Abstract
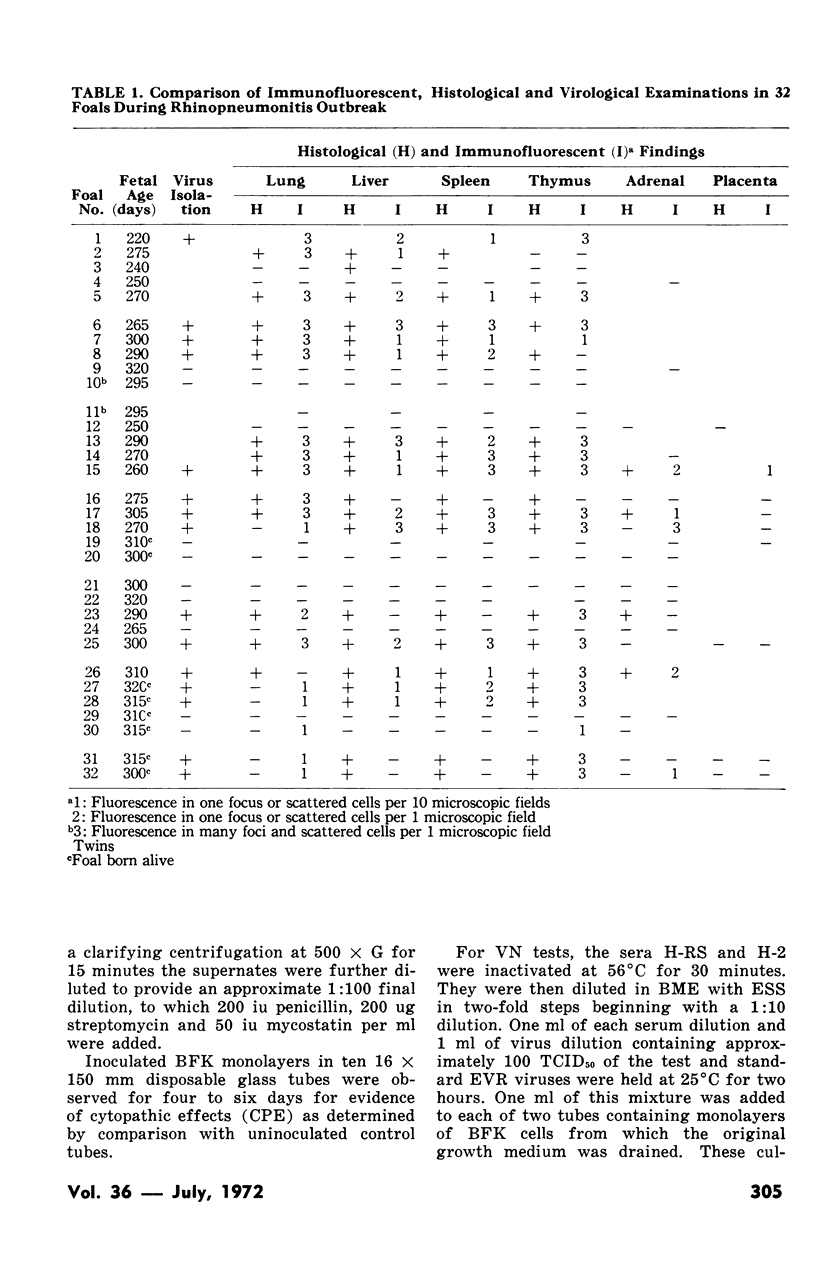
Using two known positive equine viral rhinopneumonitis (EVR) sera, conjugates were prepared with fluorescein isothiocyanate and tested for specificity using EVR infected tissue culture cells. The conjugate was then applied to selected tissues from 32 aborted fetuses and foals submitted during a natural outbreak of EVR. Antigen was detected in various tissues by immunofluorescence in 20 cases (62.5%). In 24 cases bovine fetal kidney cell monolayers were inoculated with a pool of lung and liver and EVR virus was isolated from 15 (62.5%). Histological examination of various tissues from 29 cases resulted in the diagnosis of EVR in 19 (65.5%), based upon the presence of focal areas of necrosis and intranuclear inclusion bodies. Correlation of results was not obtained in two cases. One was diagnosed positive histologically and negative on fluorescence, the other was negative histologically and by virus isolation but showed fluorescence. The distribution of fluorescence in various infected fetal tissues indicated that the combined examination of lung and thymus gland was most likely to provide a positive diagnosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulanger P., Bannister G. L., Greig A. S., Gray D. P., Ruckerbauer G. M., Willis N. G. African swine fever. IV. Demonstration of the viral antigen by means of immunofluorescence. Can J Comp Med Vet Sci. 1967 Jan;31(1):16–23. [PMC free article] [PubMed] [Google Scholar]
- CORNER A. H., MITCHELL D., MEADS E. B. Equine virus abortion in Canada. I. Pathological studies on aborted fetuses. Cornell Vet. 1963 Jan;53:78–88. [PubMed] [Google Scholar]
- DOLL E. R., MCCOLLUM W. H., WALLACE M. E., BRYANS J. T., RICHARDS M. G. Complement-fixation reactions in equine virus abortion. Am J Vet Res. 1953 Jan;14(50):40–45. [PubMed] [Google Scholar]
- Duxbury A. E., Oxer D. T. Isolation of equine rhinopneumonitis virus from an epidemic of acute respiratory disease in horses. Aust Vet J. 1968 Feb;44(2):58–63. doi: 10.1111/j.1751-0813.1968.tb04956.x. [DOI] [PubMed] [Google Scholar]
- GIRARD A., GREIG A. S., MITCHELL D. Equine virus abortion in Canada. II. Isolation of viruses and detection of antibodies in tissue culture. Cornell Vet. 1963 Jan;53:88–98. [PubMed] [Google Scholar]
- Girard A., Greig A. S., Mitchell D. A virus associated with vulvitis and balanitis in the horse-- preliminary report. Can J Comp Med. 1968 Oct;32(4):603–604. [PMC free article] [PubMed] [Google Scholar]
- Kemeny L., Pearson J. E. Isolation of herpesvirus from equine leukocytes: comparison with equine rhinopneumonitis virus. Can J Comp Med. 1970 Jan;34(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- PLUMMER G., WATERSON A. P. Equine herpes viruses. Virology. 1963 Mar;19:412–416. doi: 10.1016/0042-6822(63)90083-7. [DOI] [PubMed] [Google Scholar]
- Ruckerbauer G. M., Gray D. P., Girard A., Bannister G. L., Boulanger P. Studies on bluetongue. V. Detection of the virus in infected materials by immunofluorescence. Can J Comp Med Vet Sci. 1967 Jul;31(7):175–181. [PMC free article] [PubMed] [Google Scholar]