Type 2 diabetes is growing in prevalence at different rates in different populations. In developed countries, where rates are rising relatively slowly, the age-adjusted prevalence of diabetes ranges from about 5% among people of European ancestry to 40% or higher in Aboriginal populations. In developing countries, progressive westernization is followed by a rapid rise in diabetes prevalence. For example, according to current trends, the prevalence of diabetes in India and China is expected to double by 2030, whereas globally it is expected to rise by only 61%.1 This growth is due in part to a rise in prevalence among children and young adults in the developing world. (The estimated prevalence and age distribution of diabetes in developed and developing countries in 1995 and 2000 are presented in an online table available at www.cmaj.ca/cgi/content/full/174/1/25/DC1.)
According to the “thrifty gene hypothesis,” the global diabetes epidemic is occurring because our hunter-gatherer ancestors evolved in an environment with uncertain food availability and high demands for physical activity, whereas we live in a world with an abundance of food and decreased requirement for physical activity. However, this hypothesis does not account for the marked regional and ethnic differences in diabetes prevalence in different societies.
Consideration of hyperglycemia rates among domesticated mammals suggests that ethnic and national heterogeneity in diabetes prevalence is rooted in more recent events. Domesticated mammals are the result of generations of selective breeding that targeted a phenotype best suited for a particular task. Thus, for thousands of years, domesticated pigs and cows were selectively bred for their ability to efficiently accumulate and store energy for later consumption by humans, whereas dogs and rodent-catching cats were selected to maximize physical work and minimize consumption of food supplied by humans. Pigs and cows should therefore be protected against the toxic effects of a “diabetogenic” environment (i.e., one that favours inactivity and energy abundance), whereas dogs and cats would have no such protection when suddenly placed in a diabetogenic environment. Indeed, hyperglycemia is either unreported or extremely rare in pigs and cows and other domesticated mammals bred to accumulate energy, whereas it is relatively common in dogs and cats, which as pets are now exposed to a different environment from that for which they were bred. This suggests that it is possible for environmental pressures to select for protection against the metabolic consequences of a diabetogenic environment.
Observations from pig studies suggest that susceptibility and resistance to diabetes may be related to recent (and not remote) environmental effects. About 500 years ago, a colony of domesticated pigs was released on Ossabaw Island, off the coast of Georgia. Since then, they have lived in an environment characterized by an uncertain food supply and high physical demands. Despite the shared ancestry, when these pigs are captured and raised together with their “food-producing cousins” in a high-calorie, low-activity environment, they experience obesity and hyperglycemia, whereas the other pigs do not.2
But how does this explain why people of European descent are more resistant to diabetes than most other ethnic groups in a westernized environment that is inherently diabetogenic? Europeans have been exposed to a westernized environment the longest (they “invented” it). They may therefore have been “selected” for an ability to thrive in such an environment. Indeed, for at least 300 years Europeans have enjoyed a relatively stable food supply and increasingly available labour-saving devices.3 Other populations that are newly exposed to such an environment may have little resistance to its diabetogenic effects and will experience rising diabetes rates.
This “selection and adaptation” hypothesis can be true only if diabetes reduces reproductive success and the ability to pass diabetes susceptibility to future generations. Several lines of reasoning now support this view. First, the observation from studies that diabetes is rare among Europeans less than 45 years old is precisely what one would expect if selective pressures eliminated individuals who were susceptible to the disease at an earlier age (i.e., during the reproductive years): susceptibility for diabetes would linger in the post-reproductive years. Second, in the developing world, type 2 diabetes occurs during the reproductive years.1 Third, the development of diabetes during the reproductive years increases the risk of cardiovascular disease and premature death, which may impair reproductive success. Fourth, dysglycemic women are known to have a high risk of irregular menstrual periods and polycystic ovary syndrome — conditions that reduce fertility. Fifth, anthropological observations suggest that healthy grandparents may be important for the health of their grandchildren, especially in developing societies (e.g., by participating in child care and freeing parents to earn income). If the grandparents' health is compromised, the viability of their grandchildren may be affected because of the diversion of valuable family resources to the care of the grandparents.
Finally, as with the pigs on Ossabaw Island, these selective pressures may only need to act for a few hundred years to change the phenotype of a population. Using a simple genetic model,4 we are able to estimate that, if people with type 2 diabetes are just 10% less likely to reproduce than are people without diabetes, only 12–25 generations would be required for the prevalence of diabetes to fall from 30%–40% currently seen in Aboriginal populations to the prevalence of 5%–10% observed in populations of European descent (Fig. 1). Thus, changes in human societies as recent as 300–400 years ago can account for resistance to diabetes in ethnic groups exposed to a diabetogenic environment during those 300–400 years, and for susceptibility in newly exposed groups. This perspective is summarized in Fig. 2.
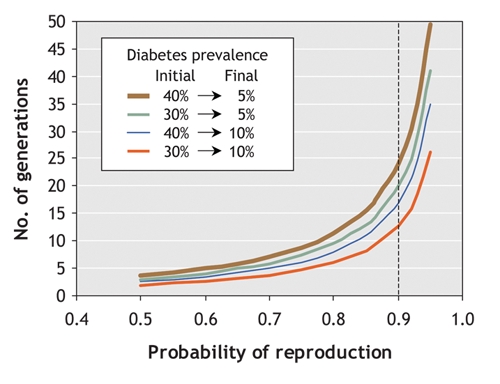
Fig. 1: Model showing estimated effect of reproductive fitness on the prevalence of diabetes. In this model, if people with type 2 diabetes have a reproductive success rate that is 10% lower than that among people without diabetes (represented by dotted line), it will take 12–25 generations for the prevalence of diabetes to fall from 30%–40% (currently seen in Aboriginal populations) to 5%–10% (currently seen in populations of European descent).
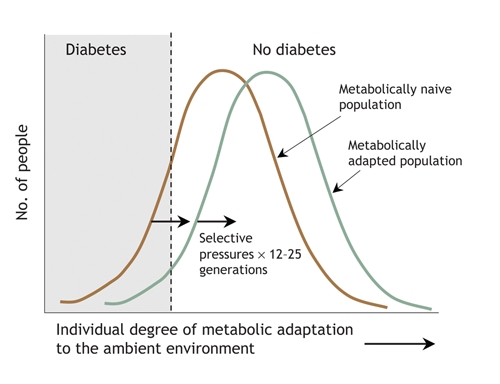
Fig. 2: Prevalence of diabetes in populations according to their ability to adapt genetically to the environment. When the environment becomes diabetogenic (i.e., promotes inactivity and increased food consumption), the number of people who are not metabolically adapted (naive) to the new environment increases; diabetes and its antecedents will develop more frequently and earlier in these people than in their peers, and their reproductive success will be reduced. Natural selection will favour the remaining individuals, and after 12–25 generations, the normal distribution of the population's ability to cope metabolically with the environment will shift to the right, and the prevalence of diabetes will fall. A further change in the environment, or the introduction of a naive population to the existing environment, starts the process over.
Thus, the current diabetes epidemic in developing countries may be occurring because populations in those countries have not yet had time to genetically adapt to their new diabetogenic environments. Moreover, the recent increase in the incidence of diabetes in developed countries may be the result of an ever more diabetogenic environment that is taxing even the “adapted” populations of European descent. Careful prospective studies of glucose metabolism and fertility in populations both before and after westernization could successfully test such a hypothesis.
In the meantime, these considerations suggest that the global solution that will have the greatest impact on slowing or stopping the diabetes epidemic in both developed and developing countries does not rest with the susceptible individual. Moreover, both the rate at which the environment is changing and the benefits of modern health care preclude any ability of populations to adapt further to the environment. Rather, the solution lies in the hands of our policy__makers and governments, who can and should change the economic and environmental structure of westernized society from one that is constantly pushing the abilities of even highly motivated individuals to cope metabolically, to one that promotes sensible physical activity and healthy dietary choices and reduces everybody's metabolic stress.
Supplementary Material
Footnotes
This article has been peer reviewed.
Competing interests: Hertzel Gerstein holds the McMaster University Population Health Institute Chair in Diabetes Research (sponsored by Aventis).
REFERENCES
- 1.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047-53. [DOI] [PubMed]
- 2. Whitfield J. Fat pigs ape obese humans. Nature Science Update 2003 Aug 6.
- 3.Diamond J. The double puzzle of diabetes. Nature 2003;423:599-602. [DOI] [PubMed]
- 4. Hartl DL, Clark AG. Principles of population genetics. 3rd ed. Sunderland (MA): Sinauer Associates, Inc.; 1997.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


