Abstract
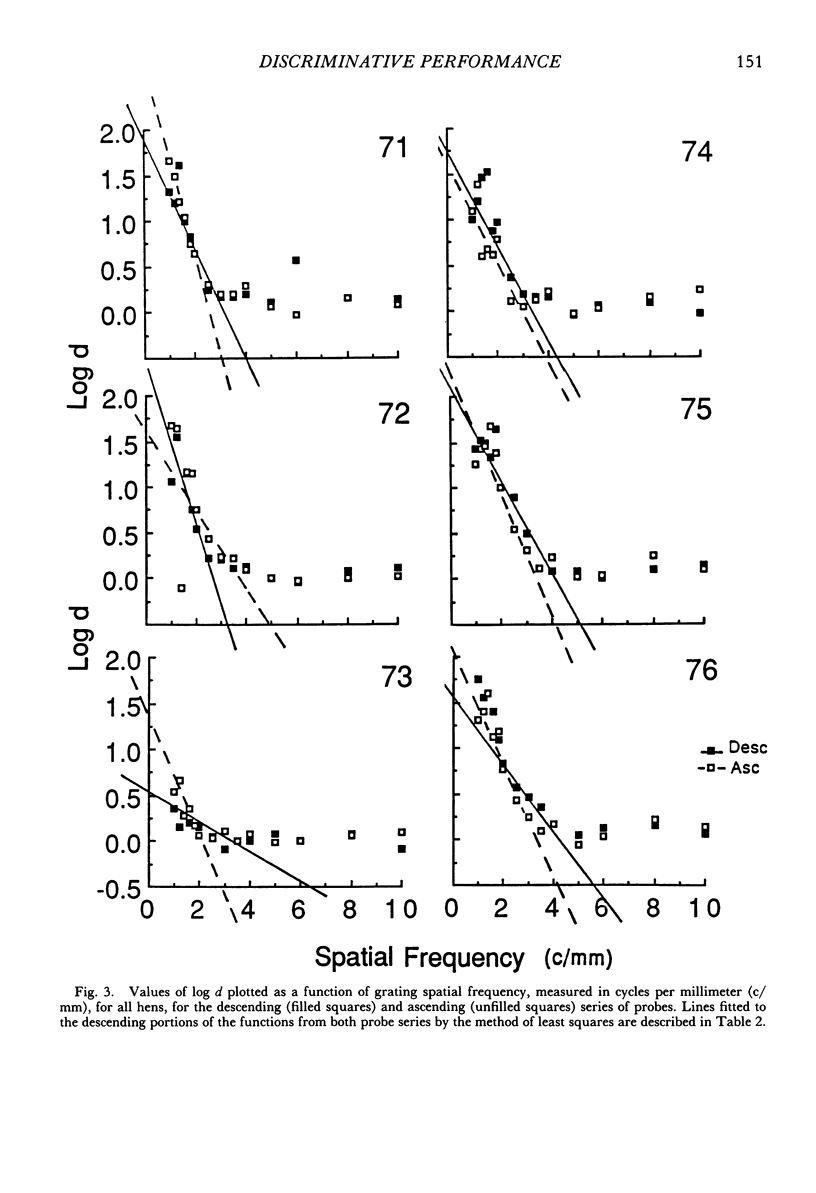
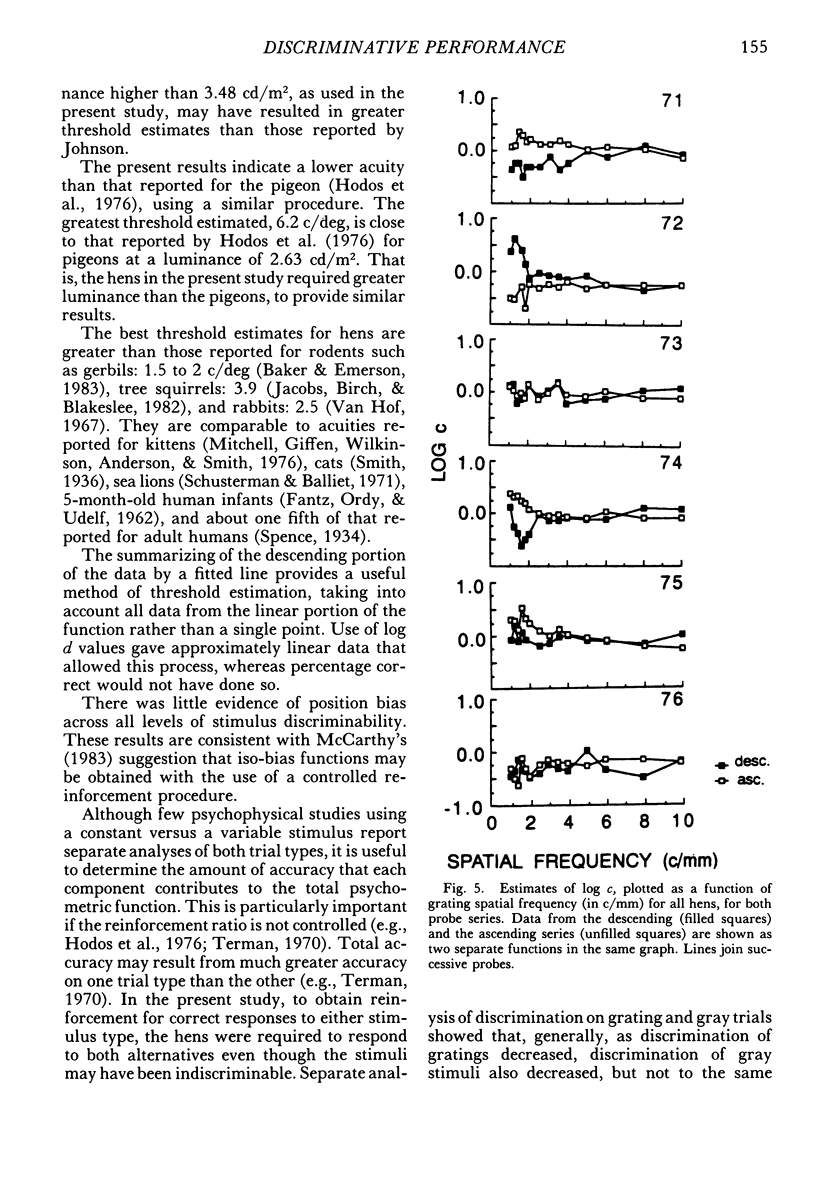
The performance of 6 domestic hens on a visual acuity task was studied using a spatial discrete-trial conditional discrimination procedure. Gray stimuli and vertical square-wave gratings, ranging in spatial frequency from 1 to 10 cycles per millimeter, were presented in a descending and ascending series of probes. On each trial, either a grating or gray stimulus was presented. Only one spatial frequency of grating was presented during each session. Between probe sessions, training continued at the coarsest grating value. Stimulus discriminability, measured as values of log d, changed with changes in grating spatial frequency for both probe series. Fitted lines described the linear portion of the psychometric functions. Thresholds estimated from the lines generally ranged from four to six cycles per degree, with slightly greater estimates from data from the descending probe series. There were no systematic changes in response bias as a function of grating spatial frequency.
Keywords: discrimination, visual acuity, threshold, signal detection, key peck, hens
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A. G., Emerson V. F. Grating acuity of the Mongolian gerbil (Meriones unguiculatus). Behav Brain Res. 1983 May;8(2):195–209. doi: 10.1016/0166-4328(83)90054-2. [DOI] [PubMed] [Google Scholar]
- Baum W. M. On two types of deviation from the matching law: bias and undermatching. J Exp Anal Behav. 1974 Jul;22(1):231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough P. M. The visual acuity of the pigeon for distant targets. J Exp Anal Behav. 1971 Jan;15(1):57–67. doi: 10.1901/jeab.1971.15-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Knowles A. The visual pigments and oil droplets of the chicken retina. Vision Res. 1977;17(7):755–764. doi: 10.1016/0042-6989(77)90117-1. [DOI] [PubMed] [Google Scholar]
- Davison M. C. Preference for fixed-interval schedules: effects of unequal initial links. J Exp Anal Behav. 1976 May;25(3):371–376. doi: 10.1901/jeab.1976.25-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M. C., Tustin R. D. The relation between the generalized matching law and signal-detection theory. J Exp Anal Behav. 1978 Mar;29(2):331–336. doi: 10.1901/jeab.1978.29-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich D. Regional specialization of the chick retina as revealed by the size and density of neurons in the ganglion cell layer. J Comp Neurol. 1981 Feb 1;195(4):643–657. doi: 10.1002/cne.901950408. [DOI] [PubMed] [Google Scholar]
- Hodos W., Leibowitz R. W., Bonbright J. C., Jr Near-field visual acuity of pigeons: effects of head location and stimulus luminance. J Exp Anal Behav. 1976 Mar;25(2):129–141. doi: 10.1901/jeab.1976.25-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D., Davison M. Independence of sensitivity to relative reinforcement rate and discriminability in signal detection. J Exp Anal Behav. 1980 Nov;34(3):273–284. doi: 10.1901/jeab.1980.34-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D., Davison M. On the discriminability of stimulus duration. J Exp Anal Behav. 1980 Mar;33(2):187–211. doi: 10.1901/jeab.1980.33-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. Measures of response bias at minimum-detectable luminance levels in the pigeon. J Exp Anal Behav. 1983 Jan;39(1):87–106. doi: 10.1901/jeab.1983.39-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millodot M. Influence of the subjects criterion on the visual resolution of a grating. Percept Mot Skills. 1973 Feb;36(1):155–158. doi: 10.2466/pms.1973.36.1.155. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Giffin F., Wilkinson F., Anderson P., Smith M. L. Visual resolution in young kittens. Vision Res. 1976;16(4):363–366. doi: 10.1016/0042-6989(76)90197-8. [DOI] [PubMed] [Google Scholar]
- Over R., Moore D. Spatial acuity of the chicken. Brain Res. 1981 May 4;211(2):424–426. doi: 10.1016/0006-8993(81)90967-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F., Howland H. C., Farkas L. Natural accommodation in the growing chicken. Vision Res. 1986;26(12):1977–1993. doi: 10.1016/0042-6989(86)90123-9. [DOI] [PubMed] [Google Scholar]
- Stubbs D. A., Pliskoff S. S. Concurrent responding with fixed relative rate of reinforcement. J Exp Anal Behav. 1969 Nov;12(6):887–895. doi: 10.1901/jeab.1969.12-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs D. A. Response bias and the discrimination of stimulus duration. J Exp Anal Behav. 1976 Mar;25(2):243–250. doi: 10.1901/jeab.1976.25-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple W., Foster T. M., O'Donnell C. S. Behavioural estimates of auditory thresholds in hens. Br Poult Sci. 1984 Oct;25(4):487–493. doi: 10.1080/00071668408454890. [DOI] [PubMed] [Google Scholar]
- Terman M. Discrimination of auditory intensities by rats. J Exp Anal Behav. 1970 Mar;13(2):145–160. doi: 10.1901/jeab.1970.13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hof M. W. Visual acuity in the rabbit. Vision Res. 1967 Sep;7(9):749–751. doi: 10.1016/0042-6989(67)90037-5. [DOI] [PubMed] [Google Scholar]


