Abstract
The effects of a neonatal calf diarrhea virus on cell cultures were investigated. Bovine embryonic kidney cell cultures were the most satisfactory for production of virus. Cytoplasmic changes detected after inoculation with a high multiplicity of virus were: 1) cytoplasmic vacuoles; 2) some eosinophilic cytoplasmic inclusions; and 3) some degeneration of cells and detachment from the monolayer. Cultures stained with fluorescein-labeled antibody showed cytoplasmic fluorescence as early as four hr after infection with the maximum fluorescence at five days. No cross reactions were observed between the neonatal calf diarrhea virus and reovirus type 1 or type 3 by the fluorescent antibody technique. Plaques were small and were not produced consistently. The optimal adsorption time was one to two hr. The maximum titer was reached at 18 hr, with the cell-associated titer remaining higher than the cell-free titer until that time. An interferon was produced by cultures infected with either ultraviolet-inactivated or untreated virus.
Full text
PDF




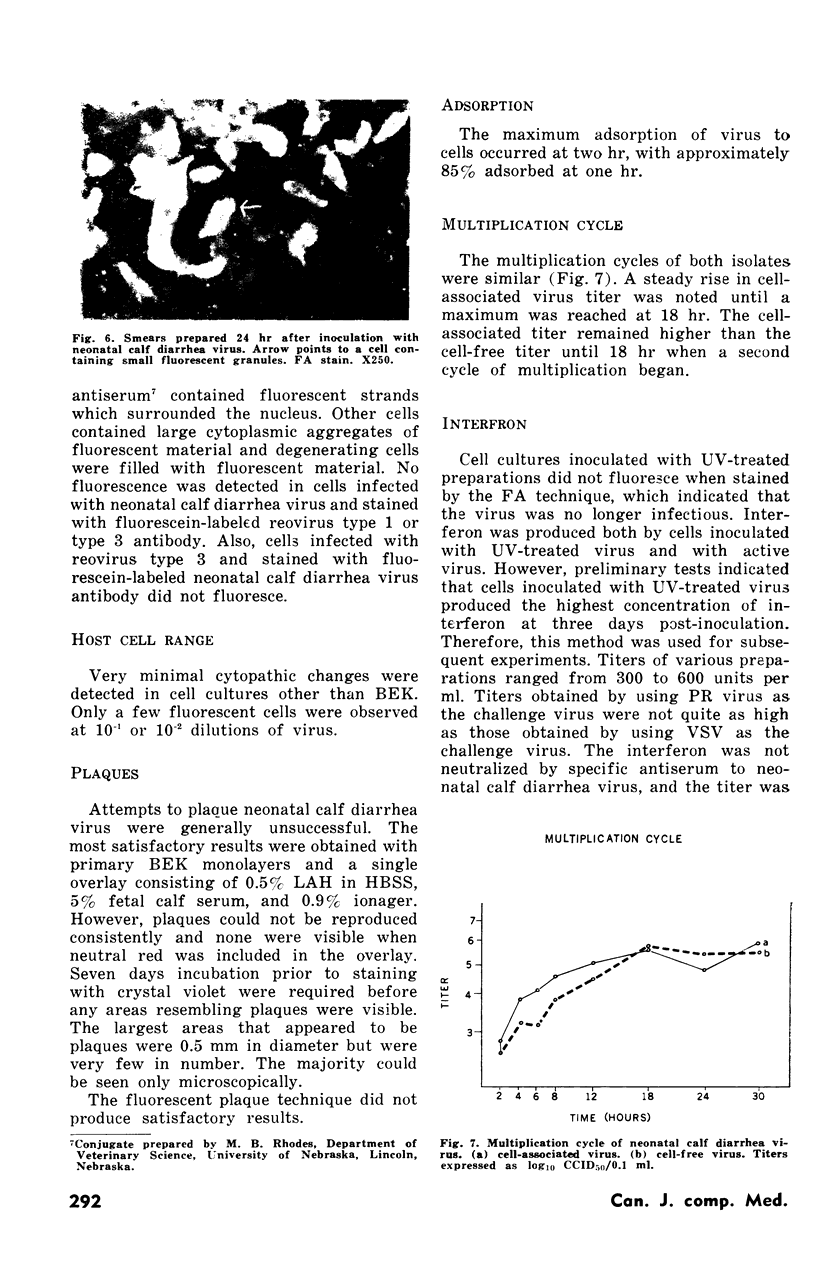


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fernelius A. L., Ritchie A. E., Classick L. G., Norman J. O., Mebus C. A. Cell culture adaptation and propagation of a reovirus-like agent of calf diarrhea from a field outbreak in Nebraska. Arch Gesamte Virusforsch. 1972;37(1):114–130. doi: 10.1007/BF01241157. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADIN S. H., ANDRIESE P. C., DARBY N. B. The in vitro cultivation of tissues of domestic and laboratory animals. Am J Vet Res. 1957 Oct;18(69):932–941. [PubMed] [Google Scholar]
- MAYOR H. D. Cytochemical and fluorescent antibody studies on the growth of poliovirus in tissue culture. Tex Rep Biol Med. 1961;19:106–122. [PubMed] [Google Scholar]
- Mebus C. A., Kono M., Underdahl N. R., Twiehaus M. J. Cell culture propagation of neonatal calf diarrhea (scours) virus. Can Vet J. 1971 Mar;12(3):69–72. [PMC free article] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Inhibition of infectious and hemagglutinating properties of type 2 dengue virus by aqueous Agar extracts. Virology. 1963 Jan;19:49–57. doi: 10.1016/0042-6822(63)90023-0. [DOI] [PubMed] [Google Scholar]
- SPENDLOVE R. S., LENNETTE E. H. A simplified immunofluorescent plaque method. J Immunol. 1962 Jul;89:106–112. [PubMed] [Google Scholar]
- Welch A. B. Purification, morphology and partial characterization of a reovirus-like agent associated with neonatal calf diarrhea. Can J Comp Med. 1971 Jul;35(3):195–202. [PMC free article] [PubMed] [Google Scholar]
- White R. G., Mebus C. A., Twiehaus M. J. Incidence of herds infected with a neonatal calf diarrhea virus (NCDV). Vet Med Small Anim Clin. 1970 May;65(5):487–490. [PubMed] [Google Scholar]