Abstract
Before synaptogenesis, early excitability implicating voltage-dependent and transmitter-activated channels is known to be crucial for neuronal development. We previously showed that preplate (PP) neurons of the mouse neocortex express functional Na+ channels as early as embryonic day 12. In this study, we investigated the role of these Na+ channels in signaling during early development. In the neocortex of embryonic-day-13 mice, activation of Na+ channels with veratridine induced a large Ca2+ response throughout the neocortex, even in cell populations that lack the Na+ channel. This Na+-dependent Ca2+ activity requires external Ca2+ and is completely blocked by inhibitors of Na+/Ca2+ exchangers. Moreover, veratridine-induced Ca2+ increase coincides with a burst of exocytosis in the PP. In parallel, we show that Na+ channel stimulation enhances glutamate secretion in the neocortical wall. Released glutamate triggers further Ca2+ response in PP and ventricular zone, as indicated by the decreased response to veratridine in the presence of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor and NMDA-receptor inhibitors. Therefore, the combined activation of the Na+ channel and the Na+/Ca2+ exchanger triggers Ca2+ signaling in the PP neurons, leading to glutamate secretion, which amplifies the signal and serves as an autocrine/paracrine transmitter before functional synapses are formed in the neocortex. Membrane depolarization induced by glycine receptors activation could be one physiological activator of this Na+ channel-dependent pathway.
Keywords: Ca2+ signaling, Na+/Ca2+ exchanger, exocytosis, neurogenesis
Neurons in the cerebral cortex are generated in a proliferative region known as the ventricular zone (VZ) (1). The first phase of neurogenesis occurs at embryonic days 11-13 (E11-E13) in mice and gives rise to a group of neurons that form the preplate (PP) above the VZ (2).
During development, ligand- and voltage-gated ionic currents appear in a complex and progressive pattern, generating cell excitability. This early excitability has a widespread and important role in transmission of information and development of the nervous system. Combinations of ion channels usually generate Ca2+ influx, which influences differentiation and regulates channel expression (for review, see ref. 3). Neurotransmitters are reported to be present very early during neurogenesis (4). GABA and glutamate are both present in the neocortical wall and can regulate neuronal progenitor proliferation (4, 5) and neuronal migration (6). Taurine and glycine are also present and have been reported to be the most abundant neurotransmitters at E13 in mouse neocortex (7). Little is known about the channels and pathways that are involved in this early activity, and particularly little is known about how these neurotransmitters are secreted before the appearance of voltage-dependent Ca2+ channels (8, 9).
Although cells are devoid of synaptic connection, spontaneous Ca2+ activity is already present at E13 (J.-C.P. and M.A., unpublished data). Electrical and Ca2+ spontaneous activities are known to be interconnected and to work together in developmental signaling (3, 10). At E13, although all neurons and proliferative cells express a delayed outward K+ current, only a subpopulation of PP neurons also express an inward Na+ current (11). Moreover, no voltage-dependent Ca2+ current can be recorded at this stage, in either the VZ or the PP cells (8, 9). Therefore, the only depolarizing voltage-dependent inward current that is expressed at this stage is found in a subset of neuronal PP cells, which led us to study the role of the PP Na+ currents in this Ca2+ signaling in early neurogenesis.
By using veratridine, a specific voltage-dependent Na+ channel agonist (12), we show that the Na+ channel activation of PP neurons triggers Ca2+ signaling via a plasma membrane Na+/Ca2+ exchanger. This Ca2+ rise extends to most of PP cells, as well as some cells in the VZ, suggesting the secretion of a diffusible messenger. The veratridine-induced Ca2+ rise triggers an exocytosis in PP cells, as measured with the fluorescent dye FM1-43. Also, we show that this exocytosis coincides with glutamate secretion in the neocortex. Hence, glutamate may be responsible for activation of a population of cells that are devoid of Na+ channels by paracrine communication. Our results demonstrate that new, immature Na+ and Ca2+ excitabilities are established in the first generated neurons and trigger neurotransmitter secretion, which is involved in paracrine communication between neocortical cells.
Materials and Methods
Calcium Imaging. Neocortical brain slices were prepared from E13 embryos of C57/Bl6 mice (Iffa Credo, Lyon, France), as described in ref. 11. Slices were loaded with the Ca2+ indicator dye Fluo-4 by bath application for 60-90 min at room temperature in artificial cerebrospinal fluid (ACSF; 119 mM NaCl/2.5 mM KCl/1.3 mM MgCl2/2.5 mM CaCl2/1 mM NaH2PO4/26.2 mM NaHCO3/11 mM d-glucose), containing 5 μM Fluo-4 acetoxymethyl ester (AM) (Molecular Probes), 0.005% Pluronic F-127 (Molecular Probes), and 0.1% DMSO (Sigma). Slices were then placed in a constantly perfused chamber (ACSF bubbled with 95% O2/5% CO2) at room temperature on the stage of an upright compound microscope (Eclipse E600FN, Nikon) equipped with a ×40 water-immersion objective (numerical aperture, 0.8) and a confocal head (PCM 2000, Nikon). Drugs were perfused in the bath by using a multivalve system (VC-6M, Harvard Apparatus). Relative changes in intracellular Ca2+ concentration ([Ca2+]i) were assessed in the dorsomedial neocortex part. The fluorescent dye was excited with light at 488 nm from an argon laser. Emitted light was filtered with a 515 ± 15-nm filter. Stacks of 15-μm-spaced images were acquired every 4 s in a time-lapse sequence with ez 2000 software (Nikon).
Quantification and Analysis. We developed a computer program (named calsignal) for automatic detection of cells and measurement of their intracellular Ca2+ activity (J.-C.P., M.A., A.D., S.B., M.V., and J.B., unpublished data). This software made it possible to automatically detect and track hundreds of cells per slice and to carry out statistical analysis for numerous parameters. For each series of images, F0 is the mean fluorescence intensity measured throughout all of the regions of interest, and F is the mean fluorescence intensity in a single cell. Relative changes in [Ca2+] over time are expressed as F/F0. The percentage of activated cells was calculated as the number of cells displaying a significant agonist-induced (e.g., veratridine) fluorescence intensity increase (>20% F/F0 increase upon application) divided by the total number of detected cells. The amplitude was determined as the difference between the peak maximum and initial peak intensities in the recorded cell. This program is freely accessible on request. Data were exported to sigmastat software (Systat) for statistical analysis.
All results are presented as mean ± SEM. Statistical analyses were carried out with nonparametric Wilcoxon (Fig. 1, paired data) or Mann-Whitney (unpaired data) tests. *, P < 0.05; **, P < 0.01; and ***, P < 0.001, compared with control.
Fig. 1.
Activation of Na+ channels leads to Ca2+ activity in mouse neocortical slices. (A)Na+ channel staining (red) labels cells in the upper PP. Neuronal cells are identified with TuJ1 staining (green). The upper meninges layer is TuJ1-negative, and the dotted white line indicates the ventricular border of the slice. (B) Example of a fluorescence increase induced by 50 μM veratridine applied on a E13 cortex slice loaded with 5 μM Fluo-4 AM (C) Corresponding fluorescence variations measured in two typical cells (one in the PP and one in the VZ) of the slice shown in B. (D) Percentage of responding cells upon veratridine stimulation in the PP and VZ (n = 16 slices; 1,281 and 2,511 cells in the PP and in the VZ, respectively).
FM1-43 Optical Measurements. We measured exocytosis with the fluorescent-membrane probe FM1-43 (Molecular Probes), which was added to the ASCF perfusion medium at a concentration of 2 μM at 1 min before the recording. The membrane trafficking cycle was monitored by the two approaches described in ref. 13: (i) without washing FM1-43, in which exocytotic stimulation should trigger an increase in fluorescence; and (ii) after a 5 min wash of external FM1-43, in which stimulation should trigger a decrease of the remaining (internal) fluorescence.
Measurements were made with the Ca2+-imaging setup, with stimulation at 488 nm and emission filtered with a 595 ± 35-nm filter. The signal is sampled every 4 s. We then quantified the fluorescence in both PP and VZ by using regions of interest corresponding to these two areas. Results are expressed as fluorescence ratios (in percentage), comparing fluorescence intensity levels before (stabilized basal level) and 2 min after FM1-43 addition in the absence of added drugs or 1 min after the addition of veratridine (applied 1 min after FM1-43). Also, for destaining experiments, fluorescence-intensity levels were compared before and 1 min after veratridine addition.
Immunostaining. E13 brains were fixed in cold 4% paraformaldehyde in ACSF for 1 h, washed with PBS (3.16 mM NaH2PO4/6.84 mM Na2HPO4/0.15 mM NaCl, pH 7.2), and cryoprotected by incubation in 15% sucrose in PBS for 1 h. Brains were then frozen in Tissue-Tek (Torrance, CA) embedding medium, and 20-μm sections were cut with a cryostat. Sections were blocked with 1% polyvinylpyrrolidone (PVP-40, Sigma)/0.2% Triton X-100 in PBS (PBSTP) for 30 min. Sections were then incubated overnight with the following primary antibodies diluted in PBSTP: antineuronal class III β-tubulin antibody (TuJ1; Babco, Richmond, CA; 1:500 dilution); anti-Pan Na channel antibody (S8809, Sigma; 1:200 dilution); anti-Na+/Ca2+ exchangers (NCX) antibodies R3F1 and W1C3 (gifts from H. Porzig, Univerität Bern, Bern, Switzerland; 1:100 and 1:25 dilutions, respectively); anti-NCX3 (gift from K. Philipson, University of California, Los Angeles; 1:100 dilution); or anti-glutamate antibody (mAb 2D7, Swant, Bellinzona, Switzerland; 1:500 dilution). Sections were washed in PBS plus 0.2% Triton X-100 and then incubated for 2 h with the appropriate secondary antibody (Alexa Fluor 488- or Alexa Fluor 546-conjugated (Molecular Probes) diluted in PBSTP (1:1,000 dilution). Sections were washed in PBS plus 0.2% Triton X-100 and mounted in Vectashield (Vector Laboratories). Images were acquired with a TCS-SP2 confocal setup (Leica, Deerfield, IL) and then merged in photoshop (version 7.0; Adobe Systems, San Jose, CA).
Glutamate, Aspartate, and GABA Assays. The 300-μm slices were microdissected to retain only the neocortical part. Batches of five such neocortex sections were placed in 1.5-ml tubes. All incubations were performed at room temperature. At t = 0, 100 μl of ACSF with or without 50 μM veratridine were added to each tube, with gentle shaking. We collected 10 μl of homogenate every 1 min for 5 min, and it was immediately frozen (-80°C). The concentrations of aspartate, glutamate, and GABA in the collected volumes were determined by HPLC with laser-induced fluorescence detection, as described in ref. 14. With this procedure, a 4-μl sample allows the simultaneous measurement of the concentrations of aspartate, glutamate, and GABA, with detection limits of 46, 42, and 60 pM, respectively (signal/noise = 3).
Results
Na+ Channels and Calcium Signaling. A subpopulation of the mouse neuronal cells appearing in the neocortex at E11-E13 and forming the PP zone, express tetrodotoxin (TTX)-sensitive Na+ channel (11). At E13, expression of this Na+ channel is restricted to the upper part of the PP zone (red staining in Fig. 1 A). Double staining with TuJ1 antibody directed against neuronal β-tubulin confirms that only some of the neuronal cells (green staining in Fig. 1 A) possess this Na+ channel.
Our preliminary observations indicated the presence of Ca2+ activity in mouse neocortex as early as E13. Given the essential role of the pioneer PP neurons in corticogenesis, we investigated the role of the Na+ channel in calcium signaling at this stage. Ca2+ imaging combined with automated individual cell analysis (see Materials and Methods) allow the simultaneous monitoring of hundreds of cells (237 ± 15 cells per slice, n = 16 slices). Application of 50 μM veratridine (a specific agonist of voltage-dependent Na+ channels) on E13 coronal slices during 60 s, triggers a large Ca2+ increase in the PP that extends to some VZ cells (Fig. 1 B and C). Because the Na+ channel is restricted to the upper part of the PP (Fig. 1 A), these data strongly suggest that there is also a Ca2+ rise in cells lacking the Na+ channel. Hence, veratridine may induce a direct effect on the Na+ channel-expressing cells, probably relayed by an indirect effect, which takes place in both PP and VZ.
Quantification and statistical analysis of responding cells show that there are significantly more PP cells responding to veratridine (58.0 ± 6.8%) than VZ cells (26.3 ± 4.7%; n = 16; P < 0.001; see Fig. 1D). Moreover, the amplitude of Ca2+ rises (expressed as F/F0 ratios) in PP cells is significantly higher than in VZ cells (109.4 ± 7.6 vs. 53.4 ± 3.4; n = 16; P < 0.001).
Preincubation of slices in ACSF-0 Na+ N-methyl-d-glucamine (data not shown) or in 1 μM TTX (Fig. 2A, 2.7 ± 0.3% responding cells vs. 58.0 ± 6.8% in ACSF, n = 6, P < 0.001) prevents the Ca2+ rise induced by veratridine. This set of data demonstrates that veratridine triggers an Na+ influx, via a voltage-dependent Na+ channel, which is responsible for the subsequent Ca2+ rise.
Fig. 2.
Na+/Ca2+ exchange is responsible for Na+-mediated Ca2+ signaling. (A) Percentage of responding PP cells after 60 s of 50 μM veratridine application in the presence of ACSF (control; n = 16 slices per 1,281 cells); TTX (1 μM TTX; n = 6 slices per 335 cells); Thapsi (2 μM thapsigargin; n = 15 slices per 1,242 cells); 0 mM Ca2+ (ACSF-0 mM Ca2+/1 mM EGTA; n = 4 slices per 240 cells); Ni2+ plus Cd2+ (200 μM NiCl2 plus 200 μM CdCl2; n = 3 slices per 201 cells); KB-R (1 μM KB-R7943; n = 6 slices per 655 cells). *, significantly different from control (vera). (B) Immunofluorescence labeling of each of the three Na+/Ca2+ exchanger subtypes (NCX1, NCX2, and NCX3) in green. NCX1 and NCX2 are coexpressed with Na+ channel staining (red) in most cells.
A Na+/Ca2+ Exchange Is Responsible for the Veratridine-Induced Ca2+ Rise. We first aimed at identifying the mechanism between Na+ channel activation and Ca2+ response. We previously showed that ryanodine receptor and inositol trisphosphate receptor are specifically present and functional in PP cells at E13 (15). Incubation of slices with 2 μM thapsigargin (a Ca2+ store-depleting agent) significantly decreases the number of PP cells that are activated by veratridine (30.3 ± 5.7% vs. 58.0 ± 6.8% in ACSF; n = 15; P < 0.05; Fig. 2 A) with no change in signal amplitude (99.9 ± 10.7 vs. 109.4 ± 7.6 in ACSF; n = 15). This observation suggests that Ca2+ stores may be partially involved in the Na+-induced Ca2+ rise but do not constitute the main route for veratridine-induced Ca2+ influx. However, removal of external Ca2+ from the bath (ACSF-0 mM Ca2+/1 mM EGTA) strongly prevents the veratridine-induced Ca2+ increase (7.3 ± 2.4% responding cells, n = 4, Fig. 2 A), suggesting that external Ca2+ is the primary source of Na+-induced Ca2+ rise.
Although voltage-dependent Ca2+ channels cannot be recorded electrophysiologically in the neocortex at E13 (8, 11), they may be present at a low density or only in thin cellular processes in which they could go undetected. In the presence of 200 μM Ni2+ + 200 μM Cd2+, broad-range potent inhibitors of voltage-dependent Ca2+ channels, veratridine elicits a response in 40.0 ± 14.8% of PP cells (n = 3), which is not significantly different from control (Fig. 2 A). Hence, the Ca2+ rise associated with Na+ channel opening is not mediated by voltage-dependent Ca2+ channels.
Alternatively, Ca2+ may enter the cell via a Na+/Ca2+ exchanger. Such exchangers are involved in the regulation of Ca2+ homeostasis by exchanging three Na+ ions for one Ca2+ ion and may operate in either direction (16). They are known to be present in differentiating neurons in rat embryonic neocortex, where they contribute to the maintenance of basal intracellular Ca2+ concentration ([Ca2+]i) levels (17, 18). We investigated whether a Na+/Ca2+ exchanger could be involved in the veratridine-induced Ca2+ rise, by incubating slices with a reverse mode Na+/Ca2+ exchanger antagonist, KB-R7943, at a concentration of 1 μM (19). In these conditions, veratridine failed to induce Ca2+ increase in PP cells (2 ± 1% responding cells vs. 58.0 ± 6.8%; n = 6; P < 0.001, Fig. 2 A, KB-R). This complete inhibition suggests that the observed Ca2+ influx is mediated primarily by Na+/Ca2+ exchangers. These observations were confirmed using benzamil, which is another potent inhibitor of the reverse mode of the brain Na+/Ca2+ exchanger, binding to the exclusive Na+ transport site (20). Benzamil (100 μM) also prevents veratridine-induced Ca2+ response (3.0 ± 0.8% responding cells vs. 58.0 ± 6.8%; n = 4; P < 0.001; data not shown). Upon veratridine stimulation, Na+ accumulates in the cytosolic compartment and exchanges for external Ca2+, leading to Ca2+ increase in cells expressing voltage-gated Na+ channels.
Double-staining experiments with anti-Na+ channel antibodies together with anti-NCXs antibodies (21) directed against the three identified subtypes of the Na+/Ca2+ exchanger (NCX1, NCX2, and NCX3) in cortical slices show that Na+ channels, NCX1 and NCX2 are expressed in the same subpial area of the PP zone (Fig. 2B) and that most of the cells coexpress the Na+ channel and the exchangers. The NCX3 exchanger is even more confined to the very upper PP cell layer. Thus, these observations are in favor of a functional partnership between Na+ channels and Na+/Ca2+ exchangers in the corresponding neurons of the PP zone.
Na+ Channel Activation Leads to Exocytosis in PP Cells. Expression of Na+/Ca2+ exchangers and Na+ channels is restricted to the external part of the PP (Fig. 2B). Therefore, they cannot be involved directly in the Ca2+ rise occurring in cells of the inner PP and VZ. Therefore, we hypothesized that veratridine-triggered Ca2+ influx in Na+ channel-expressing PP cells may induce the release of a diffusible messenger responsible for the Ca2+ rise observed in VZ and more internal PP cells. We used the fluorescent dye FM1-43 to investigate a possible role of veratridine in exocytosis (for review, see ref. 13). Addition of 2 μM FM1-43 in the perfusion medium of slices results in faint staining of the whole slice (Fig. 3 A Left and B, ACSF). To quantify the fluorescence increase, we calculated the fluorescence signal ratio at 2 min after addition of FM1-43 vs. the initial fluorescence level (t = 0). In the control condition, the level of FM1-43 staining shows a 62.7 ± 3.1% increase in the PP (Fig. 3B, n = 4). This basal staining depends on external Ca2+, because it is totally abolished with prior incubation in ASCF-0 mM Ca2+/1 mM EGTA (data not shown). Hence, spontaneous FM1-43 fluorescence increase is probably due to spontaneous basal exocytosis. Application of 50 μM veratridine at 1 min after FM1-43 perfusion increased the intensity of FM1-43 staining in the whole PP zone (Fig. 3 A Right and B; 242.8 ± 58.1%; n = 8), indicating the occurrence of a significantly larger exocytosis than in ACSF conditions (P < 0.01; Fig. 3B). This massive exocytosis was completely inhibited by addition of 1 μM KB-R7943 in the bathing medium (14.0 ± 7.1%; P < 0.001; n = 3; Fig. 3B), indicating that Ca2+ influx after Na+ channel activation is crucial to exocytosis in PP cells. Also, we found that the spontaneous fluorescence increase is smaller in the presence of KB-R7943 than in ACSF (P = 0.057), suggesting that the Na+/Ca2+ exchanger may also be involved in spontaneous exocytosis in E13 neocortical slices.
Fig. 3.
Veratridine-mediated Na+ channel activation induces exocytosis in PP cells. (A) FM1-43 fluorescence in slices treated with veratridine. Left shows the basal fluorescence at 30 s after the beginning of FM1-43 perfusion. Right shows the same slice at 1 min after application of 50 μM veratridine. (B) FM1-43 fluorescence increase in the whole PP measured 120 s after the beginning of the FM1-43 perfusion. KB-R7943 is preincubated 2 min before and during FM1-43 perfusion. (*, significantly different from ACSF; #, significantly different from veratridine). (C) Residual fluorescence after a 5-min wash of external FM1-43 measured after 1 min in ACSF or in 50 μM veratridine.
To confirm that the FM1-43 fluorescence increase described above was due to exocytosis, we removed FM1-43 from the external medium after 5 min of incubation. The application of 50 μM veratridine then significantly decreased fluorescence, demonstrating the washing off of internalized FM1-43 [20.7 ± 4.6% decrease 1 min after veratridine application (n = 5) compared with 0% decrease (n = 5) in ACSF; P < 0.05; Fig. 3C]. Thus, these data demonstrate that stimulation of the Na+ channel with 50 μM veratridine triggers exocytosis in PP cells. This phenomenon depends on external Ca2+ and requires the involvement of Na+/Ca2+ exchange.
Na+ Channel Stimulation in PP Cells Triggers Glutamate Secretion. At E13, synapses have not been formed yet (22), and gap junctions are not present in differentiated neuronal cells (8, 23), suggesting that Ca2+ increases in cells devoid of Na+ channels probably involve extracellular signaling. GABA and glutamate are good candidates because (i) they are present in the mouse neocortex as early as E10 (4), (ii) they both lead to a Ca2+ increase (5), and (iii) they are known to be involved in early corticogenesis (24).
Thus, we quantified glutamate and GABA levels by HPLC measurements in the bathing medium of slices that were exposed to veratridine. Microdissected neocortices were used in these experiments, and external medium samples were collected every minute. Glutamate secretion into the medium considerably increases after incubation with 50 μM veratridine up to 3 min after application (1,198 ± 533% vs. 119 ± 29% in ACSF; P < 0.01; n = 4 and 11, respectively). In the same samples, there was no significant difference in GABA levels (nor in aspartate levels; data not shown) between ACSF and veratridine treatments (Fig. 4A).
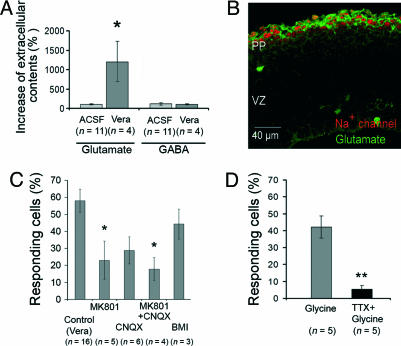
Fig. 4.
Na+ channel activation leads to glutamate secretion and can be physiologically activated by GlyRs. (A) HPLC measurement of extracellular glutamate and GABA from neocortices incubated in 50 μM veratridine vs. ACSF for 3 min. All results are expressed as percentage of measures done at t = 0. (B) Glutamate staining (green) in E13 cortex slices coincides with the Na+ channel staining (red) in the PP. (C) Percentage of responding PP cells after 60 s of 50 μM veratridine application in the presence of ACSF (control; n = 16 slices and 1,281 cells); CNQX (10 μM; n = 6 slices and 454 cells), MK801 (100 μM; n = 5 slices and 422 cells); CNQX+MK801 (n = 4 slices and 337 cells); BMI (20 μM; n = 3 slices and 230 cells). *, significantly different from control (vera). (D) Percentage of responding PP cells after 60 s of 2 mM glycine application in ACSF (control, n = 5 slices and 274 cells) or in presence of 1 μM TTX (n = 5 slices and 317 cells).
The presence of glutamate at this stage of development was confirmed by immunohistochemical data. High levels of glutamate were detected in the PP of E13 slices (Fig. 4B; see also ref. 6), and glutamate staining in the PP coincides with staining of Na+ channel (Fig. 4B).
NMDA Receptor (NMDAR) and α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) Receptor (AMPAR) Relay the Veratridine-Induced Ca2+ Rise. AMPA/kainate and NMDAR are expressed in mouse neocortex (5, 6). To check whether released glutamate could propagate the veratridine-induced Ca2+ rise, we applied veratridine in the presence of glutamate receptor antagonists. Addition of 10 μM CNQX (to block AMPARs) depressed the veratridine-induced Ca2+ response (Fig. 4C; 28.8 ± 7.9% responding cells vs. 58.0 ± 6.8% in ACSF, n = 6; P = 0.051). Blocking NMDAR with 100 μM MK801 significantly inhibited the veratridine-induced Ca2+ increase in PP cells (23.0 ± 11.2% vs. 58.0 ± 6.8% in ACSF, n = 5; P < 0.05). These results suggest that both NMDAR and AMPAR are involved in the Ca2+ response. Concomitant application of CNQX and MK801 does not trigger an additional effect on the percentage of residual active PP cells. Both inhibitors also affect the signal amplitude of the remaining PP responding cells (47.2 ± 10.8 in CNQX+MK801 vs. 109.4 ± 7.6 in ACSF; P < 0.01), suggesting an autocrine effect on the initially activated cells, which possess (i) Na+ channels, (ii) Na+/Ca2+ exchangers, and (iii) NMDAR and/or AMPAR. Last, the tested inhibitors also significantly reduce the number of active VZ cells (11.5 ± 2.4% vs. 26.3 ± 4.7% in ACSF; P < 0.05) and the signal amplitude in the remaining (34.5 ± 6.1 vs. 53.4 ± 3.4 in ACSF; P < 0.05), suggesting the involvement of NMDAR and AMPAR in the veratridine-induced Ca2+ signal recorded in the VZ. Metabotropic glutamate receptors seem to be poorly involved at this stage because l-AP3 (50 μM) had no inhibitory effect on the veratridine-induced Ca2+ signal (data not shown).
GABAARs are also expressed in mouse neocortical cells, and their activation can lead to a Ca2+ increase (5), which can be blocked by bicuculline methiodide (BMI). In agreement with the absence of GABA release induced by veratridine, BMI (20 μM) does not inhibit the veratridine-induced Ca2+ rise (Fig. 4C), as assessed by the absence of significant effect on the number of active PP cells (44.3 ± 8.8% in BMI vs. 58.0 ± 6.8% in ACSF; n = 3) and on the signal amplitude (92.6 ± 7.1 in BMI vs. 109.4 ± 7.6 in ACSF; n = 3; data not shown).
Taurine and Glycine Acting on Glycine Receptors (GlyRs) Are Possible Physiological Activators of Na+ Channels. To assess the physiological relevance of the pathway described above, we look to the possibility of physiological activators of the Na+ channel in PP cells. Glycine and/or taurine appear as potential candidates because they are abundantly present in embryonic neocortex (7, 25), together with their target receptor, the GlyR (25). Activation of GlyRs during embryonic stages is excitatory, leading to membrane depolarization (25), which can, in turn, activate Na+ channels.
Application of 2 mM glycine (or 2 mM taurine) induces a Ca2+ increase in PP cells (42.2 ± 6.6% responding cells; n = 5; Fig. 4D). This effect is totally blocked by 20 μM strychnine but not by 20 μM bicuculline, indicating the specific activation of GlyRs (data not shown). This Ca2+ rise implies activation of the Na+ channels because it is totally blocked by the concomitant application of 1 μM TTX (5.4 ± 2.2% vs. 42.2 ± 6.6% responding cells; P < 0.005; n = 5; Fig. 5). This result indicates that depolarization induced by GlyRs stimulation triggers Na+ channels activation, leading to Ca2+ rise. Glycine also induces a Ca2+ increase in VZ cells (24.2 ± 6.7% responding cells; n = 5), an effect similar to that produced by veratridine (26.3 ± 4.7%; n = 16). Most important, the effect of glycine on VZ cells is blocked by TTX (9.0 ± 1.4%; P < 0.05; n = 5; data not shown), indicating that GlyR activation is sufficient to trigger both the activation of PP cells that express Na+ channels and the propagation of the Ca2+ signal to other cells in a manner that is similar to that observed with direct activation of the Na+ channels with veratridine.
Fig. 5.
Schematic representation of the chain reaction triggered by activation of PP neurons Na+ channels in E13 neocortex. 1, Stimulation of the Na+ channel results in a transient influx of Na+. 2, Exchange of this Na+ with extracellular Ca2+ via a Na+/Ca2+ exchanger induces a Ca2+ rise in the corresponding PP neurons. 3, The Ca2+ increase triggers exocytosis in these cells. 4, Exocytosis leads to glutamate secretion in the extracellular medium. 5, Glutamate activates cells in the PP and VZ, through autocrine and/or paracrine communication mechanisms. This pathway results in Ca2+ increase in a larger neocortical wall cell population. GlyR may be the primary Na+ channel activator in vivo.
Discussion
In mice, voltage-dependent Na+ channels appear very early in development, in the first generated neocortical neurons at least 2 days before the expression of voltage-dependent Ca2+ channels (8, 11). In this article, we show that stimulation of the PP Na+ channels by veratridine induces Ca2+ signaling via a Na+/Ca2+ exchanger. The Ca2+ rise resulting from the activation of Na+ channels leads to exocytosis in PP cells and glutamate secretion. The resulting paracrine stimulation extends the Ca2+ signal to neocortical cells devoid of Na+ channels.
Veratridine is a plant alkaloid that specifically activates Na+ channels at the concentration that we used (50 μM), inducing a state of low conductance, which is associated with a lack of inactivation of Na+ channel (12, 26). In the neocortex of E13 mice, veratridine induces a Ca2+ rise, which depends mostly on external Ca2+. However, voltage-dependent Ca2+ channels are not yet expressed at E13 (8, 11). It can be hypothesized that depolarization resulting from Na+ entry might release the block of NMDA in the presence of ambient glutamate, which is not the case because NMDAR antagonists only partially inhibit the response to veratridine. Hence, Na+/Ca2+ exchange may be responsible for Ca2+ influx. The Na+/Ca2+ exchanger is known to regulate intracellular Ca2+ ion concentration in neurons (27, 28) even if its role in Ca2+ entry may be minor in regard to that of voltage-dependent Ca2+ channels (29). KB-R7943 is a thiourea derivative that selectively blocks the reverse mode of the Na+/Ca2+ exchanger without affecting other ion transporters at concentrations of ≤30 μM (19). This drug completely inhibits the veratridine-induced Ca2+ rise in PP cells at E13. Specific implication of the exchanger is also confirmed by the use of benzamil (20), another selective and potent inhibitor of the Na+/Ca2+ exchangers. Immunofluorescence double stainings demonstrate that NCXs and Na+ channels are located in the same PP cell population, thus confirming their potential partnership. Thus, in these cells, Na+ accumulated in response to veratridine is exchanged with external Ca2+, leading to Ca2+ influx. Intracellular Ca2+ stores are known to be functional in the neocortex at this stage (15). Thapsigargin partially blocks the veratridine effect, indicating that intracellular stores may contribute to signal amplification, probably by means of calcium-induced calcium release. Alternatively, thapsigargin-induced Ca2+ release could activate the Na+/Ca2+ exchanger, leading to Ca2+ efflux and Na+ influx (30), which would account for a decreased effect of veratridine in the presence of thapsigargin.
The spatial distribution of Na+ channels and Na+/Ca2+ exchangers is consistent with functional recordings of INa in the PP (11), but is far more limited than the widespread Ca2+ response to veratridine. This observation suggests that there might be an additional and indirect mechanism involved in the spreading of the response. At this early stage of neurogenesis, synapses have not yet formed (22), and gap junctions are not present in differentiated neuronal cells (8, 23). Radial glial cells could take part in communication between PP and VZ cells as it was demonstrated later in development (31). At E13, some of the PP pioneer cells send processes toward the VZ (32). Cells may also communicate by paracrine means as reported in immature CA1 pyramidal neurons before synapse formation (33). Our pharmacological studies, fluorescence visualization of exocytosis, immunolabeling, and direct glutamate measurements provide converging evidence that Na+ channel stimulation leads to glutamate secretion from PP cells and that this glutamate contributes to the Ca2+ signal observed in PP and VZ cells.
It is widely accepted that Na+ channels must open before they can bind veratridine (12), assuming that Na+ channels are basically and physiologically active at E13 and opening the question of the identity of a physiological activator of the Na+ channel in PP cells. GABA and glycine are both excitatory transmitters during development (25, 34). In early mouse neocortex, GlyRs are known to be physiologically activated by nonsynaptically released taurine (25) and GABAARs are expressed in embryonic Cajal-Retzius cells (35). We show that GlyRs activation leads to a Ca2+ rise through a TTX-dependent pathway, thereby implicating Na+ channels. Interestingly, 100 μM muscimol also induced a Ca2+ rise, which is not prevented in the presence of TTX and, therefore, does not involve Na+ channel activation (data not shown). Moreover, Ca2+ signaling that is activated by glycine spreads to other cells in PP and VZ in a manner that is undistinguishable to that evoked by veratridine. At E13, glycine and taurine contents are ≥10-fold higher than GABA in neocortex (7), consistent with a major GlyR implication during cortical development. These observations underline the physiological relevance of the pathway described in this work, setting GlyRs as potential actors implicated in early Na+ channel activation, leading to the whole cascade of events described here.
This pathway is shown in summary in Fig. 5. GlyR-dependent membrane depolarization or stimulation by veratridine induces a Na+ entry into PP pioneer cells expressing a Na+ channel. In these cells, Na+ exchanges for Ca2+ via a Na+/Ca2+ exchanger. The increase in intracellular Ca2+ triggers the release of vesicular glutamate into the extracellular medium. In turn, glutamate activates other PP and VZ cells, through paracrine, and possibly autocrine, communication. The absence of voltage-dependent Ca2+ channel expression at this developmental stage makes it possible to demonstrate unambiguously the direct involvement of the Na+/Ca2+ exchanger in Na+ channel-mediated Ca2+ signaling. This is direct evidence for the involvement of a Na+/Ca2+ exchanger in Na+-dependent exocytosis.
Patterns of electrical and Ca2+ activity have a crucial significance in neurotransmitter expression in embryonic neurons (36). Consistently, our work shows that Na+ channels emerge as soon as the first neurons are generated and that their immature activity controls neurotransmitter secretion in neocortex. Secreted neurotransmitters may then serve to regulate morphogenetic events, such as proliferation, growth, migration, differentiation, and survival of neural precursor cells (24, 37). In mice, it has been shown that glutamate, distributed as a gradient in the neocortical wall from the pia to the VZ, stimulates proliferation in the VZ (4). As reported for mouse cerebellar granule cell migration (38, 39), glutamate probably also acts as a neuronal chemoattractant in neocortex, as well as in cultured murine embryonic cortical cells (6). Moreover, this putative glutamate role would imply that both proliferative and neuronal migrating cells could potentially be involved in the Ca2+ signal recorded in the VZ.
Hence, before generating action potentials, Na+ channels may carry out unusual functions in specific neuronal cells, perhaps in the interim before voltage-dependent Ca2+ channel expression. Preliminary evidence suggests that Na+ channels are involved in spontaneous Ca2+ activity at E13 (J.-C.P. and M.A., unpublished data), suggesting a physiological role for Na+ channels in both Ca2+ activity and cellular communication. Characterizing this role and the involved mechanisms may be a necessary step to fully unraveling the functional implication of PP cells in cortical embryonic development.
Acknowledgments
We thank Prof. Bill Moody for introduction in the brain development field and stimulating discussions, Prof. Nick Spitzer and Dr. Nicolas Hussy for valuable suggestions and incisive discussions, Dr. M. De Waard for unvaluable support, and Profs. H. Porzig and K. Philipson for providing anti-NCXs antibodies. This work was supported by the University Joseph Fourier, the Commissariat à l'Energie Atomique (Grenoble), and the Institut National de la Santé et de la Recherche Médicale. J.-C.P. was supported by an Emergence fellowship from the Rhône-Alpes Region.
Author contributions: J.-C.P., A.D., J.B., M.V., and M.A. designed research; J.-C.P., S.B., A.P., M.S., and M.A. performed research; J.-C.P., S.B., A.D., J.B., M.S., and M.A. analyzed data; and J.-C.P., A.D., J.B., M.V., and M.A. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACSF, artificial cerebrospinal fluid; PP, preplate; VZ, ventricular zone; GlyR, glycine receptor; En, embryonic day n; TTX, tetrodotoxin; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AMPAR, AMPA receptor; NMDAR, NMDA receptor; NCX, Na+/Ca2+ exchanger; BMI, bicuculline methiodide.
References
- 1.McConnell, S. K. (1995) Neuron 15, 761-768. [DOI] [PubMed] [Google Scholar]
- 2.Marin-Padilla, M. (1998) Trends Neurosci. 21, 64-71. [DOI] [PubMed] [Google Scholar]
- 3.Spitzer, N. C., Kingston, P. A., Manning, T. J. & Conklin, M. W. (2002) Curr. Opin. Neurobiol. 12, 315-323. [DOI] [PubMed] [Google Scholar]
- 4.Haydar, T. F., Wang, F., Schwartz, M. L. & Rakic, P. (2000) J. Neurosci. 20, 5764-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LoTurco, J. J., Owens, D. F., Heath, M. J., Davis, M. B. & Kriegstein, A. R. (1995) Neuron 15, 1287-1298. [DOI] [PubMed] [Google Scholar]
- 6.Behar, T. N., Scott, C. A., Greene, C. L., Wen, X., Smith, S. V., Maric, D., Liu, Q. Y., Colton, C. A. & Barker, J. L. (1999) J. Neurosci. 19, 4449-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benitez-Diaz, P., Miranda-Contreras, L., Mendoza-Briceno, R. V., Pena-Contreras, Z. & Palacios-Prü, E. (2003) Dev. Neurosci. 25, 366-374. [DOI] [PubMed] [Google Scholar]
- 8.Picken-Bahrey, H. L. & Moody, W. J. (2003a) Cereb. Cortex 13, 239-251. [DOI] [PubMed] [Google Scholar]
- 9.Picken Bahrey, H. L. & Moody, W. J. (2003b) J. Neurophysiol. 89, 1761-1773. [DOI] [PubMed] [Google Scholar]
- 10.Corlew, R., Bosma, M. M. & Moody, W. J. (2004) J. Physiol. 560, 377-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albrieux, M., Platel, J. C., Dupuis, A., Villaz, M. & Moody, W. J. (2004) J. Neurosci. 24, 1719-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulbricht, W. (1998) Rev. Physiol. Biochem. Pharmacol. 133, 1-54. [DOI] [PubMed] [Google Scholar]
- 13.Cochilla, A. J., Angleson, J. K. & Betz, W. J. (1999) Annu. Rev. Neurosci. 22, 1-10. [DOI] [PubMed] [Google Scholar]
- 14.Windels, F., Bruet, N., Poupard, A., Urbain, N., Chouvet, G., Feuerstein, C. & Savasta, M. (2000) Eur. J. Neurosci. 12, 4141-4146. [DOI] [PubMed] [Google Scholar]
- 15.Faure, A. V., Grunwald, D., Moutin, M. J., Hilly, M., Mauger, J. P., Marty, I., De Waard, M., Villaz, M. & Albrieux, M. (2001) Eur. J. Neurosci. 14, 1613-1622. [DOI] [PubMed] [Google Scholar]
- 16.Blaustein, M. P. & Lederer, W. J. (1999) Physiol. Rev. 79, 763-854. [DOI] [PubMed] [Google Scholar]
- 17.Maric, D., Maric, I., Chang, Y. H. & Barker, J. L. (2000a) Cereb. Cortex 10, 729-747. [DOI] [PubMed] [Google Scholar]
- 18.Maric, D., Maric, I. & Barker, J. L. (2000b) Cereb. Cortex 10, 561-573. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto, T., Watano, T. & Shigekawa, M. (1996) J. Biol. Chem. 271, 22391-22397. [DOI] [PubMed] [Google Scholar]
- 20.Smith J. P., Cunningham, L. A. & Partridge, L. D. (2000) Brain Res. 887, 98-109. [DOI] [PubMed] [Google Scholar]
- 21.Thurneysen, T., Nicoll, D. A., Philipson, K. D. & Porzig, H. (2002) Mol. Brain Res. 107, 145-156. [DOI] [PubMed] [Google Scholar]
- 22.Molnar, Z. & Blakemore, C. (1995) Trends Neurosci. 18, 389-397. [DOI] [PubMed] [Google Scholar]
- 23.Lo Turco, J. J. & Kriegstein, A. R. (1991) Science 252, 563-566. [DOI] [PubMed] [Google Scholar]
- 24.Cameron, H. A., Hazel, T. G. & McKay, R. D. (1998) J. Neurobiol. 36, 287-306. [PubMed] [Google Scholar]
- 25.Flint, A. C., Liu, X. & Kriegstein, A. R. (1998) Neuron 20, 43-53. [DOI] [PubMed] [Google Scholar]
- 26.Romey, G. & Lazdunski, M. (1982) Nature 297, 79-80. [DOI] [PubMed] [Google Scholar]
- 27.Mulkey, R. M. & Zucker, R. S. (1992) J. Neurosci. 12, 4327-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch, R. A. & Barish, M. E. (1994) J. Neurosci. 14, 2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meder, W., Fink, K., Zentner, J. & Gothert, M. (1999) J. Pharmacol. Exp. Ther. 290, 1126-1131. [PubMed] [Google Scholar]
- 30.Chernaya, G., Vasquez, M. & Reeves, J. P. (1996) J. Biol. Chem. 271, 5378-5385. [DOI] [PubMed] [Google Scholar]
- 31.Weissman, T. A., Riquelme, P. A., Ivic, L., Flint, A. C. & Kriegstein, A. R. (2004) Neuron 43, 647-661. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, G., Soria, J. M., Martinez-Galan, J. R., Martin-Clemente, B. & Fairen, A. (1998) J. Comp. Neurol. 397, 493-518. [PubMed] [Google Scholar]
- 33.Demarque, M., Represa, A., Becq, H., Khalilov, I., Ben-Ari, Y. & Aniksztejn, L. (2002) Neuron 36, 1051-1061. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Ari, Y., Cherubini, E., Corradetti, R. & Gaiarsa, J. L. (1989) J. Physiol. 416, 303-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mienville, J. M. (1998) J. Physiol. 512, 809-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borodinsky, L. N., Root, C. M., Cronin, J. A., Sann, S. B., Gu, X. & Spitzer, N. C. (2004) Nature 429, 523-530. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen, L., Rigo, J. M., Rocher, V., Belachew, S., Malgrange, B., Rogister, B., Leprince, P. & Moonen, G. (2001) Cell. Tissue Res. 305, 187-202. [DOI] [PubMed] [Google Scholar]
- 38.Komuro, H. & Rakic, P. (1993) Science 260, 95-97. [DOI] [PubMed] [Google Scholar]
- 39.Rossi, D. J. & Slater, N. T. (1993) Neuropharmacology 32, 1239-1248. [DOI] [PubMed] [Google Scholar]