Abstract
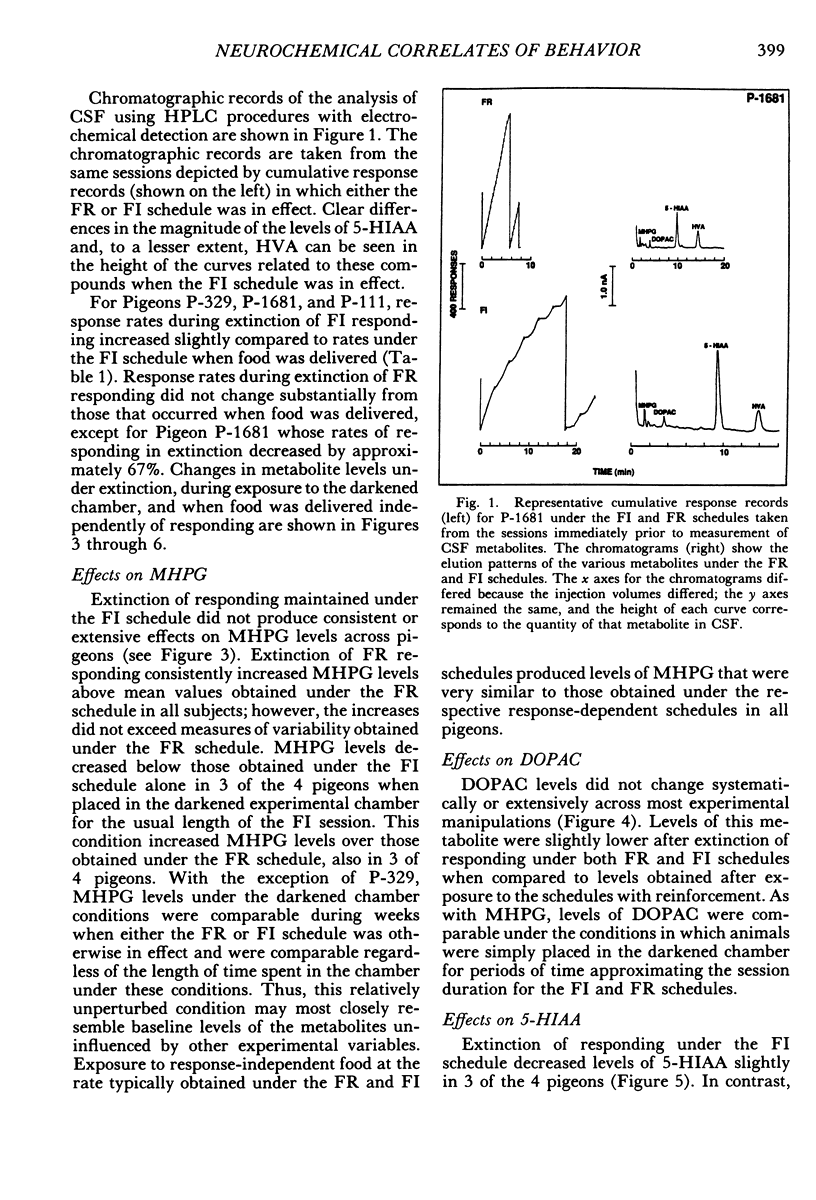
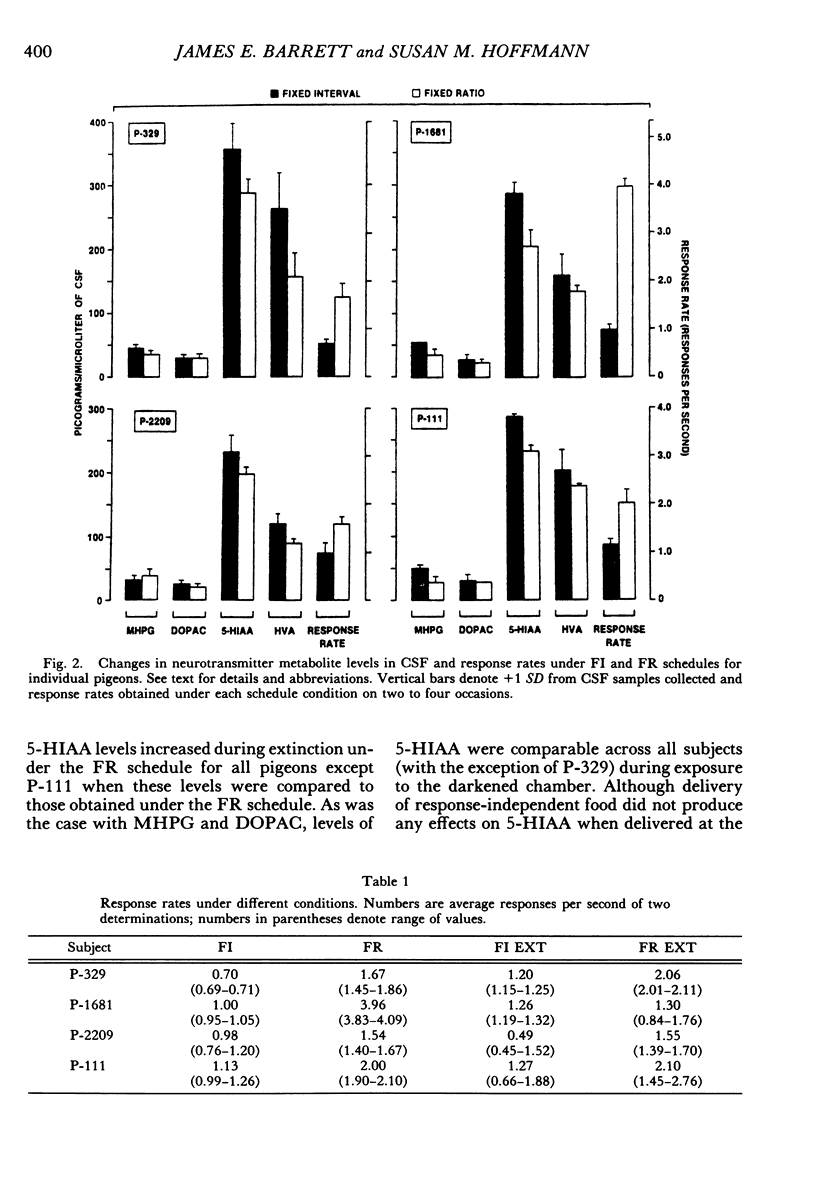
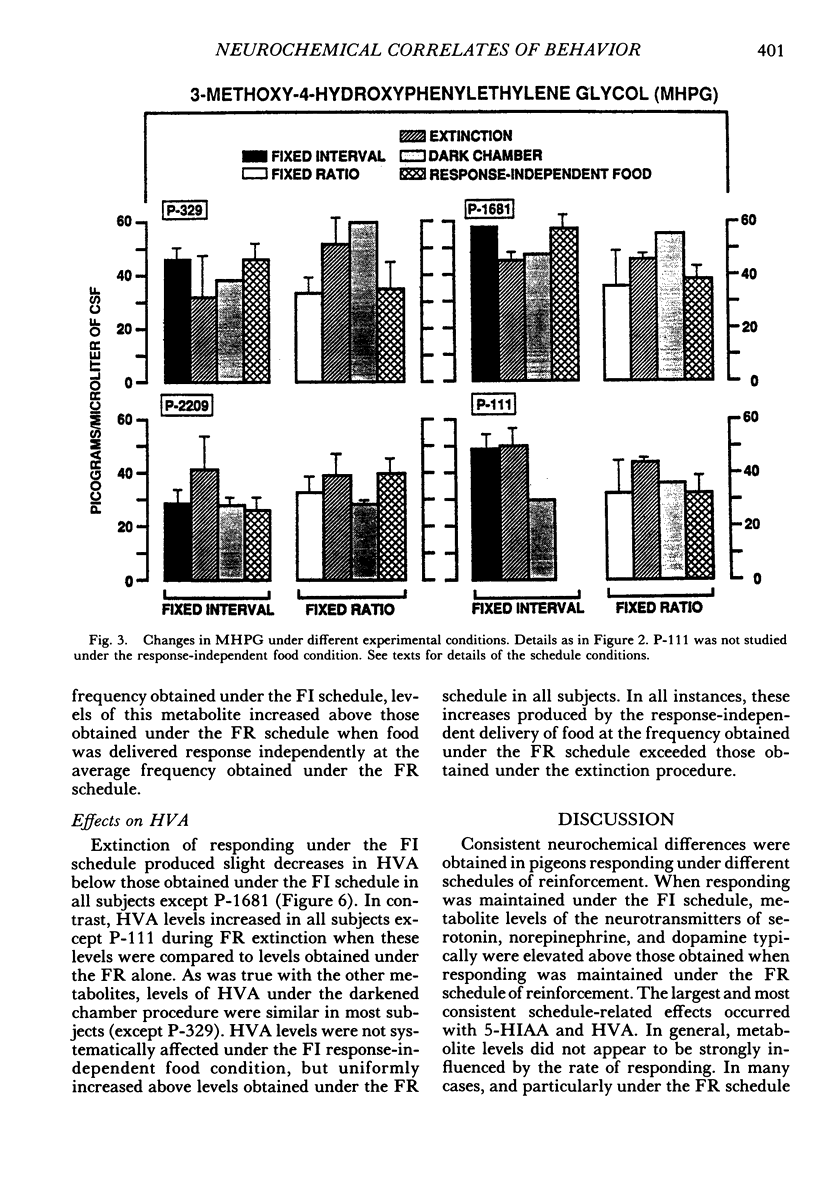
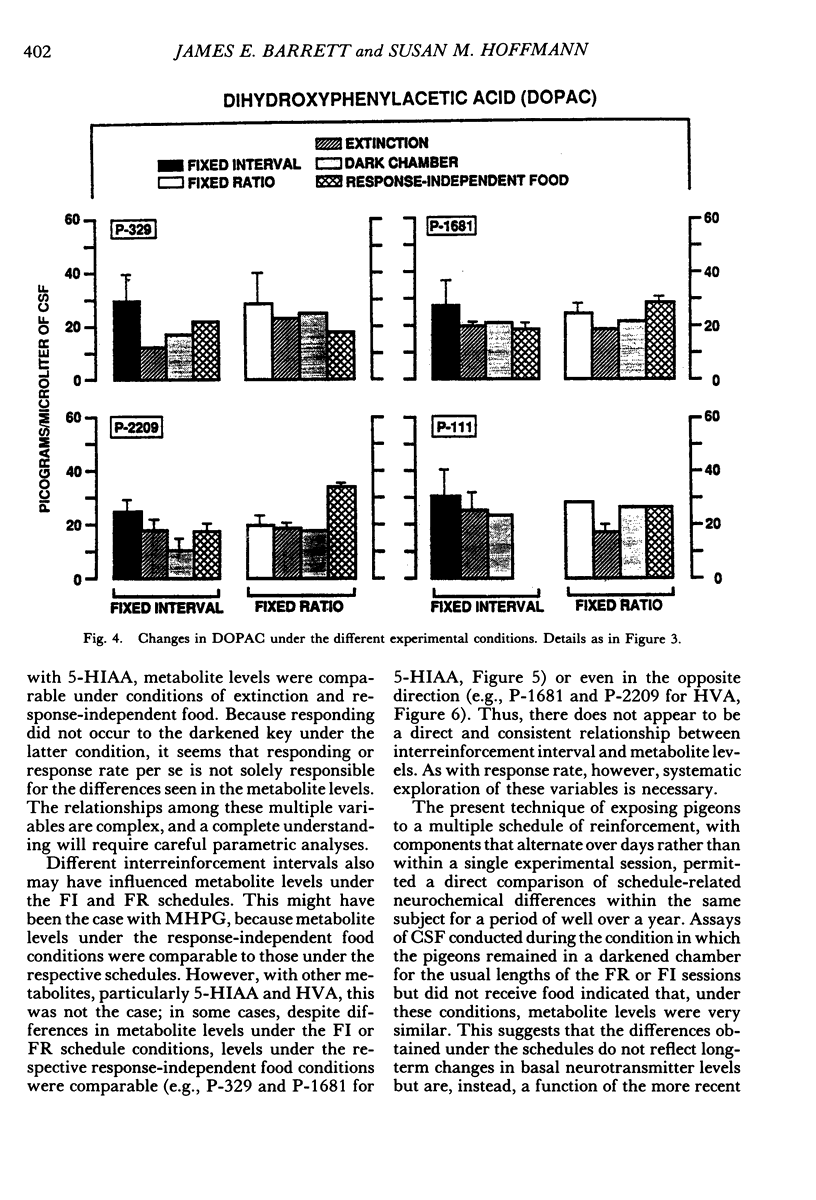
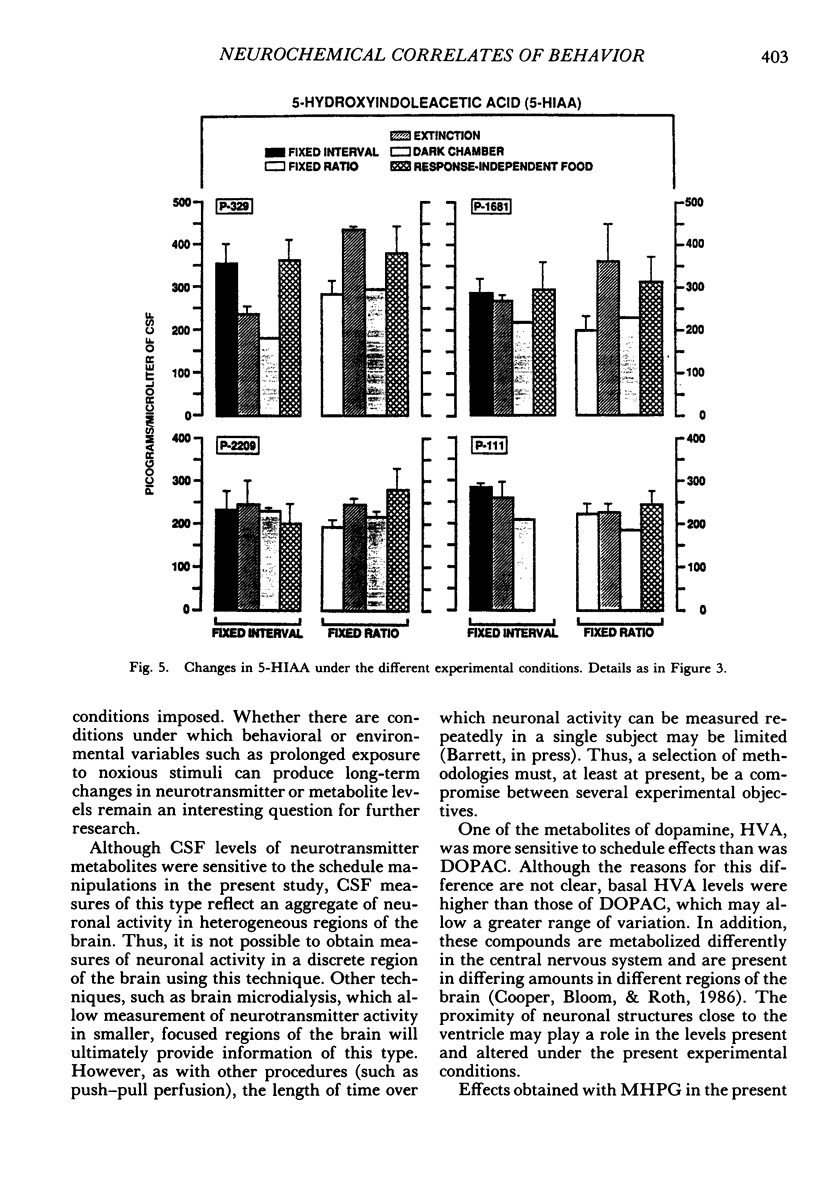
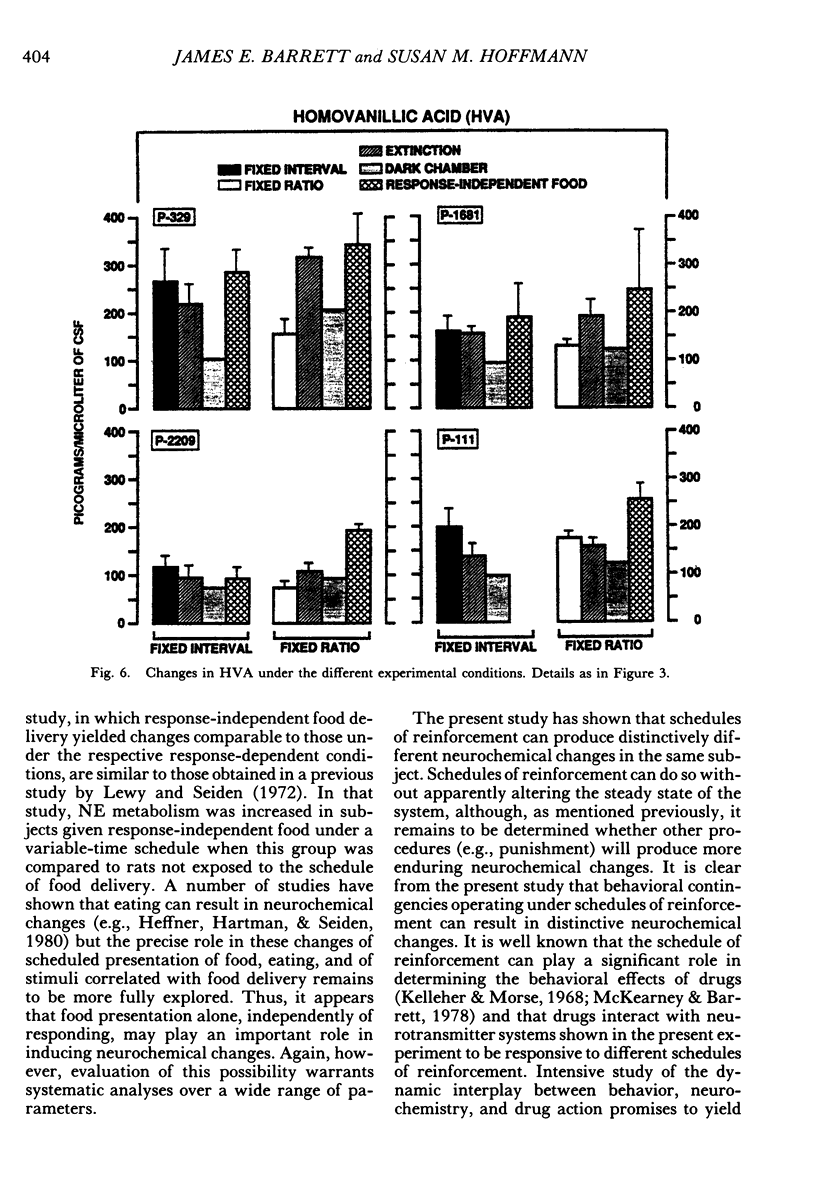
Key pecking of 4 pigeons was maintained under a multiple 3-min fixed-interval, 30-response fixed-ratio schedule of food presentation. Only one schedule was in effect during an experimental session, and each was correlated with a different keylight stimulus and location (left vs. right). The different schedule components alternated across days or weeks. Cerebrospinal fluid was collected from chronically implanted intracerebroventricular cannulae following sessions with the different schedules, as well as following sessions in which reinforcement was withheld (extinction), when response-independent food was delivered, and when the experimental chamber was dark and there were no scheduled events. Metabolites of the neurotransmitters serotonin, norepinephrine, and dopamine were assayed in cerebrospinal fluid using high-performance liquid chromatography with electrochemical detection. Compared to the fixed-ratio condition, responding maintained under the fixed-interval schedule resulted in consistently higher levels of the serotonin metabolite 5-hydroxyindoleacetic acid and of the dopamine metabolite homovanillic acid in all pigeons. Levels of 3-methoxy-4-hydroxyphenylethylene glycol, a metabolite of norepinephrine, and dihydroxyphenylacetic acid, another dopamine metabolite, were also higher in 3 of the 4 pigeons following exposure to the fixed-interval schedules when compared to levels of these metabolites after exposure to the fixed-ratio schedule. Extinction of fixed-ratio responding resulted in large increases in 5-hydroxyindoleacetic acid compared to levels of this metabolite under the fixed-ratio schedule, whereas this serotonin metabolite decreased during extinction of responding under the fixed-interval schedule. Control procedures suggested that the neurochemical changes were not related to the rate of responding but were a function of the specific experimental conditions. Distinctive neurochemical changes that accompany schedule-controlled responding show the sensitivity of the neurochemical environment to behavioral contingencies and demonstrate further the profound impact that such contingencies have on biobehavioral processes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. E. Behavioral history as a determinant of the effects of d-amphetamine on punished behavior. Science. 1977 Oct 7;198(4312):67–69. doi: 10.1126/science.408925. [DOI] [PubMed] [Google Scholar]
- DEWS P. B. Studies on behavior. I. Differential sensitivity to pentobarbital of pecking performance in pigeons depending on the schedule of reward. J Pharmacol Exp Ther. 1955 Apr;113(4):393–401. [PubMed] [Google Scholar]
- DEWS P. B. Studies on behavior. IV. Stimulant actions of methamphetamine. J Pharmacol Exp Ther. 1958 Jan;122(1):137–147. [PubMed] [Google Scholar]
- FERSTER C. B. The use of the free operant in the analysis of behavior. Psychol Bull. 1953 Jul;50(4):263–274. doi: 10.1037/h0055514. [DOI] [PubMed] [Google Scholar]
- Heffner T. G., Hartman J. A., Seiden L. S. Feeding increases dopamine metabolism in the rat brain. Science. 1980 Jun 6;208(4448):1168–1170. doi: 10.1126/science.7375926. [DOI] [PubMed] [Google Scholar]
- Kelleher R. T., Morse W. H. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Lewy A. J., Seiden L. S. Operant behavior changes norepinephrine metabolism in rat brain. Science. 1972 Jan 28;175(4020):454–456. doi: 10.1126/science.175.4020.454. [DOI] [PubMed] [Google Scholar]
- Schoenfeld R. I., Seiden L. S. Effect of alpha-methyltyrosine on operant behavior and brain catecholamine levels. J Pharmacol Exp Ther. 1969 Jun;167(2):319–327. [PubMed] [Google Scholar]
- Seiden L. S., MacPhail R. C., Oglesby M. W. Catecholamines and drug-behavior interactions. Fed Proc. 1975 Aug;34(9):1823–1831. [PubMed] [Google Scholar]
- Sparber S. B. Neurochemical changes associated with schedule-controlled behavior. Fed Proc. 1975 Aug;34(9):1802–1812. [PubMed] [Google Scholar]
- Sparber S. B., Tilson H. A. Schedule controlled and drug induced release of norepinephrine-7- 3 H into the lateral ventricle of rats. Neuropharmacology. 1972 Jul;11(4):453–464. doi: 10.1016/0028-3908(72)90001-9. [DOI] [PubMed] [Google Scholar]
- Tilson H. A., Sparber S. B. Studies on the concurrent behavioral and neurochemical effects of psychoactive drugs using the push-pull cannula. J Pharmacol Exp Ther. 1972 Jun;181(3):387–398. [PubMed] [Google Scholar]


