Abstract
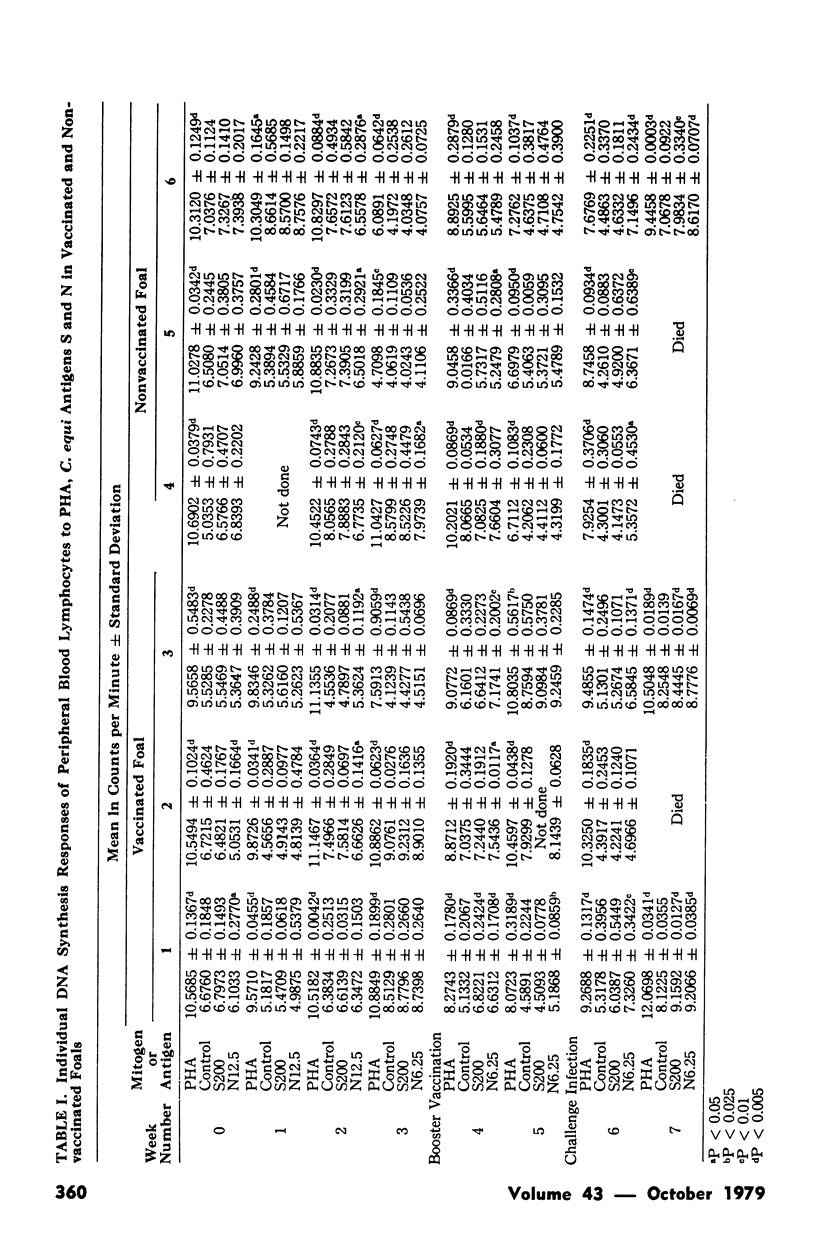
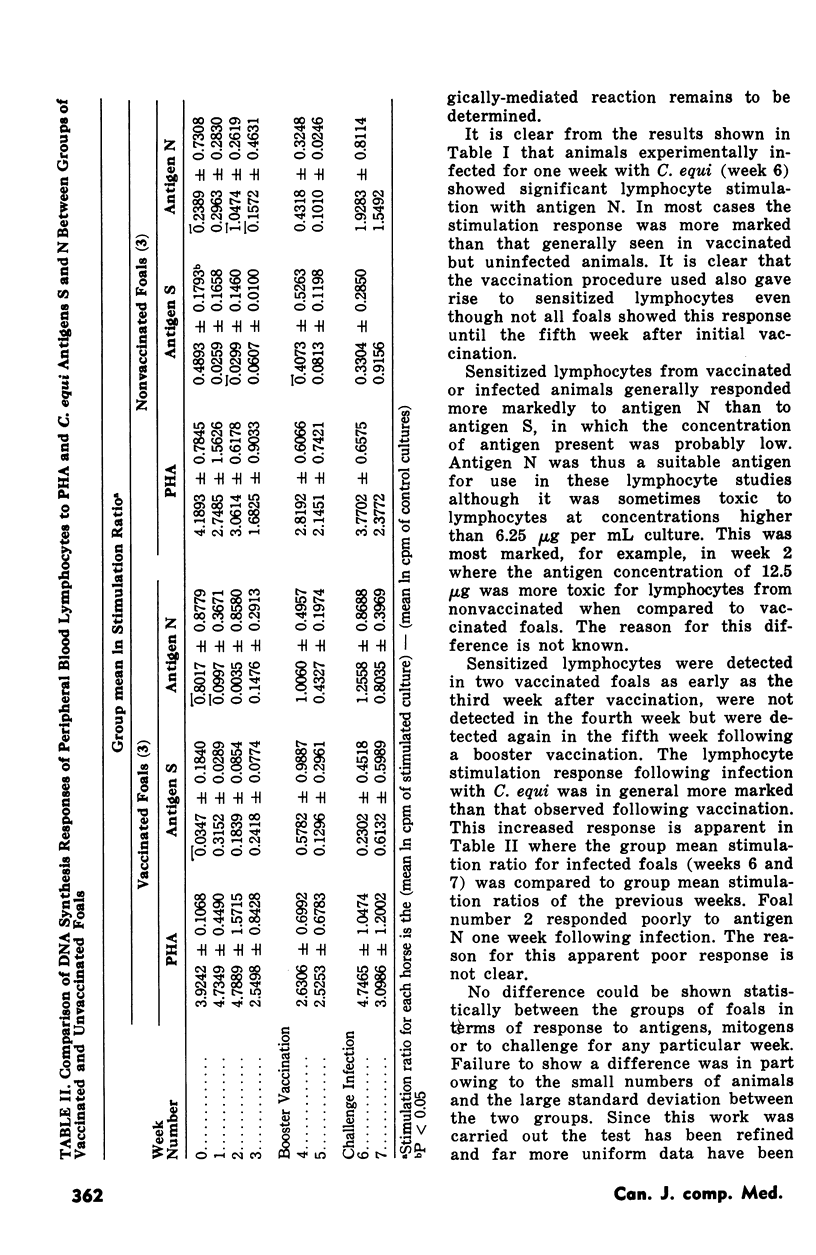
Transformation of peripheral blood lymphocytes from pony foals vaccinated and subsequently infected with Corynebacterium equi was studied. Three foals were vaccinated on two occasions using a formalinized C. equi vaccine with aluminum hydroxide as an adjuvant. Three nonvaccinated foals served as controls. Foals were challenged intratracheally with 9 x 10(9) C. equi six weeks after the initial vaccination.Foals survived this infection for one to two weeks. Significant lymphocyte transformation in response to C. equi antigens was detected in two vaccinated foals at the third week after initial vaccination and in all vaccinated animals at the fifth week. No statistically significant transformation was seen in nonvaccinated foals before infection. Vaccinated and nonvaccinated foals showed responsive lymphocytes following challenge. Vaccination offered no obvious protection against experimental challenge but this failure was probably due to an excessive infective dose of organisms. Low levels of humoral antibodies were detected in some challenged foals. The pathological changes in the lungs of infected animals were comparable with, but more fulminating than, changes observed in the natural disease.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addo P. B., Dennis S. M. Ovine pneumonia caused by Corynebacterium equi. Vet Rec. 1977 Jul 23;101(4):80–80. doi: 10.1136/vr.101.4.80. [DOI] [PubMed] [Google Scholar]
- Berg R., Chmel H., Mayo J., Armstrong D. Corynebacterium equi infection complicating neoplastic disease. Am J Clin Pathol. 1977 Jul;68(1):73–77. doi: 10.1093/ajcp/68.1.73. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carter G. R., Hylton G. A. An indirect hemagglutination test for antibodies to Corynebacterium equi. Am J Vet Res. 1974 Nov;35(11):1393–1395. [PubMed] [Google Scholar]
- Cimprich R. E., Rooney J. R. Corynebacterium equi enteritis in foals. Vet Pathol. 1977 Mar;14(2):95–102. doi: 10.1177/030098587701400201. [DOI] [PubMed] [Google Scholar]
- Hartzman R. J., Bach M. L., Bach F. H., Thurman G. B., Sell K. W. Precipitation of radioactively labeled samples: a semi-automatic multiple-sample processor. Cell Immunol. 1972 Jun;4(2):182–186. doi: 10.1016/0008-8749(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Holtman D. F. Corynebacterium equi in Chronic Pneumonia of the Calf. J Bacteriol. 1945 Feb;49(2):159–162. doi: 10.1128/jb.49.2.159-162.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. S., Lock A., Biberstein E. L. A cat with Corynebacterium equi lymphadenitis clinically simulating lymphosarcoma. Cornell Vet. 1975 Apr;65(2):232–239. [PubMed] [Google Scholar]
- Knight H. D. Corynebacterial infections in the horse: problems of prevention. J Am Vet Med Assoc. 1969 Jul 15;155(2):446–452. [PubMed] [Google Scholar]
- McCandlish I. A., Thompson H., Wright N. G. Vaccination against canine bordetellosis using an aluminum hydroxide adjuvant vaccine. Res Vet Sci. 1978 Jul;25(1):51–57. [PubMed] [Google Scholar]
- Reddy C. A., Kao M. Value of acid metabolic products in identification of certain corynebacteria. J Clin Microbiol. 1978 May;7(5):428–433. doi: 10.1128/jcm.7.5.428-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



