Abstract
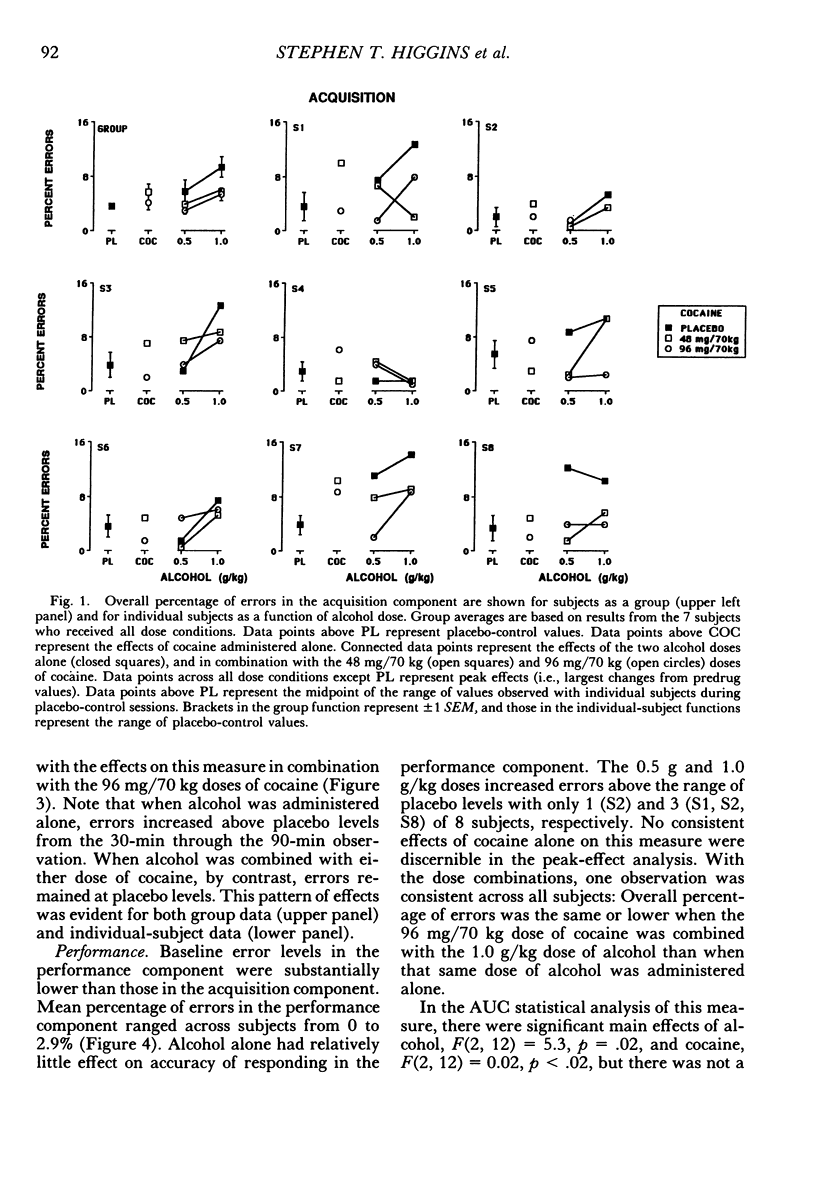
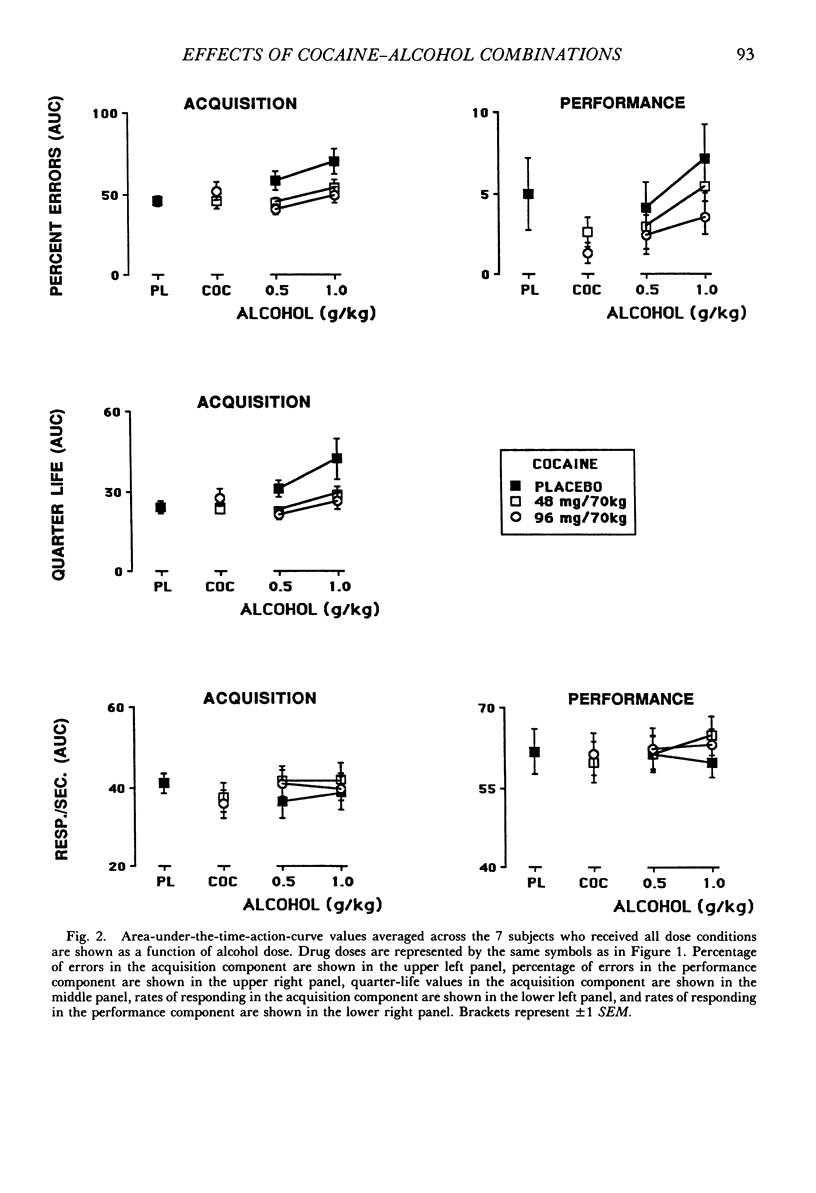
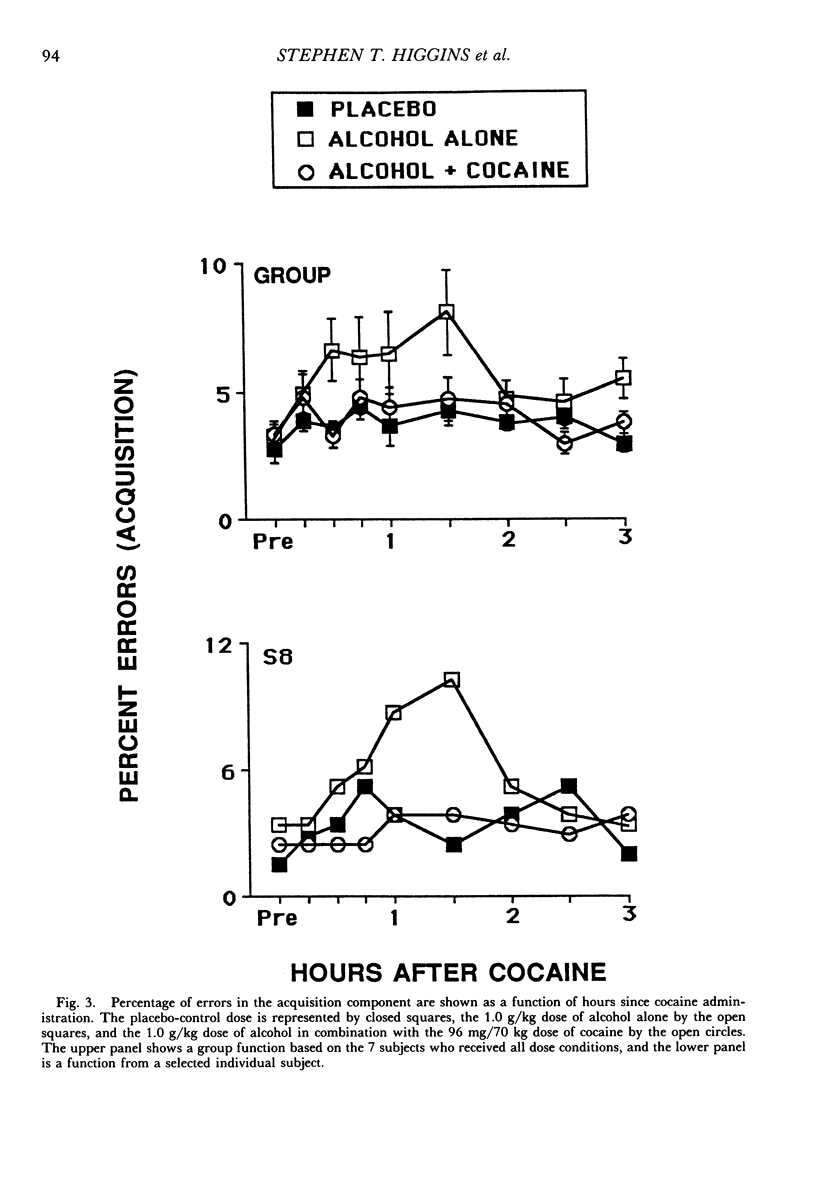
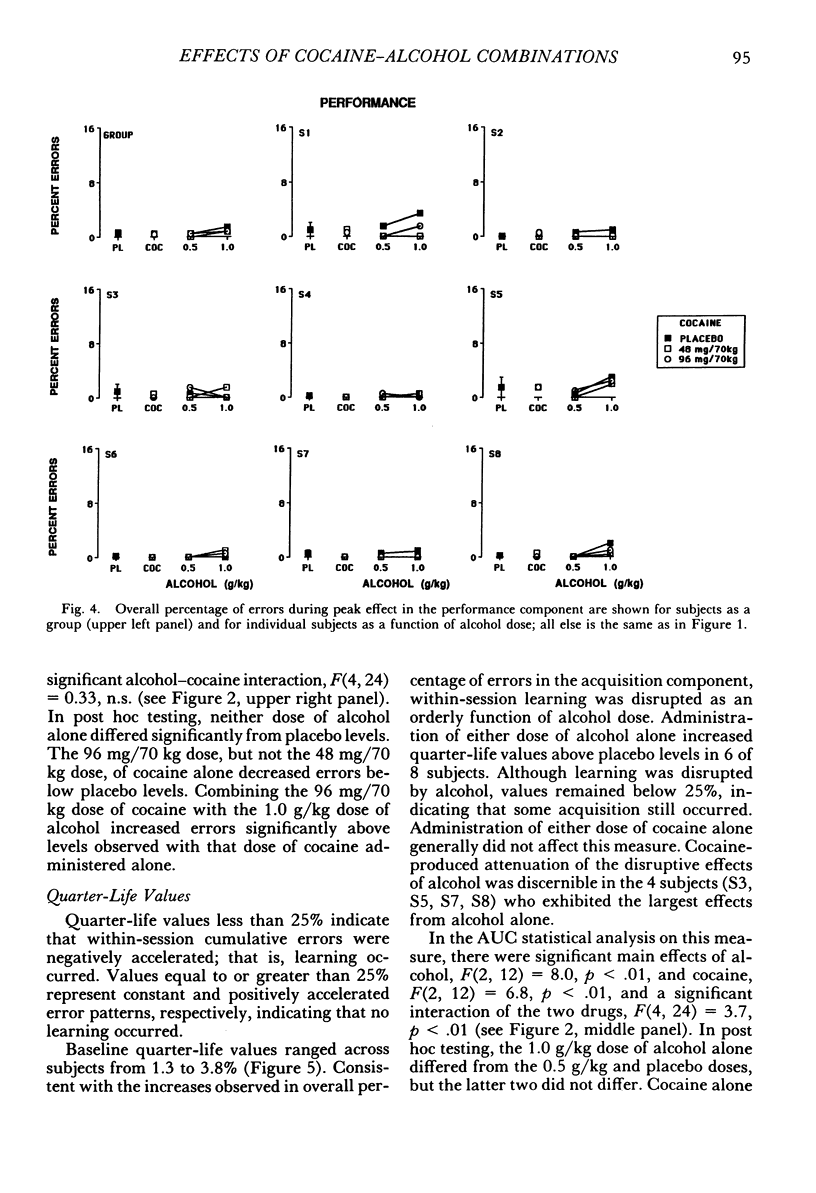
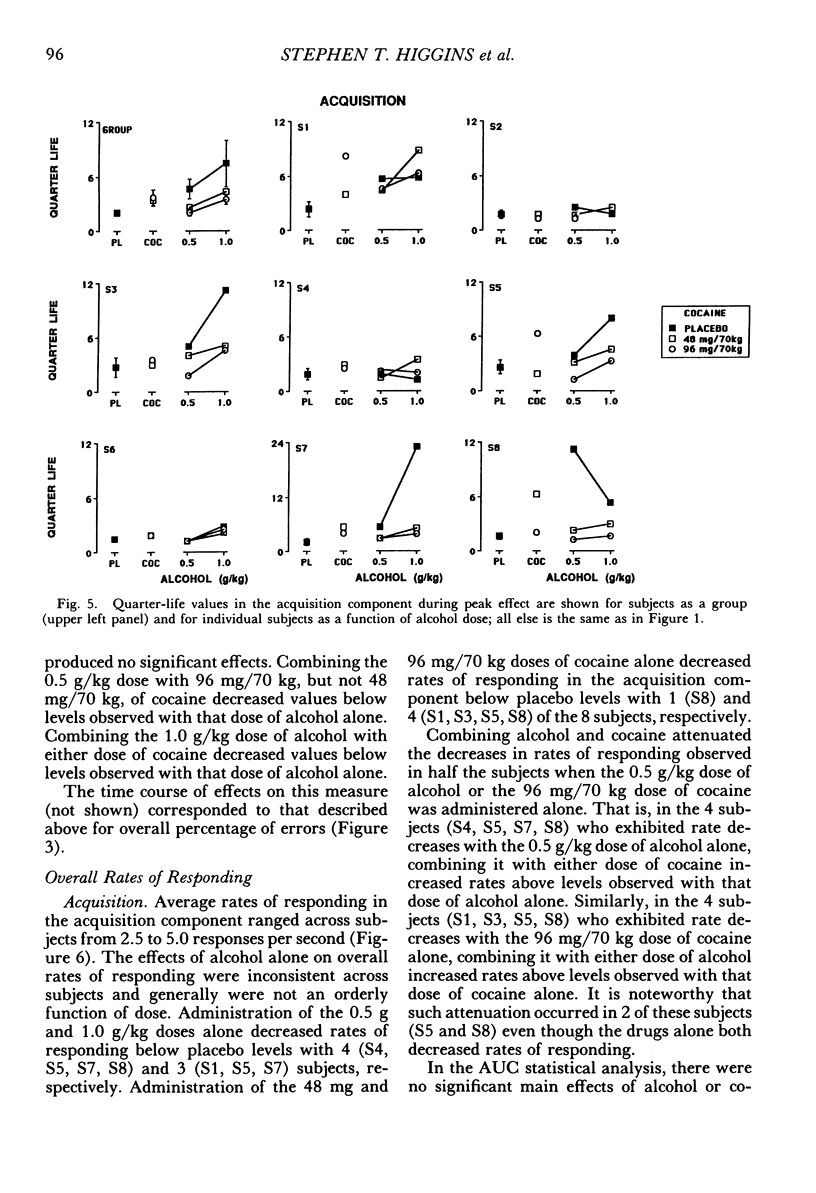
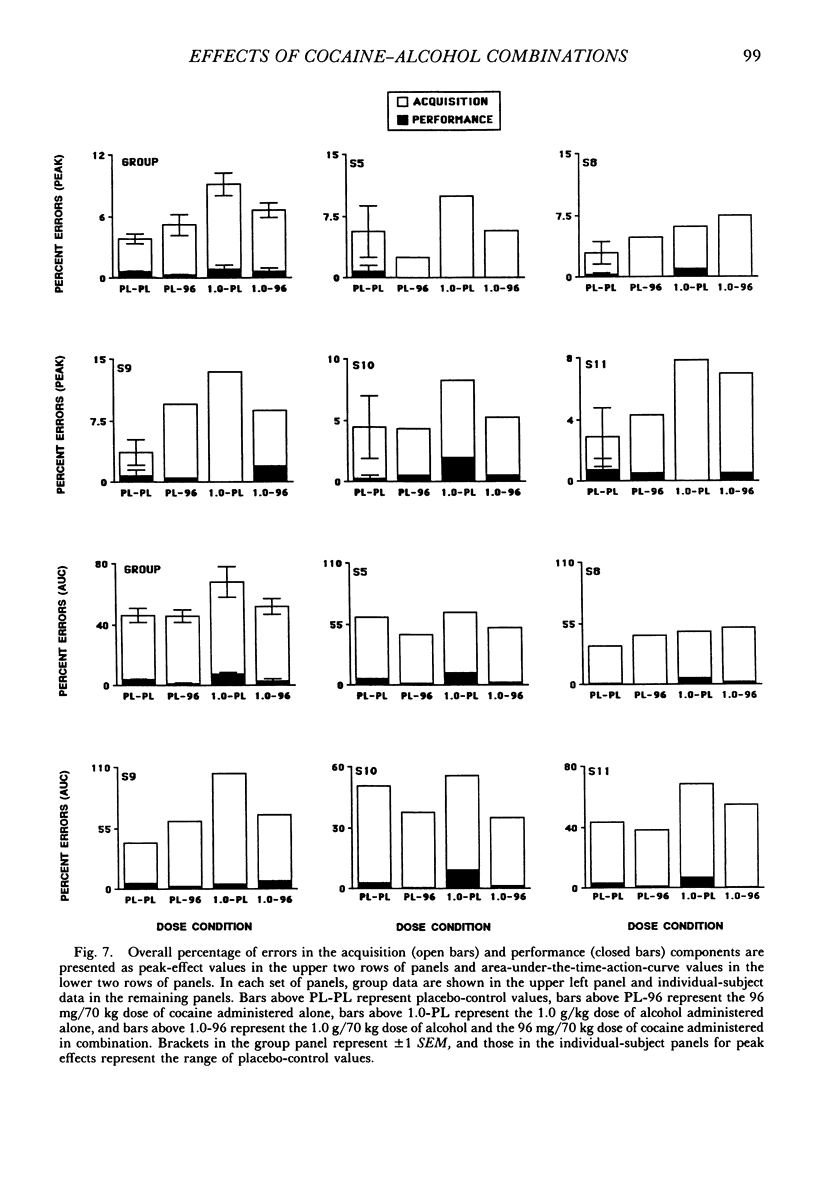
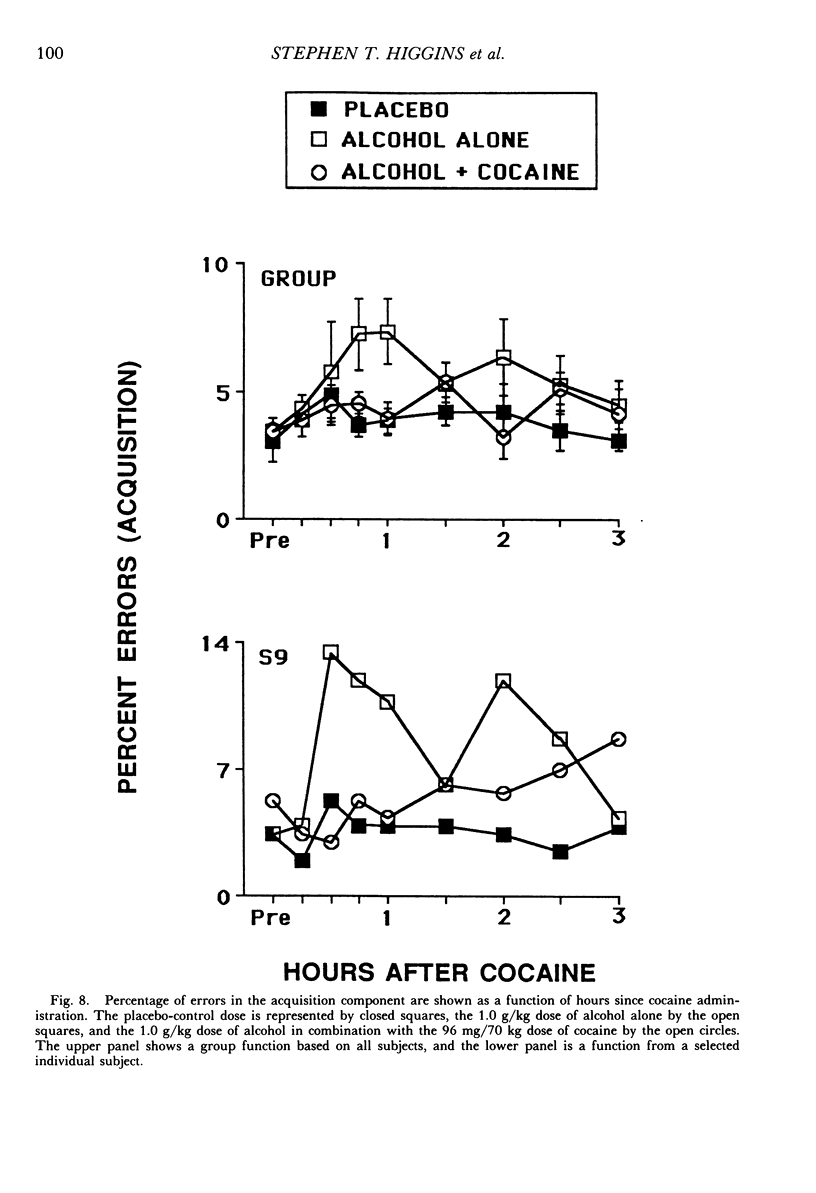
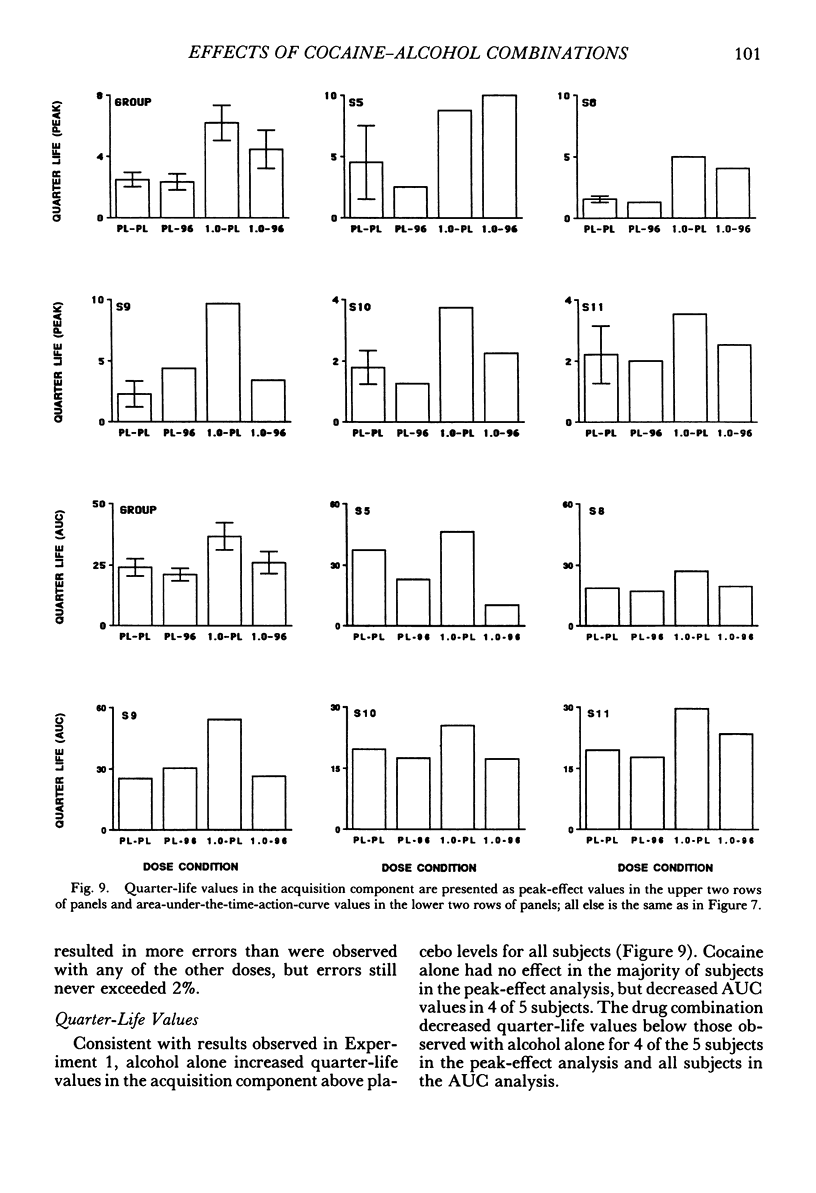
The acute effects of cocaine hydrochloride (4 to 96 mg/70 kg) and alcohol (0 to 1.0 g/kg), administered alone and in combination, were assessed in two experiments with human volunteers responding under a multiple schedule of repeated acquisition and performance of response chains. Subjects were intermittent users of cocaine and regular drinkers who were not cocaine or alcohol dependent. Alcohol was mixed with orange juice and ingested in six drinks within 30 min; cocaine was administered intranasally 45 min after completion of drinking. In each component of the multiple schedule, subjects completed response sequences using three keys of a numeric keypad. In the acquisition component, a new sequence was learned each session. In the performance component, the response sequence always remained the same. Results were consistent in both experiments, despite variations in the order in which the drugs were tested alone and in combination. Alcohol administered alone increased overall percentage of errors and decreased rates of responding in the acquisition component, whereas responding in the performance component generally was unaffected. Cocaine administered alone decreased rates of responding but did not affect accuracy of responding in the acquisition component, and enhanced accuracy of responding without affecting rates of responding in the performance component. The combined doses of cocaine and alcohol attenuated the effects observed with alcohol and cocaine alone. These results suggest that, under the conditions investigated in this study, (a) alcohol produces greater behavioral disruption than cocaine or cocaine-alcohol combinations, (b) cocaine and alcohol each attenuate effects of the other, and (c) such attenuation is most pronounced for cocaine attenuating the disruptive effects of alcohol.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aston-Jones S., Aston-Jones G., Koob G. F. Cocaine antagonizes anxiolytic effects of ethanol. Psychopharmacology (Berl) 1984;84(1):28–31. doi: 10.1007/BF00432019. [DOI] [PubMed] [Google Scholar]
- Barthalmus G. T., Leander J. D., McMillan D. E. Combined effects of ethanol and diazepam on performance and acquisition of serial position sequences by pigeons. Psychopharmacology (Berl) 1978 Sep 15;59(1):101–102. doi: 10.1007/BF00428039. [DOI] [PubMed] [Google Scholar]
- Bickel W. K., Higgins S. T., Griffiths R. R. Repeated diazepam administration: effects on the acquisition and performance of response chains in humans. J Exp Anal Behav. 1989 Jul;52(1):47–56. doi: 10.1901/jeab.1989.52-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P. J., Moerschbaecher J. M., Thompson D. M., Thomas J. R. Intravenous diazepam in humans: effects on acquisition and performance of response chains. Pharmacol Biochem Behav. 1982 Nov;17(5):1055–1059. doi: 10.1016/0091-3057(82)90493-2. [DOI] [PubMed] [Google Scholar]
- Fischman M. W., Schuster C. R. Cocaine effects in sleep-deprived humans. Psychopharmacology (Berl) 1980;72(1):1–8. doi: 10.1007/BF00433800. [DOI] [PubMed] [Google Scholar]
- Foltin R. W., Fischman M. W. Ethanol and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1988 Dec;31(4):877–883. doi: 10.1016/0091-3057(88)90399-1. [DOI] [PubMed] [Google Scholar]
- GOLLUB L. R. THE RELATIONS AMONG MEASURES OF PERFORMANCE ON FIXED-INTERVAL SCHEDULES. J Exp Anal Behav. 1964 Sep;7:337–343. doi: 10.1901/jeab.1964.7-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Harford T. C. Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend. 1990 Feb;25(1):97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- Hearn W. L., Flynn D. D., Hime G. W., Rose S., Cofino J. C., Mantero-Atienza E., Wetli C. V., Mash D. C. Cocaethylene: a unique cocaine metabolite displays high affinity for the dopamine transporter. J Neurochem. 1991 Feb;56(2):698–701. doi: 10.1111/j.1471-4159.1991.tb08205.x. [DOI] [PubMed] [Google Scholar]
- Higgins S. T., Bickel W. K., Hughes J. R., Lynn M., Capeless M. A., Fenwick J. W. Effects of intranasal cocaine on human learning, performance and physiology. Psychopharmacology (Berl) 1990;102(4):451–458. doi: 10.1007/BF02247124. [DOI] [PubMed] [Google Scholar]
- Higgins S. T., Bickel W. K., O'Leary D. K., Yingling J. Acute effects of ethanol and diazepam on the acquisition and performance of response sequences in humans. J Pharmacol Exp Ther. 1987 Oct;243(1):1–8. [PubMed] [Google Scholar]
- Higgins S. T., Delaney D. D., Budney A. J., Bickel W. K., Hughes J. R., Foerg F., Fenwick J. W. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991 Sep;148(9):1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins S. T., Woodward B. M., Henningfield J. E. Effects of atropine on the repeated acquisition and performance of response sequences in humans. J Exp Anal Behav. 1989 Jan;51(1):5–15. doi: 10.1901/jeab.1989.51-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatlow P., Elsworth J. D., Bradberry C. W., Winger G., Taylor J. R., Russell R., Roth R. H. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci. 1991;48(18):1787–1794. doi: 10.1016/0024-3205(91)90217-y. [DOI] [PubMed] [Google Scholar]
- Javaid J. I., Fischman M. W., Schuster C. R., Dekirmenjian H., Davis J. M. Cocaine plasma concentration: relation to physiological and subjective effects in humans. Science. 1978 Oct 13;202(4364):227–228. doi: 10.1126/science.694530. [DOI] [PubMed] [Google Scholar]
- Jonsson J., Lewander T. Effects of diethyldithiocarbamate and ethanol on the in vivo metabolism and pharmacokinetics of amphetamine in the rat. J Pharm Pharmacol. 1973 Jul;25(7):589–591. doi: 10.1111/j.2042-7158.1973.tb09168.x. [DOI] [PubMed] [Google Scholar]
- Katz J. L., Terry P., Witkin J. M. Comparative behavioral pharmacology and toxicology of cocaine and its ethanol-derived metabolite, cocaine ethyl-ester (cocaethylene). Life Sci. 1992;50(18):1351–1361. doi: 10.1016/0024-3205(92)90286-x. [DOI] [PubMed] [Google Scholar]
- Masur J., Souza-Formigoni M. L., Pires M. L. Increased stimulatory effect by the combined administration of cocaine and alcohol in mice. Alcohol. 1989 May-Jun;6(3):181–182. doi: 10.1016/0741-8329(89)90015-3. [DOI] [PubMed] [Google Scholar]
- Miller N. S., Belkin B. M., Gold M. S. Multiple addictions: co-synchronous use of alcohol and drugs. N Y State J Med. 1990 Dec;90(12):596–600. [PubMed] [Google Scholar]
- Moerschbaecher J. M., Thompson D. M. Effects of d-amphetamine, cocaine, and phencyclidine on the acquisition of response sequences with and without stimulus fading. J Exp Anal Behav. 1980 May;33(3):369–381. doi: 10.1901/jeab.1980.33-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech R. H., Vomachka M. K., Rickert D. E. Interactions between depressants (alcohol-type) and stimulants (amphetamine-type). Pharmacol Biochem Behav. 1978 Feb;8(2):143–151. doi: 10.1016/0091-3057(78)90331-3. [DOI] [PubMed] [Google Scholar]
- Thompson D. M. Development of tolerance to the disruptive effects of cocaine on repeated acquisition and performance of response sequences. J Pharmacol Exp Ther. 1977 Nov;203(2):294–302. [PubMed] [Google Scholar]


