Abstract
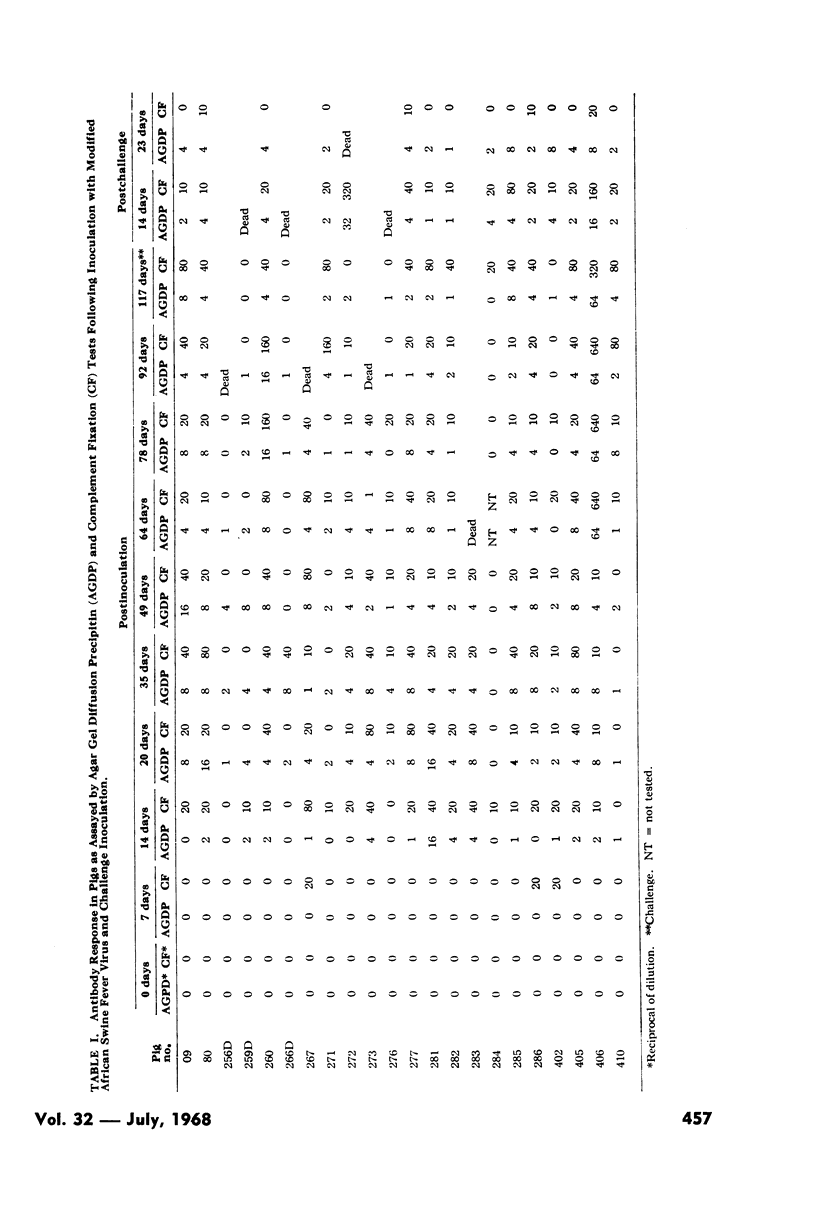
Pigs were inoculated with a modified isolate of African swine fever virus (ASFV). Complement-fixing (CF) and agar gel diffusion precipitin (AGDP) antibodies could be detected in the serums of most pigs from 14-days postinoculation (DPI) until their immunity was challenged with virulent ASFV at 117 DPI.
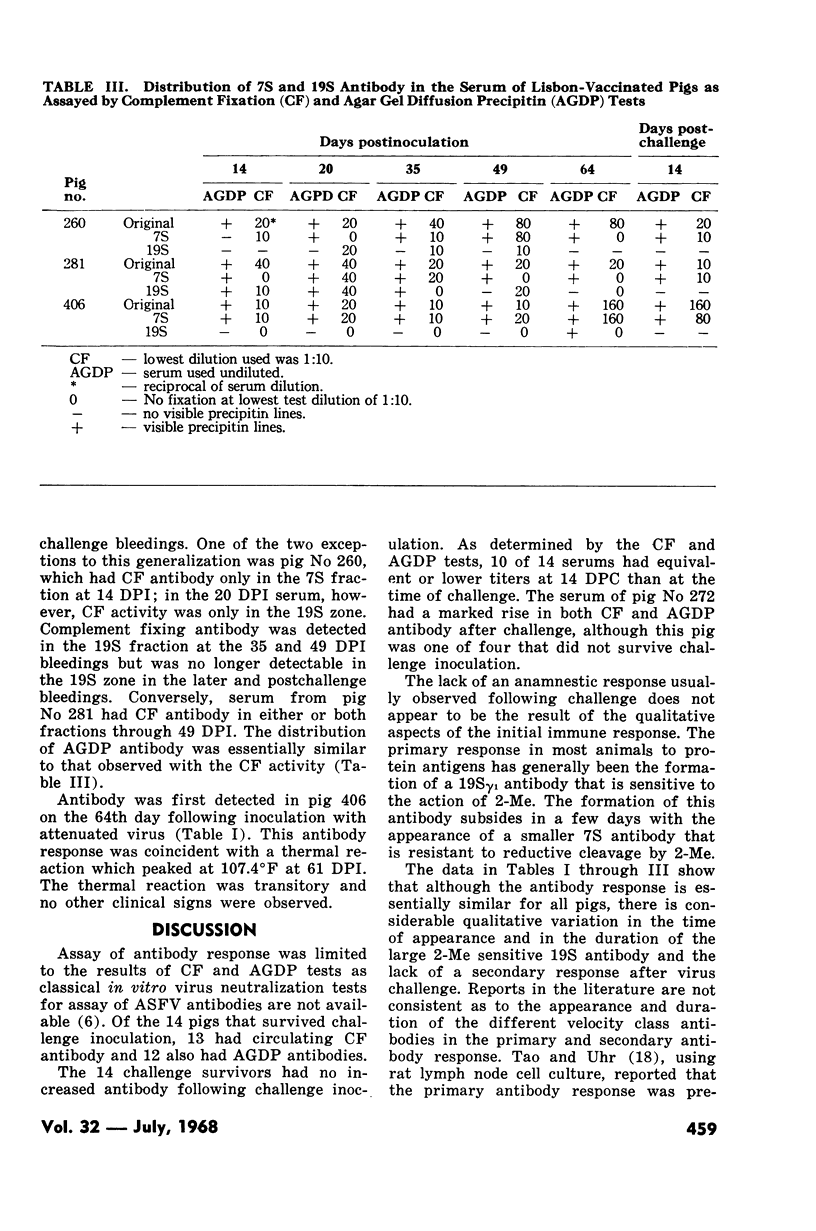
Reductive cleavage with 2-mercaptoethanol showed that serums collected at 14 to 35 DPI contained 19S antibody, but that the 7S antibody was dominant at 35 and 117 DPI. This distribution of antibody was confirmed by sucrose-gradient centrifugation. Nearly all of the early serums also contained 7S antibodies which fixed complement and reacted in the AGDP test. Pigs whose serums contained both CF and AGDP antibodies at time of challenge failed to develop acute disease while pigs without CF antibodies were usually not protected.
Pigs surviving challenge with virulent virus showed no increase in antibody titers, or reversion to 19S antibody.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COWAN K. M. Immunologic studies on African swine fever virus. II. Enhancing effect of normal bovine serum on the complement-fixation reaction. Am J Vet Res. 1963 Jul;24:756–761. [PubMed] [Google Scholar]
- Cohen I. R., Norins L. C. Natural human antibodies to gram-negative bacteria: immunoglobulins G, A, and M. Science. 1966 May 27;152(3726):1257–1259. doi: 10.1126/science.152.3726.1257. [DOI] [PubMed] [Google Scholar]
- DETRAY D. E. Persistence of viremia and immunity in African swine fever. Am J Vet Res. 1957 Oct;18(69):811–816. [PubMed] [Google Scholar]
- EDELMAN G. M., KUNKEL H. G., FRANKLIN E. C. Interaction of the rheumatoid factor with antigen-antibody complexes and aggregated gamma globulin. J Exp Med. 1958 Jul 1;108(1):105–120. doi: 10.1084/jem.108.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS W. R., COX B. F., HEUSCHELE W. P., STONE S. S. PROPAGATION AND MODIFICATION OF AFRICAN SWINE FEVER VIRUS IN CELL CULTURES. Am J Vet Res. 1965 Jan;26:141–146. [PubMed] [Google Scholar]
- MALMQUIST W. A., HAY D. Hemadsorption and cytopathic effect produced by African Swine Fever virus in swine bone marrow and buffy coat cultures. Am J Vet Res. 1960 Jan;21:104–108. [PubMed] [Google Scholar]
- NOSSAL G. J., SZENBERG A., ADA G. L., AUSTIN C. M. SINGLE CELL STUDIES ON 19S ANTIBODY PRODUCTION. J Exp Med. 1964 Mar 1;119:485–502. doi: 10.1084/jem.119.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta-Hatano R., Hinuma Y. Mercaptoethanol-sensitive neutralizing-antibody in natural infection with coxsackievirus B5. Proc Soc Exp Biol Med. 1966 Jul;122(3):725–729. doi: 10.3181/00379727-122-31237. [DOI] [PubMed] [Google Scholar]
- Osler A. G., Mulligan J. J., Jr, Rodriguez E. Weight estimates of rabbit anti-human serum albumin based on antigen-binding and precipitin analyses: specific hemagglutinating activities of 7 S and 19 S components. J Immunol. 1966 Feb;96(2):334–344. [PubMed] [Google Scholar]
- STONE S. S., DELAY P. D. The inactivation of rinderpest virus by beta-propiolactone and its effect on homologous complement-fixing and neutralizing antibodies. J Immunol. 1961 Oct;87:464–467. [PubMed] [Google Scholar]
- Stone S. S., Hess W. R. Antibody response to inactivated preparations of African swine fever virus in pigs. Am J Vet Res. 1967 Mar;28(123):475–481. [PubMed] [Google Scholar]
- Tao T. W., Uhr J. W. Primary-type antibody response in vitro. Science. 1966 Mar 4;151(3714):1096–1098. doi: 10.1126/science.151.3714.1096. [DOI] [PubMed] [Google Scholar]


