Abstract
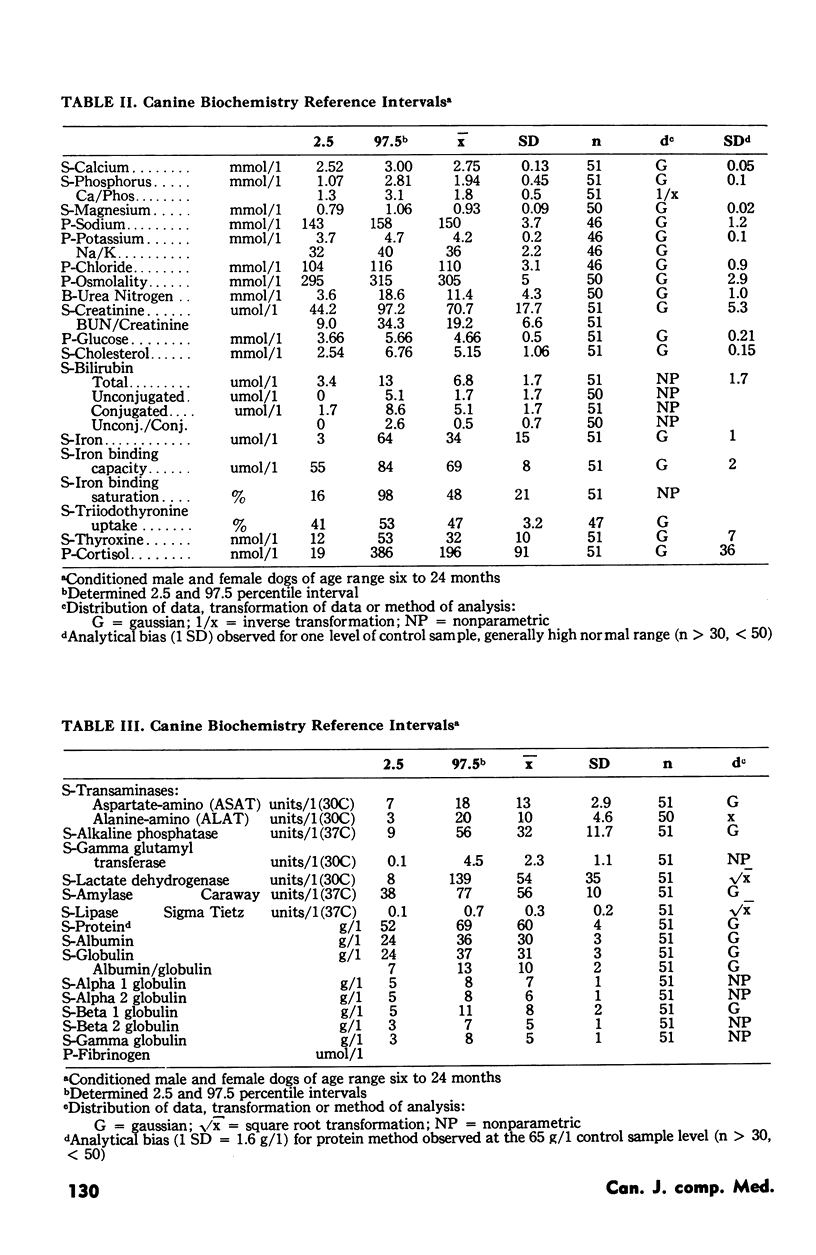
Reference hematology and biochemistry values for 53 variables are presented from 51 clinically healthy dogs, 26 female and 25 male, approximately six to 24 months of age and of mixed breed. These dogs were sampled because of their good health status and the opportunity to collect the volume of blood required to complete the variable analysis of interest. Collection of blood specimens and laboratory analysis was done in a standard described manner, the latter including a continuing quality control program. For each variable the data were examined for homogeneity and when present, outliers (n = 9) were excluded. Parametric analysis was used to calculate the reference interval for those variables which had a Gaussian distribution or could be changed to a Gaussian distribution by any of four transformations. For those variables in which Gaussian distribution was not present or attained, nonparametric analysis was used. Due to the small size of the population sample, the uncertainty of breed and the exact age of each dog, breed, age and sex effects were not examined. Reference values should be used to assist interpretation of observations obtained from an animal or animals of comparable origin, i.e. similar subpopulation, and only if the same laboratory techniques are followed. Until each laboratory is able to generate reference values using adequate sample size and current methodology for the numerous subpopulations of interest, reference intervals such as these are useful to clinicians and researchers.
Full text
PDF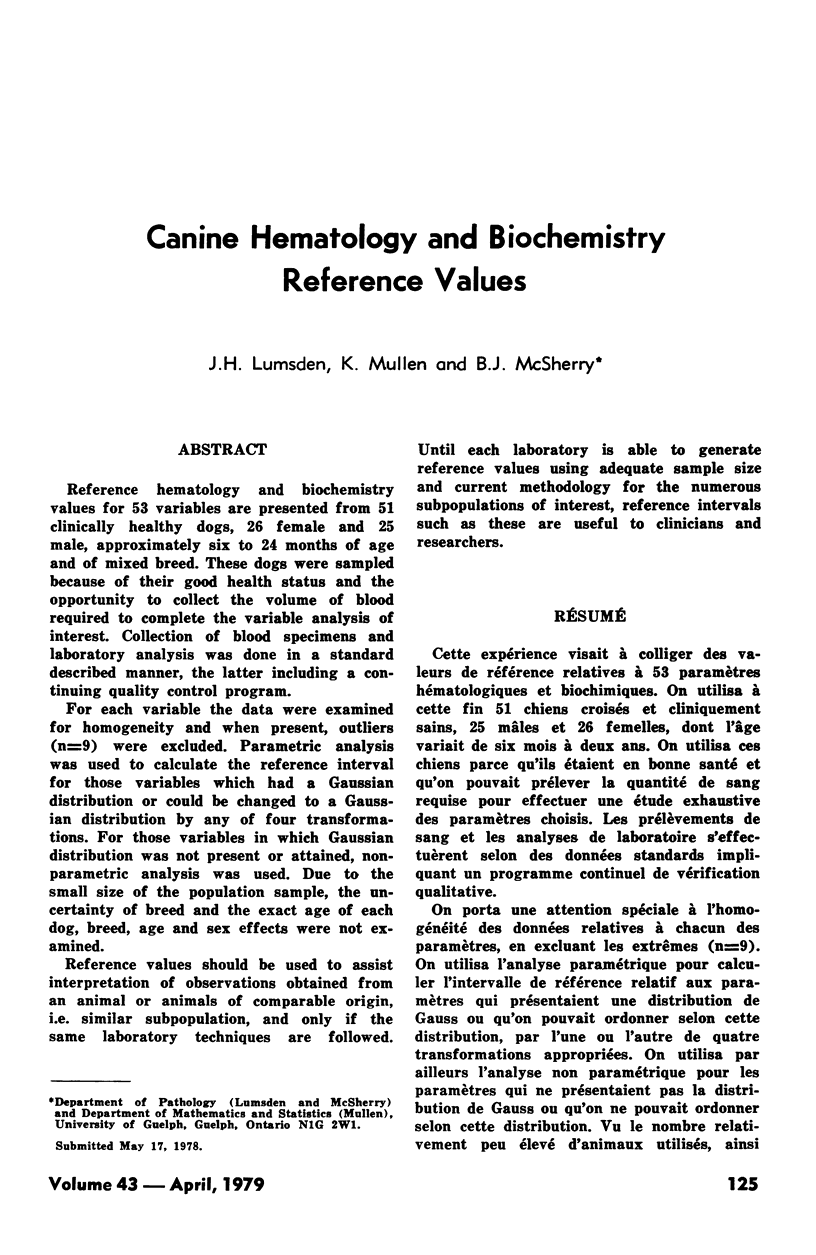





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alström T., Gräsbeck R., Hjelm M., Skandsen S. Recommendations concerning the collection of reference values in clinical chemistry and activity report by the Committee on Reference Values of the Scandinavian Society for Clinical Chemistry and Clinical Physiology. Scand J Clin Lab Invest Suppl. 1975;144:1–44. [PubMed] [Google Scholar]
- BRECHER G., SCHNEIDERMAN M. A time-saving device for the counting of reticulocytes. Am J Clin Pathol. 1950 Nov;20(11):1079–1083. doi: 10.1093/ajcp/20.11_ts.1079. [DOI] [PubMed] [Google Scholar]
- Babson A. L., Greeley S. J., Coleman C. M., Phillips G. E. Phenolphthalein monophosphate as a substrate for serum alkaline phosphatase. Clin Chem. 1966 Aug;12(8):482–490. [PubMed] [Google Scholar]
- Breznock E. M., McQueen R. D. Rapid fluorometric analysis of plasma corticosteroids as a test of adrenocortical function in the dog. Am J Vet Res. 1969 Sep;30(9):1523–1533. [PubMed] [Google Scholar]
- CARAWAY W. T. A stable starch substrate for the determination of amylase in serum and other body fluids. Am J Clin Pathol. 1959 Jul;32(1):97–99. doi: 10.1093/ajcp/32.1_ts.97. [DOI] [PubMed] [Google Scholar]
- Crocker C. L. Rapid determination of urea nitrogen in serum or plasma without deproteinization. Am J Med Technol. 1967 Sep-Oct;33(5):361–365. [PubMed] [Google Scholar]
- FETERIS W. A. A SERUM GLUCOSE METHOD WITHOUT PROTEIN PRECIPITATION. Am J Med Technol. 1965 Jan-Feb;31:17–21. [PubMed] [Google Scholar]
- Gay R. J., McComb R. B., Bowers G. N., Jr Optimum reaction conditions for human lactate dehydrogenase isoenzymes as they affect total lactate dehydrogenase activity. Clin Chem. 1968 Aug;14(8):740–753. [PubMed] [Google Scholar]
- Goldenberg H., Fernandez A. Simplified method for the estimation of inorganic phosphorus in body fluids. Clin Chem. 1966 Dec;12(12):871–882. [PubMed] [Google Scholar]
- Grams R. R., Johnson E. A., Benson E. S. Laboratory data analysis system. VI. System summary. Am J Clin Pathol. 1972 Aug;58(2):216–219. doi: 10.1093/ajcp/58.2.216. [DOI] [PubMed] [Google Scholar]
- KARMEN A., WROBLEWSKI F., LADUE J. S. Transaminase activity in human blood. J Clin Invest. 1955 Jan;34(1):126–131. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause R. D., Anand V. D., Gruemer H. D., Willke T. A. The impact of laboratory error on the normal range: a Bayesian model. Clin Chem. 1975 Mar;21(3):321–324. [PubMed] [Google Scholar]
- LADUE J. S., WROBLEWSKI F. Serum glutamic pyruvic transaminase SGP-T in hepatic disease: a preliminary report. Ann Intern Med. 1956 Nov;45(5):801–811. doi: 10.7326/0003-4819-45-5-801. [DOI] [PubMed] [Google Scholar]
- Lumsden J. H., Mullen K. On establishing reference values. Can J Comp Med. 1978 Jul;42(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem. 1969 Feb;15(2):124–136. [PubMed] [Google Scholar]
- Tietz N. W., Fiereck E. A. A specific method for serum lipase determination. Clin Chim Acta. 1966 Mar;13(3):352–358. doi: 10.1016/0009-8981(66)90215-4. [DOI] [PubMed] [Google Scholar]
- Trudeau D. L., Freier E. F. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin Chem. 1967 Feb;13(2):101–114. [PubMed] [Google Scholar]
- Weldon K. L., MacKay J. S. On improving the diagnostic value of quantitative medical laboratory tests in conventional practice. J Chronic Dis. 1972 Dec;25(12):683–696. doi: 10.1016/0021-9681(72)90004-5. [DOI] [PubMed] [Google Scholar]
- Werner M., Marsh W. L. Normal values: theoretical and practical aspects. CRC Crit Rev Clin Lab Sci. 1975 Sep;6(2):81–100. doi: 10.3109/10408367509151566. [DOI] [PubMed] [Google Scholar]
- Wybenga D. R., Pileggi V. J., Dirstine P. H., Di Giorgio J. Direct manual determination of serum total cholesterol with a single stable reagent. Clin Chem. 1970 Dec;16(12):980–984. [PubMed] [Google Scholar]


