Abstract
The first step in the secretion of most mammalian proteins is their transport into the lumen of the endoplasmic reticulum (ER). Transport of pre-secretory proteins into the mammalian ER requires signal peptides in the precursor proteins and a protein translocase in the ER membrane. In addition, hitherto unidentified lumenal ER proteins have been shown to be required for vectorial protein translocation. This requirement was confirmed in this study by using proteoliposomes that were made from microsomal detergent extracts and contained either low or high concentrations of lumenal ER proteins. Furthermore, immunoglobulin-heavy-chain-binding protein (BiP) was shown to be able to substitute for the full set of lumenal proteins and, in the case of biotinylated precursor proteins, avidin was found to be able to substitute for lumenal proteins. Thus, the polypeptide-chain-binding protein BiP was identified as one lumenal protein that is involved in efficient vectorial protein translocation into the mammalian ER.
Introduction
The initial step in the secretion of most mammalian proteins, in which it is decided whether they enter the secretory pathway, is their transport into the lumen of the endoplasmic reticulum (ER; Blobel & Dobberstein, 1975). The transport of presecretory proteins into the ER requires cleavable signal peptides at the amino termini of the precursor proteins and a transport machinery that operates co- or post-translationally. Transport occurs in a sequence of three consecutive steps: first, membrane association of the precursor protein; second, membrane insertion (assayed as signal peptide cleavage); and third, completion of translocation (assayed as sequestration). This transport requires a protein translocase, which comprises Sec61-α, Sec61-β and Sec61-γ as its central components (Görlich & Rapoport, 1993). In addition, ATP-binding proteins of the ER lumen are part of the protein translocase and facilitate the insertion of presecretory proteins into the Sec61 complex (Klappa et al., 1991; Liao et al., 1997). A functional approach, involving proteoliposomes made from microsomal detergent extracts, identified the ATP-binding proteins as the ER-resident members of the heatshock protein 70 (Hsp70) protein family, immunoglobulin-heavy-chain-binding protein (BiP)/glucose-regulated protein 78 (Grp78) and Grp170 (Dierks et al., 1996; Hamman et al., 1998). On the basis of analogy to the situation in yeast (Brodsky et al., 1995; Young et al., 2001), these Hsp70 protein family members that reside in the mammalian ER may be recruited to the Sec61 complex by the membrane-integrated Hsp40 protein family member, Sec63 (Meyer et al., 2000; Tyedmers et al., 2000). Furthermore, the reticuloplasm (the complete set of ER lumenal proteins) has been shown to be required for vectorial protein translocation into mammalian microsomes (Nicchitta & Blobel, 1993).
This latter requirement was confirmed here by using proteoliposomes that were made from microsomal detergent extracts and contained trapped reticuloplasm. Furthermore, BiP (Haas & Wabl, 1983) was shown to be able to substitute for the complete set of lumenal proteins and, only in the case of biotinylated precursor proteins, avidin was found to be able to substitute for lumenal proteins. Thus, the polypeptide-chain-binding protein BiP (Flynn et al., 1991) was identified here as one, or perhaps the only, lumenal protein that is involved in efficient vectorial protein translocation into the mammalian ER.
Results
The translocation efficiency of proteoliposomes made from either microsomal detergent extracts or from purified components is lower than that of microsomes (Nicchitta & Blobel, 1990; Görlich & Rapoport, 1993; Dierks et al., 1996). On the basis of the observation that the translocation efficiency of dog pancreatic microsomes correlates with the concentration of lumenal microsomal proteins they contain (Nicchitta & Blobel, 1993), we assumed that the lower translocation efficiency of proteoliposomes is due to their low content of lumenal proteins. Therefore, we analysed here whether the translocation efficiency of proteoliposomes made from microsomal detergent extracts could be improved by the trapping of lumenal microsomal proteins. Lumenal microsomal proteins (the reticuloplasm) were purified and concentrated. Microsomal detergent extracts were supplemented with these lumenal proteins, or were not supplemented. Subsequently, proteoliposomes were formed and isolated. Aliquots of the two populations of proteoliposomes were subjected to protease treatment in the absence or presence of detergent (Fig. 1A,B). The analysis of the two types of proteoliposomes confirmed that they were indistinguishable with respect to membrane proteins, such as Sec61-α (Fig. 1B). By contrast, only when a high concentration of lumenal microsomal proteins was present during formation of the proteoliposomes were lumenal proteins, such as PDI (protein disulphide isomerase), observed and protease-protected to any significant extent in the absence of detergent (Fig. 1A). However, the ratio between a specifc lumenal protein (such as PDI) and Sec61-α, even in these latter proteoliposomes, was at least ten times lower than the ratio between the same two proteins in microsomes (Fig. 1, compare PK in (A) to that in (B)); see the Methods section for the relevant concentrations). The two populations of proteoliposomes were then analysed with respect to their transport efficiency. The model presecretory protein preprolactin was synthesized in reticulocyte lysate in the presence of proteoliposomes. In addition, translation reactions were carried out in the absence of membranes and in the presence of microsomes. Subsequently, aliquots of these co-translational transport reactions were subjected to sequestration analysis (Fig. 1C). There was a significant increase in translocation efficiency in the presence of lumenal proteins, measured as the amount of protease-resistant prolactin as a percentage of total prolactin (Fig. 1D).
Figure 1.
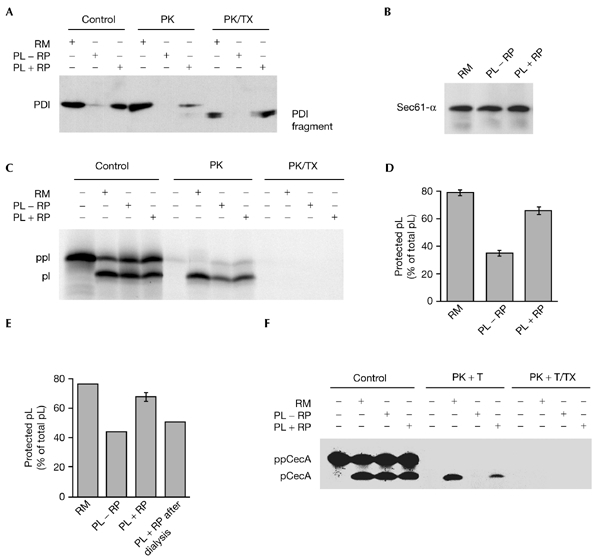
Lumenal endoplasmic reticulum proteins, trapped in proteoliposomes, stimulate translocation efficiency. (A–F) Proteoliposomes (PL) were formed in the absence (−RP) or presence (+RP) of added lumenal endoplasmic reticulum (ER) proteins (reticuloplasm), as described in the Methods. (E) For a third preparation, proteoliposomes that had been formed in the absence of added lumenal ER proteins (−RP) were supplemented with lumenal ER proteins after dialysis, but before separation by centrifugation (+RP after dialysis). (A,B) Proteoliposomes (−RP and +RP) were subjected to sequestration analysis. Proteins were separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and blotted onto polyvinylidene difluoride membranes. Blots were incubated with rabbit anti-PDI (protein disulphide isomerase) antibodies (StressGen), rabbit anti-Sec61-α antibodies and peroxidase-conjugated goat anti-rabbit IgG secondary antibodies. The antibodies were visualized by incubation of the blots in ECL chemiluminescence reagents and exposure to X-ray film. We note that reticuloplasm did not affect the reconstitution (B) and protease accessibility (not shown) of Sec61-α. (C–E) Preprolactin (ppl) was synthesized in reticulocyte lysate in the presence of [35S]methionine with buffer, microsomes (RM) or the proteoliposomes indicated. After incubation for 60 min at 30 °C, the reactions were subjected to sequestration analysis. (F) Preprocecropin A (ppCecA) was synthesized in reticulocyte lysate in the presence of [3H]proline for 15 min. After inhibition of protein synthesis, buffer, microsomes or proteoliposomes were added as indicated. After incubation for 30 min at 30 °C, the reactions were subjected to sequestration analysis. The samples were separated by SDS–PAGE and were analysed by phosphorimaging or fluorography. The sequestration efficiency was calculated as the amount of protease-resistant prolactin as a percentage of the total prolactin. Average values and standard errors of the mean (s.e.m.) are presented, and were based on n = 13 (D) and n = 2 (E). pCecA, procecropin A; PK, proteinase K; pl, prolactin; T, trypsin; TX, Triton X-100.
Similar results were obtained for co-translational transport of the pre-secretory proteins preprocecropin A and pre-immunoglobulin-κ light-chain (data not shown). Thus, with proteoliposomes, we confirmed the observations made for microsomes (Nicchitta & Blobel, 1993). Furthermore, a similar effect of lumenal proteins was seen when the translocation efficiency was analysed after transport of preprocecropin A under post-translational conditions (Fig. 1F). To ensure that the effect of the lumenal proteins was exerted on the lumenal face of the proteoliposomal membranes, a third population of proteoliposomes was formed in the absence of high concentrations of lumenal microsomal proteins, but was supplemented with high concentrations of lumenal proteins before the isolation of the proteoliposomes. The translocation efficiency of this third population of proteoliposomes was analysed after co-translational transport of preprolactin. Lumenal proteins, which were added to the cytosolic face of preformed proteoliposomes, had no stimulatory effect (Fig. 1E). Thus, the observed stimulatory effect of lumenal proteins present during the formation of proteoliposomes was indeed due to the trapped lumenal proteins. We also analysed the stage at which the transport of presecretory proteins into proteoliposomes is affected by trapped lumenal proteins. Ribosomes that contained nascent preprolactin polypeptide chains of a defined length (86 amino-acid residues, known as a ppl-86mer) were used in an experiment in which the stages of co-translational transport were analysed. In the first part of the experiment, the nascent ppl-86mer was allowed to insert into the membranes of the two types of proteoliposome, as described above. In this case, membrane insertion was assayed by analysing the crosslinking of the nascent precursor protein to Sec61-α. The crosslinking efficiency (in other words, the membrane insertion) was not affected by lumenal proteins (Fig. 2A). In the second part of the experiment, the nascent ppl-86mer was allowed to be translocated into the lumen of the two types of proteoliposomes by the addition of puromycin. Translocation efficiency was assayed by sequestration analysis. There was a significant stimulation of the sequestration efficiency by lumenal proteins (Fig. 2B). We thus confirmed, using proteoliposomes, previous observations about the role of lumenal proteins in the completion of translocation (Nicchitta & Blobel, 1993).
Figure 2.
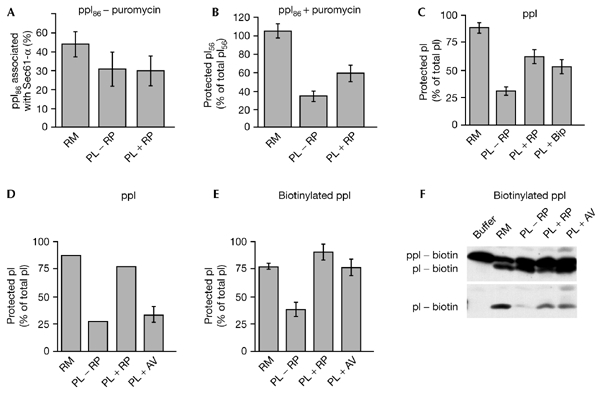
BiP can substitute for lumenal proteins, whereas avidin can substitute for lumenal proteins only when the precursor proteins are biotinylated. (A–F) Proteoliposomes (PL) were formed in the absence of added lumenal endoplasmic reticulum (ER) proteins (−RP), in the presence of added lumenal ER proteins (+RP), in the presence of added BiP (immunoglobulin-heavy-chain-binding protein; +BiP), or in the presence of avidin (+Av), as described in the Methods. We note that BiP and avidin did not affect the reconstitution and protease accessibility of Sec61-α (data not shown). (D–F) After isolation, the proteoliposomes were supplemented with biotin (final concentration 6 mM). (A,B) Nascent preprolactin polypeptide chains (ppl-86mer) were synthesized in reticulocyte lysate containing [35S]methionine in the presence of microsomes or the indicated proteoliposomes. After incubation for 20 min at 30 °C, the translation reactions were centrifuged (at 15,000g for 20 min at 2 °C). Subsequently, the pellets were resuspended in buffer and divided into two aliquots. One aliquot was left untreated and the other aliquot was incubated for 15 min at 30 °C in the presence of puromycin (1.25 mM). (A) One part of each aliquot was incubated with succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (335 μM) for 20 min at 0 °C. (B) The rest of each aliquot was subjected to sequestration analysis. The samples were separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and analysed by phosphorimaging. (A) The crosslinking efficiency was calculated for the untreated samples as the amount of Sec61-α-associated ppl-86mer as a percentage of the total ppl-86mer. (B) The sequestration efficiency was calculated for the puromycin-treated samples as the amount of protease-resistant pl-56mer (the mature form of ppl-86mer, which comprises 56 amino-acid residues) as a percentage of the total pl-56mer. (C,D) Preprolactin (ppl) was synthesized in reticulocyte lysate containing [35S]methionine, in the presence of microsomes or the proteoliposomes indicated. After incubation for 60 min at 30 °C, the translation reactions were subjected to sequestration analysis. The samples were separated by SDS–PAGE and analysed by phosphorimaging. The sequestration efficiency was calculated as the amount of protease-resistant prolactin (pl) as a percentage of the total prolactin. (E,F) Preprolactin was synthesized in reticulocyte lysate containing biotinylated lysyl transfer RNA in the presence of microsomes or the proteoliposomes indicated. After incubation for 60 min at 30 °C, the translation reactions were subjected to sequestration analysis. The samples were separated by SDS–PAGE and the proteins were transfered to a polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were incubated with peroxidase-conjugated streptavidin (F) or [125I]streptavidin (E). The biotinylated proteins were visualized by incubation of the blots in ECL chemiluminescence reagents and subsequent exposure to X-ray film (F), or directly by autoradiography (E). (E) The sequestration efficiency was calculated as the amount of protease-resistant biotinylated prolactin as a percentage of the total biotinylated prolactin. Average values and standard errors of the mean (s.e.m.) are presented, and were based on n = 2 (A,D,E), n = 4 (B) and n = 5 (C).
In the second set of experiments, we wanted to identify the lumenal protein of interest and characterize its mode of action. On the basis of the fact that BiP has been shown to mediate the completion of translocation of secretory proteins into yeast microsomes (Brodsky et al., 1995; Young et al., 2001), we analysed whether canine BiP can substitute for the full set of lumenal microsomal proteins in our proteoliposomes. Microsomal detergent extracts were left untreated, or were supplemented with reticuloplasm or purified BiP (see the Methods for the concentrations used). Subsequently, proteoliposomes were formed and isolated. The three populations of proteoliposomes were then analysed with respect to their translocation efficiency. The presecretory protein preprolactin was synthesized in reticulocyte lysate in the presence of these proteoliposomes. Subsequently, aliquots of the co-translational transport reactions were subjected to sequestration analysis. There was a similar stimulation of the sequestration efficiency by BiP and by lumenal proteins (Fig. 2C). Similar results were obtained for the presecretory protein preprocecropin A (data not shown). Thus, BiP was identified as a lumenal microsomal protein that can mediate completion of protein translocation into mammalian microsomes. We note that, in contrast to our previous observations about the membrane insertion of pre-secretory proteins (Dierks et al., 1996), Grp170 did not have any additional effect on sequestration efficiencies when it was added with BiP (not shown).
Next, we analysed which of the following activities of the molecular chaperone BiP is involved in completion of protein translocation: binding of the transport substrate or modulation of the transport machinery. To differentiate between these two possibilities, avidin was used as an artificial lumenal protein, and its effect on the translocation efficiency of biotinylated versus non-biotinylated prolactin was analysed. The microsomal detergent extracts were left untreated or were supplemented with lumenal proteins or purified avidin. Subsequently, proteoliposomes were formed and isolated. The three populations of proteoliposomes were supplemented with biotin (to saturate any external avidin, and thus allow the synthesis of biotinylated polypeptides) and analysed for their translocation efficiency under two different conditions. In the first set of conditions, the pre-secretory protein preprolactin was synthesized in reticulocyte lysate in the presence of the proteoliposomes and [35S]methionine (Fig. 2D). In the second set of conditions, preprolactin was simultaneously synthesized and biotinylated in reticulocyte lysate in the presence of proteoliposomes (Fig. 2E,F). Aliquots of all co-translational transport reactions were then subjected to sequestration analysis. The 35S-labelled proteins were visualized and quantified by phosphorimaging. Biotinylated proteins were transferred to polyvinylidene difluoride (PVDF) membranes and probed with streptavidin-coupled peroxidase (Fig. 2F) or [125I]streptavidin (Fig. 2E). The biotinylated, or biotinylated and 125I-labelled, proteins were visualized by exposure of the PVDF membranes to X-ray films and quantified by densitometry. There was a significant increase in the sequestration efficiency of biotinylated proteins by avidin (Fig. 2E). However, the sequestration of 35S-labelled proteins was not affected by avidin (Fig. 2D). Thus, the binding of translocating or newly translocated proteins in the lumen of membrane vesicles is required and sufficient to mediate efficient completion of translocation. Furthermore, this observation supports the view that the effect of the lumenal proteins, and BiP, was a direct effect on translocating or newly translocated proteins, and not an indirect effect on the stability of proteoliposomes or components of the transport machinery during reconstitution and protein transport, respectively.
Discussion
We showed that the binding of polypeptide-chain-binding proteins to incoming or newly imported secretory proteins on the lumenal face of the ER membrane is required for efficient co-translational transport into the mammalian ER. In vitro, and in the case of biotinylated precursor proteins, avidin is a suitable artificial polypeptide-chain-binding protein to provide this function in vitro. In the intact mammalian ER, BiP seems to provide this function. However, other molecular chaperones and folding catalysts, such as Grp170 (Dierks et al., 1996), the PDIs and PPIases (peptidyl prolyl cis–trans isomerases; Klappa et al., 1995; Volkmer et al., 1997), as well as calreticulin and calnexin (Ou et al., 1993; Molinari & Helenius, 2000), are additional candidates for this function. We propose that the need for this function arises from the fact that the Sec61 complex allows the bidirectional movement of polypeptide chains (Ooi & Weiss, 1992). Therefore, association with a polypeptide-chain-binding protein on the lumenal face of the membrane is needed to ensure unidirectional transport. Apparently, the binding energy can provide a driving force for the completion of translocation. This was observed previously for post-translational protein transport into yeast microsomes (Matlack et al., 1999) and across the outer membrane of mitochondria (Mayer et al., 1995).
Thus, members of the Hsp70 family that reside in the ER lumen are involved in protein transport into the mammalian ER at several stages: first, BiP and Grp170 facilitate the co- and post-translational insertion of pre-secretory proteins into the Sec61 complex (Klappa et al., 1991; Dierks et al., 1996). Second, the completion of co- and post-translational translocation is mediated by BiP (Nicchitta & Blobel, 1993; this paper). Third, BiP seals Sec61 complexes at several stages during the co-translational translocation of newly synthesized proteins (Liao et al., 1997; Hamman et al., 1998; Haigh & Johnson, 2002). At the first stage, the molecular chaperones seem to act on the transport machinery, probably the Sec61 complex; at the second stage, they act on the transport substrate; and at the third stage, they appear to act on the transport machinery and the transport substrate. It remains to be seen whether BiP and/or Grp170 need to be recruited to the Sec61 complex by Sec63 (Meyer at al., 2000; Tyedmers et al., 2000) for all three purposes, or whether one or two stages involve a different co-chaperone, such as Mtj1 (Dudek et al., 2002).
Methods
Materials.
[35S]methionine, [3H]proline, [125I]streptavidin and ECL reagents were from Amersham Biosciences. PVDF membranes and Centricon units were from Millipore. X-ray films were from Kodak. CHAPS (3-[(3-cholamido-propyl)dimethylammonio]-1-propanesulphonate) was from Calbiochem. Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate was from Pierce. Translation kits and the biotin–streptavidin chemiluminescence blotting kit with the peroxidase conjugate of streptavidin were from Roche Diagnostics. Peroxidase conjugates of anti-rabbit goat IgG antibodies and avidin were from Sigma.
Isolation of lumenal endoplasmic reticulum proteins.
Canine pancreatic microsomes were prepared as described previously (Watts et al., 1983). Microsomes were re-isolated by centrifugation and resuspended in lowsalt extraction buffer (20 mM HEPES–KOH, pH 7.5, 50 mM KCl, 1 mM EDTA, 1.5 mM MgCl2, 2 mM dithiothreitol (DTT), 0.65% CHAPS) at a concentration of 1 equivalent (eq) μl−1. The unsolubilized material was pelleted by centrifugation at 100,000 r.p.m. for 60 min at 2 °C in a Beckmann TLA 100.3 rotor. The supernatant was incubated with 0.5 g ml−1 bio-beads SM-2 (BioRad Laboratories) for 3 h at 4 °C to remove the detergent. After removal of the bio-beads by centrifugation, the supernatant was centrifuged again under the same conditions. The supernatant was concentrated to a protein concentration of 150 mg ml−1 (using Centricon 30 units) and diluted again to 1 eq μl−1 with low-salt extraction buffer, before a third centrifugation, as described above. The supernatant was concentrated to a protein concentration of 150 mg ml−1 and supplemented with KCl to a concentration of 400 mM and with sucrose to a concentration of 200 mM.
Reconstitution of microsomal proteins.
Microsomes were stripped of ribosomes (Görlich & Rapoport, 1993). After re-isolation, the stripped microsomes were resuspended in highsalt extraction buffer (20 mM HEPES–KOH, pH 7.5, 400 mM KCl, 1 mM EDTA, 1.5 mM MgCl2, 5 mM ATP, 2 mM DTT, 15% glycerol, 0.65% CHAPS) at a concentration of 0.66 eq μl−1. The unsolubilized material was pelleted by centrifugation for 30 min at 68,000 r.p.m. at 2 °C in a Beckman TLA 100.3 rotor. The supernatant was supplemented with 270 μg ml−1 egg yolk phospholipids (Avanti Polar Lipids) in the form of sonicated liposomes, and was dialysed to allow the formation of proteoliposomes, which were subsequently collected by centrifugation as above (this section). Purified lumenal ER proteins (final concentration, 10 mg ml−1;, corresponding to ∼0.85 mg BiP ml−1), canine BiP (final concentration, 2 mg ml−1) or avidin (final concentration, 11 mg ml−1) were present during the formation of proteoliposomes, as indicated in the figures. We note that these concentrations were found to be optimal in titration experiments. The proteoliposomes were used in the functional assays without going through any cycles of freezing and thawing.
Quantitative characterization of the reconstituted system.
The concentrations of various proteins in the microsomal suspensions were in the micromolar range: ∼5 μM for protein disulphide isomerase (PDI), PDIp (a pancreas-specific PDI) and BiP (Bies et al., 1999), and ∼2 μM for Sec61-α and Sec63 (Tyedmers et al., 2000). Thus, the concentrations of these proteins and their lumenal domains in the microsomal lumen may be 100- or 200-fold higher than this. Without taking into account the possibility that BiP may have become concentrated by Sec63 as compared to the surrounding solution in the lumen of the proteoliposomes (Tyedmers et al., 2000), the concentration of BiP in the proteoliposomes, proteoliposomes plus reticuloplasm, and proteoliposomes plus BiP must have been 3 μM, 15 μM and 30 μM, respectively. The concentration of avidin in the proteoliposomes plus avidin was ∼170 μM. Higher concentrations of these proteins during reconstitution seem to interfere with the formation of proteoliposomes.
In vitro translation and translocation.
Synthesis of preprolactin was carried out in reticulocyte lysates in the presence of [35S]methionine, [3H]proline or biotinylated lysyl transfer RNA (translation kit type I or type II, or biotin in vitro translation kit) in accordance with the manufacturer's instructions. Sequestration analysis was carried out as described in Dierks et al. (1996).
Where indicated, statistical analysis was carried out using the Igor Pro software (WaveMetrics, Inc.). Each bar chart represents a number (n, in the figure legends) of translation and translocation assays that were carried out separately and that each used a separate set of proteoliposomes.
Acknowledgments
We thank D. Hof for his help with the statistical analyses. This work was supported by the Deutsche Forschungsgemeinschaft (grant C1 in Sonderforschungsbereich 530) and by the Fonds der Chemischen Industrie.
References
- Bies C., Guth S., Janoschek K., Nastainczyk W., Volkmer J. & Zimmermann R. (1999) A Scj1p homolog and folding catalysts present in dog pancreas microsomes. Biol. Chem., 380, 1175–1182. [DOI] [PubMed] [Google Scholar]
- Blobel G. & Dobberstein B. (1975) Transfer of proteins across membranes. J. Cell Biol., 67, 835–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J.L., Goeckeler J. & Schekman R. (1995) BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc. Natl Acad. Sci. USA, 92, 9643–9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T., Volkmer J., Schlenstedt G., Jung C., Sandholzer U., Zachmann K., Schlotterhose P., Neifer K., Schmidt B. & Zimmermann R. (1996) A microsomal ATP-binding protein involved in efficient protein transport into the mammalian endoplasmic reticulum. EMBO J., 15, 6931–6942. [PMC free article] [PubMed] [Google Scholar]
- Dudek J. et al. (2002) A novel type of co-chaperone mediates transmembrane recruitment of Dank-like chaperones to ribosomes. EMBO J., 21, 2958–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn G.C., Pohl J., Flocco M.T. & Rothman J.E. (1991) Peptide-binding specificity of the molecular chaperone BiP. Nature, 353, 726–730. [DOI] [PubMed] [Google Scholar]
- Görlich D. & Rapoport T.A. (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell, 75, 615–630. [DOI] [PubMed] [Google Scholar]
- Haas I.G. & Wabl M. (1983) Immunoglobulin heavy chain binding protein. Nature, 306, 387–389. [DOI] [PubMed] [Google Scholar]
- Haigh N.G. & Johnson A.E. (2002) A new role for BiP: closing the aqueous translocon pore during protein integration into the ER membrane. J. Cell Biol., 156, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman B.D., Hendershot L.M. & Johnson A.E. (1998) BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell, 92, 747–758. [DOI] [PubMed] [Google Scholar]
- Klappa P., Mayinger P., Pipkorn R., Zimmermann M. & Zimmermann R. (1991) A microsomal protein is involved in ATP-dependent transport of presecretory proteins into mammalian microsomes. EMBO J., 10, 2795–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappa P., Freedman R. & Zimmermann R. (1995) Protein disulfide isomerase and a lumenal cyclophilin-type peptidyl prolyl cis-trans isomerase are in transient contact with secretory proteins during late stages of translocation. Eur. J. Biochem., 232, 755–764. [PubMed] [Google Scholar]
- Liao S., Lin J., Do H. & Johnson A.E. (1997) Both lumenal and cytosolic gating of the aqueous ER translocon pore are regulated from inside the ribosome during membrane protein integration. Cell, 90, 31–42. [DOI] [PubMed] [Google Scholar]
- Matlack K.E.S., Misselwitz B., Plath K. & Rapoport T.A. (1999) BiP acts as a molecular ratchet during posttranslational transport of prepro-α factor across the ER membrane. Cell, 97, 553–564. [DOI] [PubMed] [Google Scholar]
- Mayer A., Neupert W. & Lill R. (1995) Translocation of apocytochrome c across the outer membrane of mitochondria. J. Biol. Chem., 270, 12390–12397. [DOI] [PubMed] [Google Scholar]
- Meyer H.-A., Grau H., Kraft R., Kostka S., Prehn S., Kalies K.-U. & Hartmann E. (2000) Mammalian Sec61 is associated with Sec62 and Sec63. J. Biol. Chem., 275, 14550–14557. [DOI] [PubMed] [Google Scholar]
- Molinari M. & Helenius A. (2000) Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science, 288, 331–333. [DOI] [PubMed] [Google Scholar]
- Nicchitta C.V. & Blobel G. (1990) Assembly of translocation-competent proteoliposomes from detergentsolubilized rough microsomes. Cell, 60, 259–269. [DOI] [PubMed] [Google Scholar]
- Nicchitta C.V. & Blobel G. (1993) Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell, 73, 989–998. [DOI] [PubMed] [Google Scholar]
- Ooi C.E. & Weiss J. (1992) Bidirectional movement of a nascent polypeptide across microsomal membranes reveals requirements for vectorial translocation of proteins. Cell, 71, 87–96. [DOI] [PubMed] [Google Scholar]
- Ou W.-J., Cameron P.H., Thomas D.Y. & Bergeron J.J.M. (1993) Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature, 364, 771–776. [DOI] [PubMed] [Google Scholar]
- Tyedmers J., Lerner M., Bies C., Dudek J., Skowronek M., Haas I., Heim N., Nastainczyk W., Volkmer J. & Zimmermann R. (2000) Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc. Natl Acad. Sci. USA, 97, 7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmer J., Guth S., Nastainczyk W., Knippel P., Klappa P., Gnau V. & Zimmermann R. (1997) Pancreas specific protein disulfide isomerase, PDIp, is in transient contact with secretory proteins during late stages of translocation. FEBS Lett., 406, 291–295. [DOI] [PubMed] [Google Scholar]
- Watts C., Wickner W. & Zimmermann R. (1983) M13 procoat and pre-immunoglobulin share processing specificity but use different membrane receptor mechanisms. Proc. Natl Acad. Sci. USA, 80, 2809–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.P., Craven R.A., Reid P.J., Willer M. & Stirling C.J. (2001) Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J., 20, 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]


