Abstract
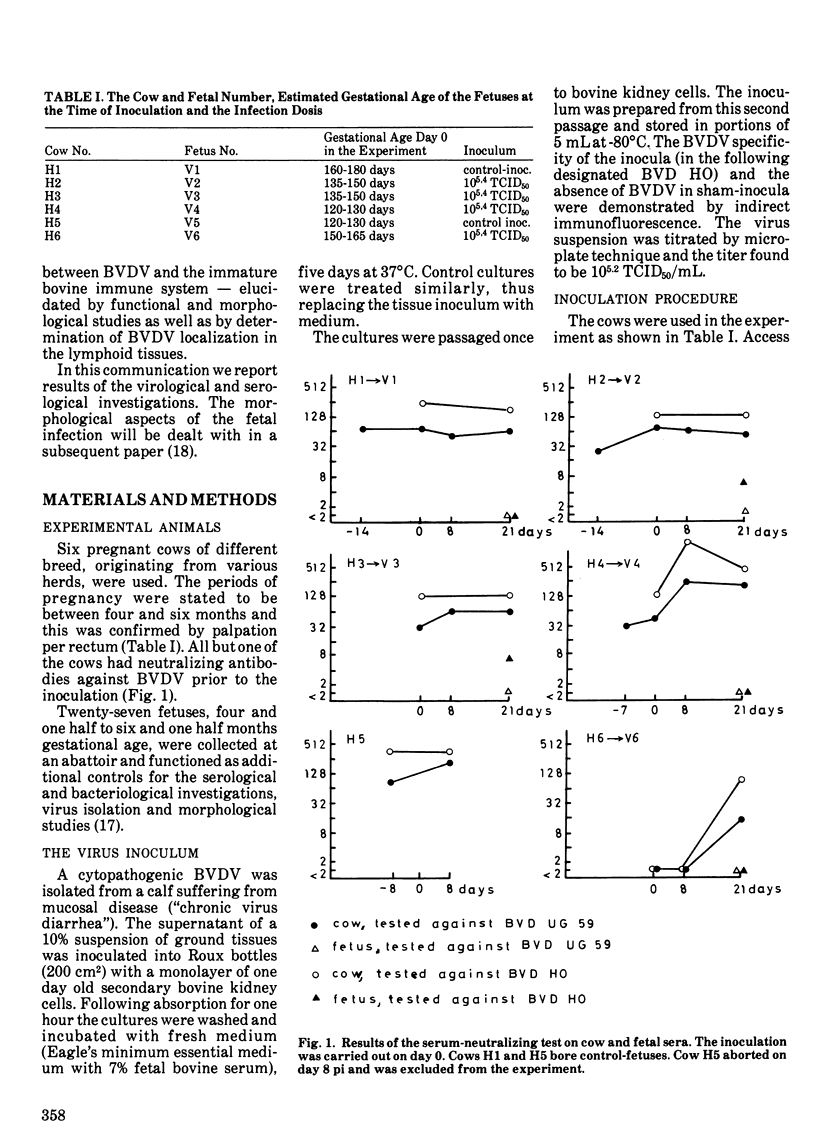
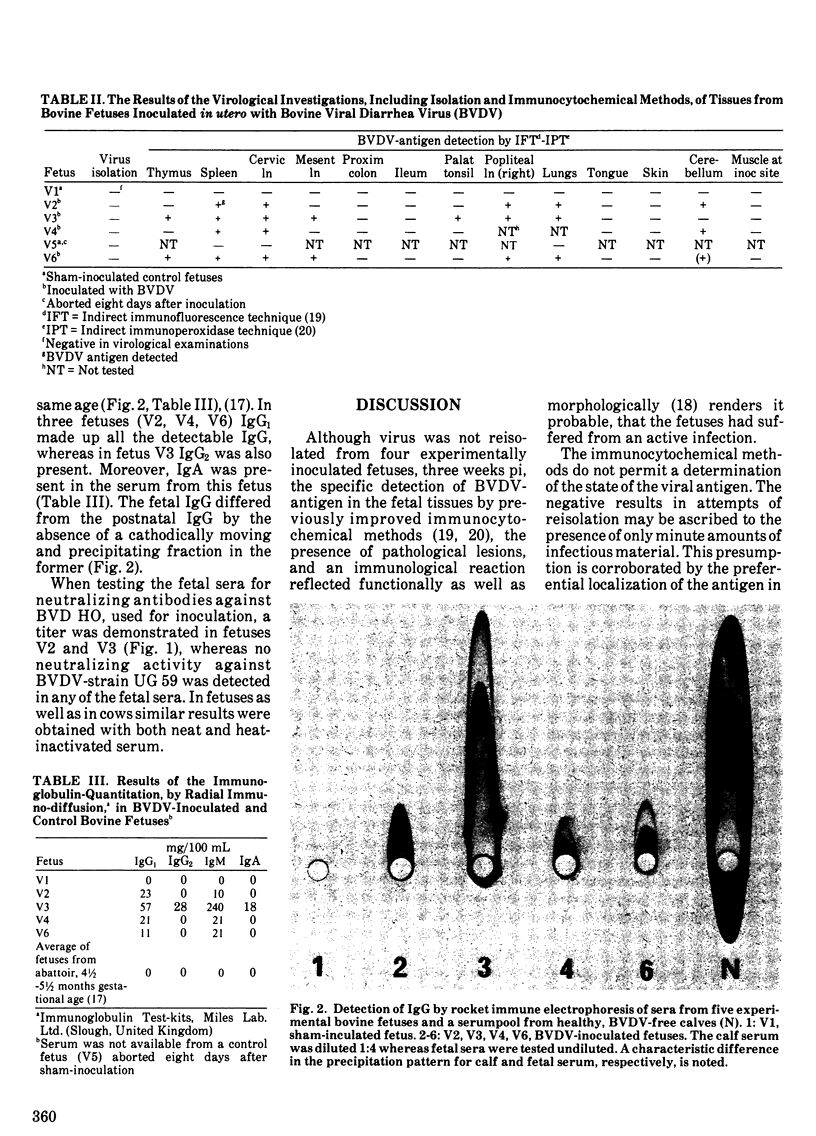
The serological and virological results of an experimental infection of bovine fetuses with bovine viral diarrhea virus are presented. Four fetuses, 120-165 days gestational age, were inoculated in utero with a second passage virus strain. Two fetuses received a sham-inoculum. A humoral immune response in the virus-inoculated fetuses, was demonstrated three weeks later. In three fetuses only IgM and IgG1 were detectable. The serum from the fourth fetus also contained IgG2 and IgA. Bovine viral diarrhea virus-neutralizing antibodies were detected in two fetuses. These two fetuses inoculated at 135-150 days gestational age, represent the youngest reported bovids, giving a specific response in three weeks following an experimental infection with bovine viral diarrhea virus. The fetal sera did not contain heat-labile factors, which could mediate the neutralization. The virus was not reisolated from any of the fetuses, but viral antigen was nevertheless demonstrated by immunocytochemical methods in sections of several of the fetal organs, primarily lymphoid tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beh K. J., Watson D. L., Lascelles A. K. Concentrations of immunoglobulins and albumin in lymph collected from various regions of the body of the sheep. Aust J Exp Biol Med Sci. 1974 Feb;52(1):81–86. doi: 10.1038/icb.1974.6. [DOI] [PubMed] [Google Scholar]
- Braun R. K., Osburn B. I., Kendrick J. W. Immunologic response of bovine fetus to bovine viral diarrhea virus. Am J Vet Res. 1973 Sep;34(9):1127–1132. [PubMed] [Google Scholar]
- Brown T. T., Jr, Schultz R. D., Duncan J. R., Bistner S. I. Serological response of the bovine fetus to bovine viral diarrhea virus. Infect Immun. 1979 Jul;25(1):93–97. doi: 10.1128/iai.25.1.93-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaro A. P., Kendrick J. W., Kennedy P. C. Response of the bovine fetus to bovine viral diarrhea-mucosal disease virus. Am J Vet Res. 1971 Oct;32(10):1543–1562. [PubMed] [Google Scholar]
- Coria M. F., McClurkin A. W. Specific immune tolerance in an apparently healthy bull persistently infected with bovine viral diarrhea virus. J Am Vet Med Assoc. 1978 Feb 15;172(4):449–451. [PubMed] [Google Scholar]
- Dalsgaard K., Overby E., Metzger J. J., Basse A. Rapid method for screening of immunoglobulins in porcine fetuses, using rocket immunoelectrophoresis. Application of an interspecies reaction between human and porcine mu-chain. Acta Vet Scand. 1979;20(3):313–320. doi: 10.1186/BF03546593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Done J. T., Terlecki S., Richardson C., Harkness J. W., Sands J. J., Patterson D. S., Sweasey D., Shaw I. G., Winkler C. E., Duffell S. J. Bovine virus diarrhoea-mucosal disease virus: pathogenicity for the fetal calf following maternal infection. Vet Rec. 1980 Jun 7;106(23):473–479. doi: 10.1136/vr.106.23.473. [DOI] [PubMed] [Google Scholar]
- Hubbert W. T., Bryner J. H., Fernelius A. L., Frank G. H., Estes P. C. Viral infection of the bovine fetus and its environment. Arch Gesamte Virusforsch. 1973;41(1):86–98. doi: 10.1007/BF01249933. [DOI] [PubMed] [Google Scholar]
- Jensen M. H. Detection of antibodies against hog cholera virus and bovine viral diarrhea virus in porcine serum. A comparative examination using CF, PLA and NPLA assays. Acta Vet Scand. 1981;22(1):85–98. doi: 10.1186/BF03547210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel B. Neutralization of animal viruses. Adv Virus Res. 1978;23:205–268. doi: 10.1016/S0065-3527(08)60101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall P. A., Stott E. J., Thomas L. H. Experimental infection of calves with two strains of bovine virus diarrhoea virus: virus recovery and clinical reactions. Res Vet Sci. 1980 Jan;28(1):91–95. [PubMed] [Google Scholar]
- OLITZKI A. L. Studies on the antigenic structure of virulent and nonvirulent brucellae with the aid of the agar gel precipitation technique. Br J Exp Pathol. 1959 Oct;40:432–440. [PMC free article] [PubMed] [Google Scholar]
- Ohmann H. B., Dalsgaard K. Indirect immunofluorescence using F(ab')2-immunoreagents for the demonstration of bovine viral diarrhea virus (BVDV) antigen in lymphoid tissue. Acta Vet Scand. 1980;21(4):705–707. doi: 10.1186/BF03546858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmann H. B. Experimental fetal infection with bovine viral diarrhea virus. II. Morphological reactions and distribution of viral antigen. Can J Comp Med. 1982 Oct;46(4):363–369. [PMC free article] [PubMed] [Google Scholar]
- Ohmann H. B. Immunoglobulin levels in non-aborted and aborted fetuses from Danish herds of cattle. Acta Vet Scand. 1981;22(3-4):428–434. doi: 10.1186/BF03548668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Isackson D. W., Overall J. C., Jr, Glasgow L. A., Brown T. T., Bistner S. I., Gillespie J. H., Scott F. W. Fetal and adult bovine interferon production during bovine viral diarrhea virus infection. Infect Immun. 1976 Sep;14(3):660–666. doi: 10.1128/iai.14.3.660-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen K. J., Askaa J. Fetal infection with porcine parvovirus in herds with reproductive failure. Acta Vet Scand. 1981;22(2):162–170. doi: 10.1186/BF03547505. [DOI] [PMC free article] [PubMed] [Google Scholar]