases of programmed frameshifting in yeast (Stahl et al., 2001).
However, an alternative explanation has been proposed for the efficient frameshifting that occurs in Ty1 and Ty3 (Ivanov et al., 2002; Baranov et al., unpublished data). As described in Sundararajan et al. (1999), the Psite codons in these frameshift sites are recognized by tRNAs that lack the ability to form a standard base pair in the wobble position in codon–anticodon duplexes. Accordingly, the rate of dissociation of these tRNAs from their P-site codons should be higher than usual. In addition, these tRNAs cannot form good base pairs with the overlapping +1 codons (especially for Ty3 decoding) and, therefore, the resulting complex cannot be stable. However, Ty1 and Ty3 frameshifting is stimulated by the presence, in the initial frame, of an A-site codon that is decoded by sparse tRNAs (Pande et al., 1995). The relative paucity of the tRNA decoding the zero-frame Asite codon, and the abundance of the tRNA decoding its overlapping +1-frame codon, are crucial for the stimulation of frameshifting (Baranov et al., 2003b). Fig. 1 shows a model for +1 frameshifting without out-of-frame tRNA binding.
Figure 1.
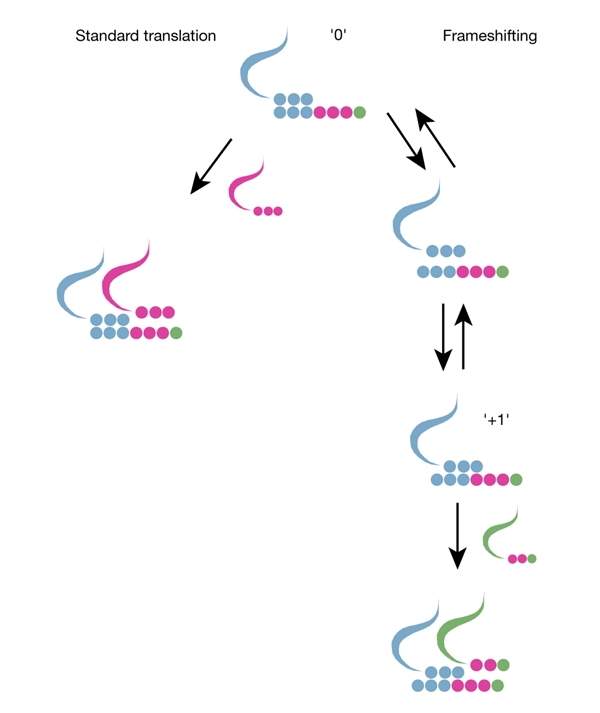
Illustration of +1 frameshifting, shown in parallel with the process of standard translation. The kinetic requirement for efficient frameshifting is the commensurability of the rates of the two processes. Usually, the rate of standard translation is significantly higher than that of frameshifting. A low concentration of incoming transfer RNA in the initial frame (magenta) makes standard translation slower than usual. Low stability of the initial P-site codon–anticodon complex ('0'), high stability of the equivalent complex in the new frame ('+1'), and high levels of the incoming tRNA corresponding to the A-site codon in the new frame (green), increase the rate of frameshifting. As a result, a high input of several stimulating factors can compensate for the lack of one of them. In Ty3, it is the lack of stable P-site codon–anticodon duplexes in the +1 shifted frame that is compensated for by three other factors.
If, conversely, frameshifting is limited by binding of the A-site tRNA to the +1-frame codon, then stimulators that promote slippage of the P-site tRNA into the +1 frame should not affect frameshift efficiency. Stimulators of this type have not been identified for +1 frameshifting in yeast; however, they are known in bacteria. We have explored the effect of this type of stimulator on frameshifting in E. coli using a model system in which the P-site tRNA cannot form good base pairs with the +1-frame codon, which is analogous to the Ty1/Ty3 situation.
Results and Discussion
The stimulator used in these experiments was first discovered because of its role in promoting the autoregulatory frameshifting required for synthesis of RF2. It is an internal Shine–Dalgarno (SD) sequence, the precise position of which is crucial for efficient frameshifting (Weiss et al., 1987, 1988; Curran & Yarus, 1988). There are at least two explanations for its action, which are not necessarily mutually exclusive. The short distance between the SD sequence and the Psite codon (three nucleotides) creates a tension between the corresponding parts of the ribosome: the anti-SD region and the decoding centre. This tension causes the P-site tRNA to slip in the +1 direction, so that the distance between the anti-SD region and the decoding centre becomes closer to that used for the 'frame-neutral' role in translation initiation (Atkins et al., 2001; Baranov et al., 2002b). The existence of such tension is supported by the fact that the SD sequence is known to stimulate −1 frameshifting when it is located ∼10–14 bases upstream of a frameshift site (discussed in detail in Atkins et al., 2001). In this case, backward movement of the ribosome–tRNA complex, relative to the messenger RNA, helps to achieve a distance between the antisD region and the decoding centre and relieves the conferred ribosomal tension. Another explanation is that SD:anti-SD complex clashes with E-site tRNA, promoting its movement towards the P-site tRNA, and also resulting in P-site tRNA slippage (Baranov et al., 2002b; K.H. Nierhaus, personal communication). It is clear that the mRNA–rRNA pairing that involves the SD sequence stimulates peptidyl-tRNA slippage.
The out-of-frame binding model was suggested for Ty3 frameshifting because tRNA located in the P site, when shifted, cannot form good base pairs with the +1 codon. Farabaugh and colleagues have noted that the P-site tRNAs that promote +1 frameshifting in yeast do not usually form canonical base pairs in the third position of the codon–anticodon duplex (Sundararajan et al., 1999). The tRNAs involved have been termed 'special' or 'near-cognate' tRNAs. Stimulators that are known to promote +1 tRNA slippage in yeast have not yet been discovered. Although a special 3′ context is important for Ty3 frameshifting (Li et al., 2001), its mechanistic function is unknown. To test the generality of the out-of-frame hypothesis, we modelled conditions of Ty3 frameshifting in a bacterial system, in which a stimulator, an internal SD sequence that promotes +1 slippage, is used. To model the Ty3 situation in bacteria, the RF2 frameshifting site was modified so that tRNAs at the Psite satisfied two requirements: first, no canonical base pair could be formed in the wobble position of the zero-frame codon–anticodon duplex. Second, after slippage, the P-site tRNAs cannot form a good base pair with the +1 P-site codon.
We investigated whether a suitably positioned SD sequence had a stimulatory effect on frameshifting at sequences where it was unclear whether out-of-frame binding of an incoming tRNA, or P-site tRNA slippage, have a causal effect. We constructed cassettes that resulted in the positioning of different codons (XYZ) adjacent to the UGA stop codon, on the 5′ side, in the RF2 frameshifting site. The sequence of the wild-type RF2 frameshift site is CUU UGA C (with the first codon in the new +1 frame underlined). The CUU shift codon was replaced by GCG, GGU, GUU, AAU, AAG, GAU, AGU, UGU or CGA. All of these codons are normally recognized by tRNAs sharing two features: they cannot form 'Watson–Crick only' base pairs with the zero-frame codon, and they cannot form more than one Watson–Crick base pair if they are shifted to the +1 overlapping codon (YZU), as in the sequence shown in Fig. 2A. The sequences containing a modified RF2 frameshifting cassette were inserted between the sequences encoding glutathione-S-transferase (GST) and maltose-binding protein (MBP), in a gst–malE fusion gene on the plasmid GHM53 (see Methods). malE is in the +1 frame relative to gst, and the complete GST–MBP fusion protein is therefore only translated if +1 frameshifting occurs. Termination at the UGA stop codon results in the synthesis of a shorter protein (Fig. 2A). The constructs were assayed for frameshifting in pulse–chase labelling experiments. Proteins were purified by affinity chroma-tography using the amino-terminal GST tag, followed by gel electrophoresis. The purification step increases the sensitivity as compared with assays using crude extracts, but is not strictly quantitative (data not shown). The identities of the frameshift and termination protein products were determined by mass spectrometry, after purification using the GST tag. In each case, the mass of the frameshift product corresponded to that expected when the amino acid at the frame junction is specified by the zero-frame codon (Fig. 2C).
Figure 2.

+1 frameshifting at the gene encoding release factor 2, wherethe CUU shift-site is replaced by GUU or AAG. (A) Constructs and their products. XYZ represents the position of CUU, which was replaced in these constructs. (B) Pulse–chase experiment. Electrophoretic separation of glutathione-S-transferase (GST)-tagged proteins on an SDS gel. The samples electrophoresed on the gel were from constructs containing a Shine–Dalgarno sequence (SD) or lacking this sequence (ΔSD). (C) Protein analysis. Mass spectra in the 67,500–71,500 Da range are shown for GST-tagged proteins that were purified from strains expressing constructs containing an SD. The major peaks are at 69,479 Da (GUU) and 69,508 Da (AAG), corresponding to frameshift products with expected masses of 69,479.6 Da (GUU) and 69,508.6 Da (AAG). A minor peak with a mass corresponding to the major product lacking one methionine is indicated (−M). An adduct of the major product is apparent in the AAG spectrum (*). (D) Codon–anticodon pairing in the +1 frame. Possible Watson–Crick (bar) and wobble (circle) base pairs are indicated. FS, product of +1 frameshifting in the insert; mRNA, messenger RNA; Term, product resulting from termination at UGA in the insert; tRNA, transfer RNA.
Of the nine codons tested, only GUU and AAG resulted in relatively high levels of frameshifting. The fact that detectable frameshifting was not promoted by all nine codons indicates that, in addition to the number of good base pairs in the codon–anticodon duplexes at the P site in the zero and shifted frames, there are other factors that are likely to influence the overall stability of mRNA–tRNA interactions. If frameshifting on these codons occurs due to slippage of the cognate P-site tRNA, the resulting +1 codon–anticodon complex should be relatively unstable, as fewer than two Watson–Crick base pairs can be formed (Fig. 2D). The E. coli valine tRNAs have the anticodons 3′-CAG-5′ and 3′-CAV-5′, where V is uridine-5-oxyacetic acid (Yaniv & Barrell, 1971). This is a permissive modification of uridine, and V can pair with U, A or G (Yokoyama et al., 1985). It is likely that both of these tRNAs usually recognize the GUU codon. In both cases, re-pairing to mRNA by the 3′-CAV-5′ or 3′-CAG-5′ anticodons through the +1-frame codon gives a Watson–Crick pair in the second position and a V:U or G:U pair in the third position, but no Watson–Crick or wobble pairing in the first position (Fig. 2D).
Protein analysis (see Methods) showed that AAG is decoded as lysine, and must therefore be decoded by the sole tRNALys. This tRNA has the anticodon 3′-UUU*-5′ (Chakraburtty et al., 1975), where U* is 5-methylaminomethyl-2-thiouridine (although some of the molecules have selenium instead of sulphur). This restrictive modification probably helps tRNALys to distinguish between Lys (AAA and AAG) and Asn (AAU and AAC) codons (Sundaram et al., 2000). Canonical base pairs cannot be formed between the anticodon of this tRNA and the codon in the +1 frame at the third position, and only a G:U pair can be formed at the second position (Fig. 2D). In both of these examples, high levels of frameshifting occur (Fig. 2B, lanes 1 and 2).
The SD sequence (AGG GGG) was destroyed in another set of constructs, in which it was replaced by ACC UCU. No frameshifting was detected when the SD sequence was destroyed, as shown in Fig. 2, lanes 3 and 4. This suggests that Psite tRNA slippage, rather than out-of-frame binding, is the reason for frameshifting in these artificial RF2 constructs. Furthermore, formation of at least two canonical base pairs between the codon and anticodon of the P-site tRNA in the +1 frame is not required for efficient frameshifting.
A similar hypothesis was recently proposed, on the basis of molecular analysis of the +1 frameshifting of eukaryotic antizyme genes (Ivanov et al., 2002). During the initial stages of analysis, it was realized that the tRNA, which is in the P site during antizyme recoding, could not form good base pairs with the overlapping +1 codon. This was even more obvious for several Psite-codon mutants, which otherwise gave almost wild-type levels of frameshifting (Matsufuji et al., 1995). On the basis of these results, it was concluded at first that +1 frameshifting in antizyme genes is due to out-of-frame binding of tRNA at the Asite. Further testing challenged this conclusion; specifically, when a mammalian antizyme 1 (one of several mammalian paralogues of antizyme) frameshift cassette was tested in Saccharomyces cerevisiae, it gave high levels of shifting to the +1 frame, but the ribosomes reached this frame mostly (90% of the time) by a −2 shift rather than by a +1 shift (10% of the time; Matsufuji et al., 1996). A similar situation was observed when the same cassette was tested in Schizosaccharomyces pombe: the ribosomes reached the +1 frame through a +1 shift 80% of the time, and through a −2 shift 20% of the time (Ivanov et al., 1998). It was clear that the −2 shift occurs only as a result of anticodon re-pairing to mRNA at the P site, and as it seems unlikely that the same sequence can induce two rare and mechanistically different events, it is likely that both shifts are the result of a single Psite re-pairing mechanism.
The examples described above do not support the idea of out-of-frame binding of A-site tRNA. Our understanding of the mechanism of translation has been advanced by recent structural analyses of ribosomes and their functional complexes (Wimberly et al., 2000; Ban et al., 2000; Schluenzen et al., 2000; Yusupov et al., 2001). The data obtained from structural studies, too, do not support the idea of out-of-frame binding. Fig. 3A shows the interactions of mRNA with Asite and P-site tRNAs in the ribosome (Ogle et al., 2001). Fig. 3B illustrates the network of ribosomal components, the decoding centre, that is responsible for the selection of Asite tRNA (Ogle et al., 2001; Lynch et al., 2003). The adenosines at positions 1,493 and 1,492 (here, and later, the E. coli numbering is used) are known to be responsible for monitoring the correct conformation of the first two base pairs in the codon–anticodon duplex at the A site (reviewed in Ramakrishnan, 2002). These two adenosines, as well as other components of the decoding centre, have a strict orientation relative to the Psite codon–anticodon duplex. If this was not the case, the ribosome would not be able to discriminate between the zero-frame A-site codon and the +1-frame codon. The 16S rRNA component that is likely to be responsible for the correct positioning of the decoding centre relative to the P-site codon–anticodon duplex is nucleotide 1,401. This nucleotide is flipped into the space between two tRNAs, as seen in the structure obtained by the diffusion of mRNA into ribosome crystals (Yusupova et al., 2001).
Figure 3.
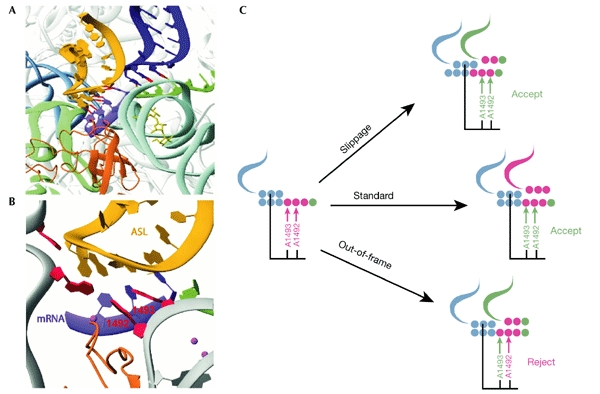
Illustration of how ribosomes prevent out-of-frame binding of incoming transfer RNA. (A) Details of the A site and P site. The A-site transfer RNA is shown in yellow; helix 6 from the neighbouring ribosome molecule that mimics P-site tRNA is shown in dark blue. (B) Details of the ribosomal components surrounding the A-site tRNA; adenosines 1,492 and 1,493 are labelled. (C) Schematic illustration of out-of-frame binding at the A site. Magenta arrows indicate that the discriminating adenosines do not sense a correct base pair; green arrows indicate that the discriminating adenosines recognize a correct base pair. Whereas standard translation and P-site tRNA slippage lead to normal recognition at the A site, out-of-frame binding does not. The illustrations in (A,B) are abstracted with permission from Ogle et al. (2001) © (2001) American Association for the Advancement of Science. ASL, anticodon stem–loop; mRNA, messenger RNA.
In the out-of-frame binding model, the first base of the A-site codon is unpaired. Normally, base pairing at this position is monitored by adenosine 1,493. The lack of correct base pairing in this position is expected to lead to the rejection of tRNAs that recognize the +1 codon. Thus, even if tRNA initially binds at the +1 codon, such binding is expected to be followed by rejection of the tRNA. This is illustrated in Fig. 3C. The recognition of a +1-frame codon is possible only if there are significant structural rearrangements within the 30S ribosomal subunit, leading to relocation of the decoding centre relative to the position of the Psite codon–anticodon duplex. It is unclear as to what forces could lead to such a relocation, but, more significantly, it is unlikely that such repositioning is possible.
A recent review suggested that “the effect of ... [tRNAs forming suboptimal codon–anticodon base pairing at the P-site] ... is to disrupt the mechanism that the ribosome uses to distinguish an in-frame from an out-of-frame amino-acid–tRNA complex in the A-site.” (Stahl et al., 2001). The data presented here suggest that the mechanism of frame control is based on, first, rigid positioning of the decoding centre relative to the Psite tRNA and, second, the stability of the complex formed between the tRNA anticodon and the mRNA codon at the P site. Taking this into account, it is clear why and how tRNAs that form less than optimal codon–anticodon base pairing at the P site disrupt the mechanism of frame control.
Methods
Plasmids and bacterial strains.
A gst–malE expression vector (GHM53), containing BamHI and EcoRI restriction sites between the coding sequences of GST and MBP has been described previously (Herr et al., 2001). Inserts made from complementary oligonucleotides (containing mutated RF2 frameshifting cassettes) were cloned between the BamHI and EcoRI sites. The oligonucleotides were based on the sequences 5′-GATCAGGGGGTATXYZTGACTAC-3′ and 5′-AATTGTAGTCAZYXATACCCCCT-3′ (italicized letters indicate complementary nucleotides; bold letters indicate nucleotides that are changed, as described in the Results and Discussion section). A derivative of E. coli strain SU1675 that lacks the F′ episome was used in all experiments (Weiss et al., 1988).
Frameshifting assay.
Overnight cultures of strains expressing the appropriate plasmid were grown in MOPS–glucose (Neidhardt et al., 1974) containing 100 μg ml−1 ampicillin and all amino acids (150 μg ml−1 each) except methionine and tyrosine, and were diluted 1:50 in 300 μl of this media. After a 2-h incubation at 37 °C, the cultures were induced for 10 min by adding 2 mM isopropyl-D-thiogalactoside (IPTG). Cells were pulsed for 2 min with 7.5 μCi [35S]methionine in 30 μl media, chased for 2 min by the addition of 30 μl of cold methionine (50 mg ml−1), chilled on ice, and harvested by centrifugation. The pellet was resuspended in 100 μl of BugBuster reagent (Novagen), shaken for 10 min and centrifuged at 12,000g for 10 min. The supernatant was transferred to 20 μl of 50% GST (AP Biotech) equilibrated in PBS (150 mM NaCl, 16 mM Na2HPO4, 4 mM NaH2PO4, pH 7.3), 0.5% Triton X-100, and shaken for 10 min. The resin was then washed in 150 μl PBS, 0.5% Triton X-100, followed by washing in 150 μl PBS, and proteins were eluted with 60 μl of 10 mM glutathione in PBS. 10 μl aliquots were loaded onto a 15% Tris–glycine–SDS polyacrylamide gel. Gels were visualized using a Molecular Dynamics PhosporImager.
Protein analysis.
Overnight cultures of strains containing the appropriate plasmids were diluted 1:50 in Terrific Broth, grown for 2 h at 37 °C, and induced with 1 mM IPTG for 4 h at 37 °C. Harvested cells were lysed using BugBuster reagent. Recombinant proteins were purified by passaging over glutathionine–sepharose (Amersham Pharmacia Biotech). Purified proteins were concentrated, and washed extensively with Nanopure H2O using a Centricon 10 unit (Millipore). Final cleanups and mass measurements were performed as described in Herr et al. (2001), except that only C4 P10 ZipTips (Millipore) were used for cleanups, and proteins were eluted with 56% (v/v) methanol, 1.5% formic acid, with three aliquots of 2 μl, which were then pooled and introduced into the mass spectrometer by infusion at 3 μl min−1.
Acknowledgments
We thank C. Nelson for important help with mass spectra analyses. This work was supported by a grant from the National Institutes of Health (GM48152) to J.F.A. and from the US Department of Energy (DE-FG03-01ER63132) to R.F.G.
References
- Asakura T. et al. (1998) Isolation and characterization of a novel actin filament binding protein from Saccharomyces cerevisiae. Oncogene, 16, 121–130. [DOI] [PubMed] [Google Scholar]
- Atkins J.F. et al. (2001) Over-riding standard decoding: implications of recoding for ribosome function and enrichment of gene expression. Cold Spring Harb. Symp. Quant. Biol., 66, 217–232. [DOI] [PubMed] [Google Scholar]
- Ban N., Nissen P., Hansen J., Moore P.B. & Steitz T.A. (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science, 289, 905–920. [DOI] [PubMed] [Google Scholar]
- Baranov P.V., Gesteland R.F. & Atkins J.F. (2000a. a) Recoding: translational bifurcations in gene expression. Gene, 286, 187–201. [DOI] [PubMed] [Google Scholar]
- Baranov P.V., Gesteland R.F. & Atkins J.F. (2000b. b) Release factor 2 frameshifting sites in different bacteria. EMBO Rep., 3, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov P.V., Gurvich O.L., Hammer A.W., Gesteland R.F. & Atkins J.F. (2003) RECODE 2003. Nucleic Acids Res., 31, 87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcourt M.F. & Farabaugh P.J. (1990) Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7-nucleotide minimal site. Cell, 62, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I. & Pennell S. (2001) Structure and function of the stimulatory RNAs involved in programmed eukaryotic −1 ribosomal frameshifting. Cold Spring Harb. Symp. Quant. Biol., 66, 233–248. [DOI] [PubMed] [Google Scholar]
- Chakraburtty K., Steinschneider A., Case R.V. & Mehler A.H. (1975) Primary structure of tRNA-Lys of E. coli B. Nucleic Acids Res., 2, 2069–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W.J. & Caskey C.T. (1986) Expression of peptide chain release factor 2 requires high-efficiency frameshift. Nature, 322, 273–275. [DOI] [PubMed] [Google Scholar]
- Craigen W.J., Cook R.G., Tate W.P. & Caskey C.T. (1985) Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc. Natl Acad. Sci. USA, 82, 3616–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J.F. (1993) Analysis of effects of tRNA:message stability on frameshift frequency at the Escherichia coli RF2 programmed frameshift site. Nucleic Acids Res., 21, 1837–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J.F. & Yarus M. (1988) Use of tRNA suppressors to probe regulation of Escherichia coli release factor 2. J. Mol. Biol., 203, 75–83. [DOI] [PubMed] [Google Scholar]
- Farabaugh P.J. & Björk G.R. (1999) How translational accuracy influences reading frame maintenance. EMBO J., 18, 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P.J., Zhao H. & Vimaladithan A. (1993) A novel programmed frameshift expresses the POL3 gene of retrotransposon Ty3 of yeast: frameshifting without tRNA slippage. Cell, 74, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr A.J., Nelson C.C., Wills N.M., Gesteland R.F. & Atkins J.F. (2001) Analysis of the roles of tRNA structure, ribosomal protein L9, and the bacteriophage T4 gene 60 bypassing signals during ribosome slippage on mRNA. J. Mol. Biol., 309, 1029–1048. [DOI] [PubMed] [Google Scholar]
- Ivanov I.P., Gesteland R.F., Matsufuji S. & Atkins J.F. (1998) Programmed frameshifting in the synthesis of mammalian antizyme is +1 in mammals, predominantly +1 in fission yeast, but −2 in budding yeast. RNA, 4, 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.P., Gurvich O.L., Gesteland R.F. & Atkins J.F. (2002) in Translation Mechanisms (eds Lapointe, J. & Braker-Gingras, L.) Landes Bioscience, Austin, Texas, USA (http://www.eurekah.com/chapter.php?chapid=841&bookid=59&catid=54). [Google Scholar]
- Li Z., Stahl G. & Farabaugh P.J. (2001) Programmed +1 frameshifting stimulated by complementarity between a downstream mRNA sequence and an error-correcting region of rRNA. RNA, 7, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S.R., Gonzalez R.L. & Puglisi J.D. (2003) Comparison of X-ray crystal structure of the 30S subunit–antibiotic complex with NMR structure of decoding site oligonucleotide–paromomycin complex. Structure, 11, 43–53. [DOI] [PubMed] [Google Scholar]
- Matsufuji S., Matsufuji T., Miyazaki Y., Murakami Y., Atkins J.F., Gesteland R.F. & Hayashi S. (1995) Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell, 80, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsufuji S., Matsufuji T., Wills N.M., Gesteland R.F. & Atkins J.F. (1996) Reading two bases twice: mammalian antizyme frameshifting in yeast. EMBO J., 15, 1360–1370. [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F.C., Bloch P.L. & Smith D.F. (1974) Culture medium for enterobacteria. J. Bacteriol., 119, 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle J.M., Brodersen D.E., Clemons W.M., Tarry M.J., Carter A.P. & Ramakrishnan V. (2001) Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science, 292, 897–902. [DOI] [PubMed] [Google Scholar]
- Pande S., Vimaladithan A., Zhao H. & Farabaugh P.J. (1995) Pulling the ribosome out of frame by +1 at a programmed frameshift site by cognate binding of aminoacyl-tRNA. Mol. Cell. Biol., 15, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan V. (2002) Ribosome structure and the mechanism of translation. Cell, 108, 557–572. [DOI] [PubMed] [Google Scholar]
- Schluenzen F. et al. (2000) Structure of functionally activated small ribosomal subunit at 3.3 Å resolution. Cell, 102, 615–623. [DOI] [PubMed] [Google Scholar]
- Stahl G., Ben Salem S., Li Z., McCarty G., Raman A., Shah M. & Farabough P.J. (2001) Programmed +1 translational frameshift in the yeast Saccharomyces cerevisiae results from disruption of translational error correction. Cold Spring Harb. Symp. Quant. Biol., 66, 249–257. [DOI] [PubMed] [Google Scholar]
- Sundaram M., Durant P.C. & Davis D.R. (2000) Hypermodified nucleosides in the anticodon of tRNALys stabilize a canonical U-turn structure. Biochemistry, 39, 12575–12584. [DOI] [PubMed] [Google Scholar]
- Sundararajan A., Michaud W.A., Qian Q., Stahl G. & Farabaugh P.J. (1999) Near-cognate peptidyl-tRNAs promote +1 programmed translational frameshifting in yeast. Mol. Cell, 4, 1005–1015. [DOI] [PubMed] [Google Scholar]
- Weiss R.B., Dunn D.M., Atkins J.F. & Gesteland R.F. (1987) Slippery runs, shifty stops, backward steps, and forward hops: −2, −1, +1, +2, +5 and +6 ribosomal frameshifting. Cold Spring Harb. Symp. Quant. Biol., 52, 687–693. [DOI] [PubMed] [Google Scholar]
- Weiss R.B., Dunn D.M., Dahlberg A.E., Atkins J.F. & Gesteland R.F. (1988) Reading frame switch caused by base-pair formation between the 3′ end of 16S rRNA and the mRNA during elongation of protein synthesis in Escherichia coli. EMBO J., 7, 1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly B.T., Brodersen D.E., Clemons W.M., Morgan-Warren R.J., Carter A.P., Vonrhein C., Hartsch T. & Ramakrishnan V. (2000) Structure of the 30S ribosomal subunit. Nature, 407, 327–339. [DOI] [PubMed] [Google Scholar]
- Yaniv M. & Barrell B.G. (1971) Sequence relationship of three valine acceptor tRNAs from Escherichia coli. Nature New Biol., 233, 113–114. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S. & Miyazawa T. (1985) Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl Acad. Sci. USA, 82, 4905–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupov M.M., Yusupova G.Z., Baucom A., Lieberman K., Earnest T.N., Cate J.H. & Noller H.F. (2001) Crystal structure of the ribosome at 5.5 Å resolution. Science, 292, 883–896. [DOI] [PubMed] [Google Scholar]
- Yusupova G.Z., Yusupov M.M., Cate J.H. & Noller H.F. (2001) The path of messenger RNA through the ribosome. Cell, 106, 233–241. [DOI] [PubMed] [Google Scholar]


