Abstract
The most devastating aspect of cancer is the emergence of metastases. Thus, identification of potentially metastatic cells among a tumor cell population and the underlying molecular changes that switch cells to a metastatic state are among the most important issues in cancer biology. Here we show that, although normal human colonic epithelial cells lack the glycosphingolipid globotriaosylceramide (Gb3), this molecule is highly expressed in metastatic colon cancer. In addition, a subpopulation of cells that are greatly enriched in Gb3 and have an invasive phenotype was identified in human colon cancer cell lines. In epithelial cells in culture, Gb3 was necessary and sufficient for cell invasiveness. Transfection of Gb3 synthase, resulting in Gb3 expression in noncancerous polarized epithelial cells lacking endogenous Gb3, induced cell invasiveness. Furthermore, Gb3 knockdown by small inhibitory RNA in colon cancer epithelial cells inhibited cell invasiveness. Gb3 is the plasma membrane receptor for Shiga toxin 1. The noncatalytic B subunit of Shiga toxin 1 causes apoptosis of human colon cancer cells expressing Gb3. Injections of the B subunit of Shiga toxin 1 into HT29 human colon cancer cells engrafted into the flanks of nude mice inhibited tumor growth. These data demonstrate the appearance of a subpopulation of Gb3 containing epithelial cells in the metastatic stage of human colon cancer and suggest their possible role in colon cancer invasiveness.
Keywords: metastases, invasion, filopodia
Colorectal cancer is the second leading cause of cancer death in the U.S. (1, 2). The high mortality associated with colorectal cancer is related to its ability to spread beyond the large intestine and to invade distant organs. One route to improve understanding of the molecular mechanisms of metastasis is by the identification of genes that are differently expressed during cancer progression and/or are responsible for acquisition of the metastatic phenotype. Although many molecular factors have been identified as contributing to metastases and represent potential targets for treatment (3), much remains to be learned about the biology of the metastatic process. Aberrant glycosylation, which has been observed in essentially all types of human cancers during cancer progression into the metastatic stage, is an example of a metastases-related change that is based, in part, on altered gene expression (4).
Aberrant glycosylation due to tumor transition into an invasive stage is also associated with changes in glycosphingolipid (GSL) composition. GSLs are abundant in the outer leaflet of plasma membranes of nearly all eukaryotic cells and have many diverse functions (5, 6). GSLs serve as receptors for viral and bacterial toxins, and microbial infections may be mediated by interactions of host GSLs with microbial membrane proteins. GSLs at the cell surface modulate transmembrane signal transduction by influencing protein kinases associated with growth factor receptors and PKC. GSL-GSL interactions and formation of so-called glycosynapses appear to provide the basis for a specific cell recognition system independent of the fibronectin/integrin or surface lectin systems (7). Surprisingly large numbers of tumor-associated antigens have been identified as GSLs. Altered GSL structures and cell surface expression patterns are associated with invasive phenotypes of some experimental tumors (7). Thus, it is well established that GM3 inhibits cell motility and invasiveness in bladder tumors (8). Gangliosides Gt1b, GD1A, GM3, and GM1 inhibit cell proliferation and epidermal growth factor receptor tyrosine phosphorylation (9, 10), whereas depletion of Gt1b and GM3 by sialidase overexpression facilitates epidermal growth factor receptor phosphorylation and cell migration (10). Oppositely, a different GSL, Gb5, strongly enhances motility of breast cancer cells (11).
We have now identified a form of abnormal glycosylation that correlates with the development of human colon cancer metastasis. Globotriaosylceramide (Gb3) is expressed in metastatic colon cancer and is virtually absent from normal colonic epithelial cells. We found that Gb3-expressing colon cancer cells represent a potentially invasive subpopulation. Molecular manipulation to increase Gb3 expression converts noninvasive epithelial cells into cells with an invasive phenotype, and the molecular alterations that lead to Gb3 up-regulation appear to be sufficient for this transformation. To explore the role of Gb3 in the growth of human colon cancer cell lines established from metastatic lesions, advantage was taken of the fact that Gb3 serves as a receptor (12, 13) for the noncatalytic B subunit of Shiga toxin 1 (Stx1B). As in other cells (14), the selective binding and uptake of the Stx1B by Gb3-positive Caco-2 cells is shown to cause apoptosis. We also show that intratumoral injection of Stx1B inhibited colonic tumor growth in the nude mouse model.
Materials and Methods
Cell Culture and Human Tissue. Caco-2, T84, OK, and HT29 cells were from the American Type Culture Collection. Samples of archived frozen human tissue included normal distal and proximal colon (n = 15), nonmalignant colonic adenomas (n = 3), colon cancer without metastases (n = 3), primary lesions of metastatic colon cancer (n = 5), and colon cancer metastases in liver (n = 2). This study was exempted under the Code of Federal Regulations Title 45 Section 46.101(b) by the Hopkins Institution Review Board.
Fluorescence Microscopy. To evaluate the distribution of Gb3, cells grown on glass coverslips were incubated for 1 h with 0.5 μg/ml Stx1B-Alexa Fluor 488 (Stx1B-488). All experiments, except immunofluorescence, were performed on living cells mounted in a perfusion chamber (Warner Instruments, Hamden, CT) with Hepes buffer, pH 7.4, at 37°C on the microscope stage, as described (Supporting Materials and Methods, which is published as supporting information on the PNAS web site) (15). The quantification of the relative amount of Gb3 in cells and tissue samples is described in detail in Supporting Materials and Methods.
Chemoinvasive Assay. Chemoinvasion was assayed in cell culture chambers by using 24-mm Transwell inserts with 8-μm pore membranes (Corning) as described in ref. 16. The membrane bottoms were precoated with 1 μg/ml laminin. Caco-2 cells or nontransformed renal proximal tubule OK cells were seeded on the top surface of the filters. After incubation for 24, 48, or 72 h, cells on both sides of the membrane were stained, and 0.5-μm confocal optical sections were taken through the whole membrane thickness with a ×100 objective lens. The distribution of cells on the top and bottom surfaces of the membrane and numbers of cells were analyzed with metamorph and volocity (Improvision, Lexington, MA) image processing software. Five fields were examined in each membrane.
Gb3 Synthase Knockdown. To affect the silencing of a specific gene, we used the pSUPER vector ligated with small inhibitory RNA (siRNA) oligonucleotides (17). By using the oligoengine siRNA design tool (OligoEngine, Inc., Seattle), five Gb3 synthase cDNA coding regions suitable for RNA interference were chosen and DNA oligos were obtained. Caco-2 cells are difficult to transfect with high efficiency by using lipophylic agents. To overcome this problem, an adenoviral vector was constructed in collaboration with the Johns Hopkins Adenoviral Preparation, Purification, and Titering Core Facility, in which the pSUPER siRNA shuttle vector with cDNA-GFP (to monitor the efficiency of viral infection) was ligated. Exposure of ≈50% confluent Caco-2 cells to 1.3 × 1010 particles per ml of adenovirus led to 50% GFP-positive cells, and this percentage did not increase significantly with higher doses of the virus. The Gb3 synthase cDNA oligo forward and reverse sequences gatccccCTTCCTGTTCATGTGTGCTCGttcaagagaCGAGCACATGAACAGGAAGtttttggaaa and agcttttccaaaaaCTTCCTGTTCATGTGCTCGtctcttgaaCGAGCACATGAACAGGAAGggg were the most effective in terms of inhibition of Gb3 synthesis, which was monitored by both specific Stx1B binding and CD77 mAb. The monolayers were inspected by fluorescence microscopy, and all GFP-positive cells were Gb3-free by 48 h after infection.
For siRNA control experiments, pSUPER vector with scrambled RNA (scRNA) duplex (catalog no. D-1200-05, Dharmacon Research, Layfayette, CO) was engineered. Caco-2 cells were infected with 1 × 1010 to 2 × 1010 particles per ml scRNA adenovirus. After 48 h, cells were used for high-performance thin-layer chromatography (HPTLC) to assess the changes in Gb3 content or for protein expression assay by Western blot.
Laser Capture Microdissection (LCM), GSLs Extraction, and HPTLC. To collect a cancer cell population enriched in epithelial cells, the tissue samples were hematoxylin stained, vacuum dried, and subjected to LCM (P.A.L.M. Microlaser Technologies, Bernried, Germany) by following manufacturer's protocols. To detect Gb3 in cancer epithelial cells, the LCM collected cells from two cases of nonmetastatic and two cases of metastatic colon cancers or from Caco-2 cells infected either with small inhibitory Gb3 or scRNA were subjected to HPTLC as described in detail (Supporting Materials and Methods) (18-20).
Nude Mouse Xenograft Model. The growth of HT29 human colonic cancer cells injected into the flanks of nude mice was determined with or without intratumor injection of a fixed amount of Stx1B administered every 2 days starting 7 days after tumor injection. Initially, effects of s.c. flank injections of various amounts of Stx1B were determined on nude mouse mortality and on liver, small intestine, and kidney histology at the light microscopy level. All experiments were conducted according to a protocol approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee.
Statistics. Data are presented as mean ± SEM. Significance was determined by using Student's t test, and P ≤ 0.05 were considered statistically significant.
Results
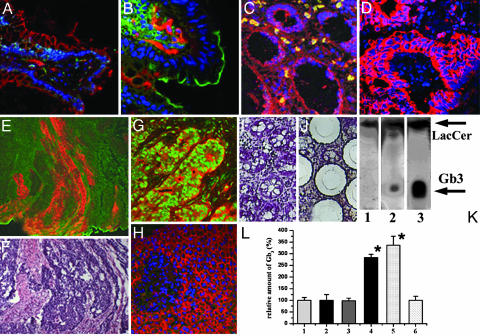
Gb3 Is Expressed in Colon Cancer Metastases but Not in Normal Colonic Tissue, Noncancer Adenomas, and Nonmetastatic Colon Cancer. To begin characterizing which types of colon cancer express Gb3, immunofluorescence staining was performed with CD77 Ab against Gb3 or fluorescently labeled Stx1B, which binds specifically to Gb3 (21). Gb3 was not expressed in normal colonic epithelial cells (Fig. 1 A and B), nonmalignant colonic adenomas (Fig. 1C), or nonmetastatic primary colon cancers (Fig. 1D). In contrast, as a positive control, another GSL GM1, the cholera toxin B subunit receptor, was readily detectable on the apical surface of colonocytes (Fig. 1B). Gb3-positive cells were present in some cells in the lamina propria (Fig. 1 A and B) and in erythrocytes (Fig. 1C). In contrast, high levels of Gb3 were expressed in epithelial cells in primary lesions of metastatic colon cancers and in the colon cancer metastases to the liver (Fig. 1 E-G). There was no detectable level of Gb3 in normal hepatocytes (Fig. 1H). To confirm the data gained by immunofluorescence, cancer cells from primary lesions of nonmetastatic colon cancer (Fig. 1 I and J) or from metastatic samples were collected by LCM. LCM was done to decrease contamination by erythrocytes, endothelial cells, or inflammatory cells, which are known to express Gb3 (22, 23) (Fig. 1). Collected cells were subjected to HPTLC, which showed the presence of Gb3 in metastatic colon cancer samples (Fig. 1K) but its virtual absence in nonmetastatic samples. In contrast, lactoceramide (LacCer), a precursor for many GSLs, including Gb3, was present in both types of samples. The relative amount of Gb3 in tissue samples was estimated from immunofluorescence images of tissue double-labeled with Stx1B or CD77 mAb to detect the Gb3 and pankeratin mAb and to ensure that exclusively epithelial cells were included in the analysis. Quantification of Gb3 fluorescence intensity, which corresponds to the relative amount of Gb3 in the sample, showed that Gb3 is up-regulated in primary lesions of metastatic colon cancer tissue ≈300% compared with the background autofluorescence of normal colonic epithelial cells, tubular adenomas, and nonmetastatic cancers (Fig. 1L). Similarly, Gb3 fluorescence intensity in colon cancer metastases to the liver was elevated >300% compared with the normal liver (Fig. 1L). This significant Gb3 elevation in metastatic colon cancer caused us to examine the potential role of Gb3 in development of tumor invasiveness.
Fig. 1.
Gb3 is expressed in the primary lesions of human metastatic colon cancer and in liver metastases. (A) Normal colonic tissue immunostained against β-catenin (red) and Stx1B-488 (green) to mark Gb3.(B) Normal colonic tissue labeled with CD77 mAb against Gb3 (red) and cholera toxin B subunit to detect GSL GM1 (green). Note that cells in lamina propria but not epithelial cells are Gb3-positive (n = 54 fields of view). (C) Noncancer colonic adenoma immunolabeled against β-catenin (red) and Stx1B-488 (green) for Gb3 (n = 12 fields of view). Note that erythrocytes (yellow, do not have nuclei) but not epithelial cells are Gb3-positive. (D) Nonmetastatic colon cancer tissue labeled against pankeratin to detect epithelial cells (red) and Gb3 by Stx1B-488 (green) (n = 12 fields). (E) Metastatic colon cancer tissue immunostained against actin (green) and Gb3 marked by Stx1B-568 (red). (F) Hematoxylin/eosin staining corresponding to sample in E (n = 29 fields). (G) Colon cancer metastases into the liver (n = 17 fields) labeled with Stx1B-488 (green) and mAb against β-catenin (red). (H) Normal liver tissue (n = 10 fields) immunostained with Stx1B-488 (green) and against β-catenin (red). Nuclei are stained with Hoechst dye (blue). (I and J) Tissue sample of hematoxylin-stained, nonmetastatic colon cancer before LCM (I) and after LCM when cancer epithelial cells were collected (J). (K) HPTLC shows the absence of Gb3 in epithelial cells collected by LCM from nonmetastatic colon cancer (lane 1) as in D, and Gb3 presence in metastatic colon cancer (lane 2) as in E. Lane 3 shows a mixture of purified markers (10 μg/ml Gb3 and 5 μg/ml LacCer). (L) Relative amount of Gb3 (percentages) in epithelial cells in human tissue normalized to normal colon or liver. Bars: 1, normal colon; 2, noncancer adenomas; 3, nonmetastatic colon cancer; 4, primary lesion of metastatic colon cancer; 5, colon cancer metastases into liver; 6, normal liver. The relative amountofGb3 in normal colonic and liver tissue was calculated from analysis of 8-bit fluorescence images as an average of fluorescence intensity per field of view and presented as 100% ± SE. The amount of Gb3 in cancer tissue was calculated similarly and expressed in percentages relative to control. The relative amount of Gb3 significantly (P < 0.05) increased in metastatic tissues compared with normal colon and liver, respectively.
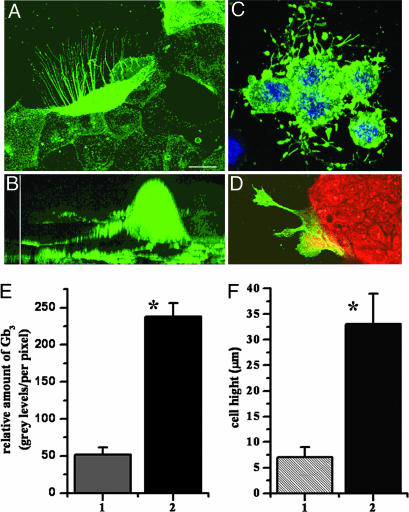
Colon Cancer Cells with Elevated Gb3 Have a Migratory Phenotype. Human intestinal epithelial Caco-2, T84, and HT29 cells derived from metastatic colon cancer were examined by immunofluorescence microscopy and FACS for the presence of Gb3-expressing cells. When 50-70% confluent, two subpopulations of cells were detected in each cell line: one with or without trace amounts of Gb3 and the other with a high amount of Gb3. The percent of Gb3-expressing cells was different in the three cell types and only ≈5% of T84 cells, ≈50% of Caco-2 cells, and ≈90% of HT29 cells were Gb3-positive. In all three cell types, Gb3-containing cells were mostly concentrated at the leading edge of cell islands. Moreover, cells enriched in Gb3 demonstrated a migratory phenotype and formed filopodia (Fig. 2 A, C, and D). In Caco-2 cells (Fig. 2A), the filopodia were very thin (≈0.2-0.5 μm), variable in number (up to 50 on a single cell), and beaded, and they contained Gb3. The filopodia could exceed 2- to 10-fold the epithelial cell diameter and projected in the direction of monolayer growth and cell spreading. In all three cell lines, there was a strong correlation between the amount of Gb3 and appearance of filopodia. Cells that formed filopodia were significantly enriched in Gb3 compared with neighboring cells without filopodia from the same leading edge (Fig. 2 A and D). Quantitative analysis of fluorescence intensity of 11 Caco-2 cells with and without filopodia showed that cells with filopodia express at least 3- to 5-fold more Gb3 per unit of surface area than cells without them (Fig. 2E). Average pixel intensity was 238 ± 18 gray levels in filopodia-containing cells vs. 52 ± 10 gray levels in cells without filopodia (P < 0.05). The filopodia-containing cells also have larger cell bodies (Fig. 2B). Measurement of the height of 20 filopodia-containing cells showed (Fig. 2F) that they are ≈3-fold taller than non-filopodia-containing cells (33 ± 6 μm vs. 7 ± 2 μm, P < 0.05).
Fig. 2.
Filopodia-containing cells are present at the leading edge of a living colon carcinoma monolayer detected by staining of the GSL Gb3.(A) x-y plane from 3D reconstruction of fluorescent confocal optical sections of representative Caco-2 cells with Gb3-containing filopodia marked by Stx1B-488. Filopodia and filopodia-containing cells are significantly enriched in Gb3-positive cells compared with the rest of the cells from the leading edge. (Scale bar, 10 μm.) (B) x-z projection from 3D reconstruction of confocal optical sections of representative Caco-2 cell with Gb3-containing filopodia, which is significantly taller than adjacent cells in the monolayer. (Vertical scale bar, 25 μm.) (C) Gb3-enriched filopodia-containing HT29 cells (blue, nuclei stained by Hoechst). (D) Gb3-positive cells with filopodia on the leading edge of T84 monolayer (red, Cyto7 dye). (E) Relative amount of Gb3 normalized per pixel in Caco-2 cells. Bars: 1, without filopodia; 2, with filopodia (P < 0.05). (F) The heights of Caco-2 cells. Bars: 1, non-filopodia-containing cells; 2, Gb3-up-regulating, filopodia-containing cells (P < 0.05 compared with bar 1).
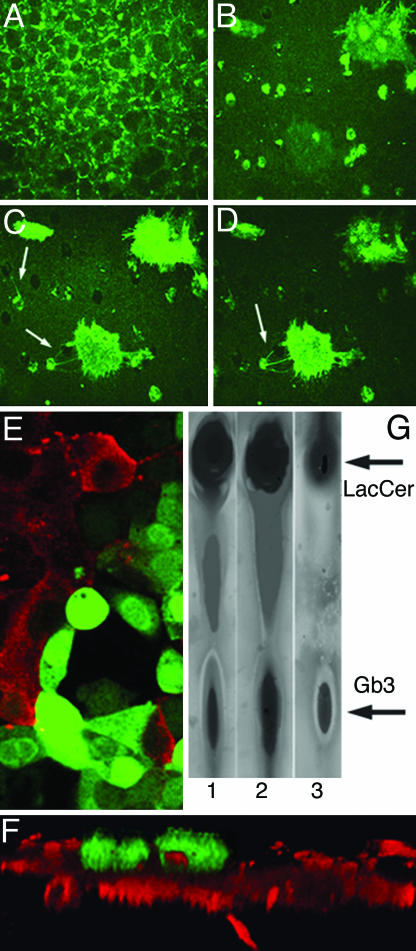
Cells Containing Gb3 and Filopodia Are Invasive. The appearance of cells with filopodia is consistent with their involvement in cell spreading, migration, or invasiveness. Because Gb3-enriched filopodia-containing cells were detected in colonic cancer cell lines and because epithelial cells with higher amounts of Gb3 were an exclusive feature of metastatic colonic cancer, we hypothesized that these cells might represent an invasive pool. To test this hypothesis, we applied a standardized chemoinvasive assay (16) that correlates with metastatic ability in vivo (24). In this assay, the movement of Caco-2 cells across a filter treated from the bottom side with laminin, a chemoattractant, was monitored. This assay demonstrated that only filopodia-containing Gb3-enriched cells migrate to the bottom surface of the filter and do so by the initial movement of filopodia through the filter pores (Fig. 3 A-D).
Fig. 3.
Gb3 is necessary for the development of the invasive cell phenotype: chemoinvasive assay. Confocal optical sections of Caco-2 and OK cells grown on Transwell filters from the top surface to bottom. Green indicates cells that were stained with Stx1B-488. (A) Confluent monolayer of Caco-2 cells on the top of filter. (B) Section through the filter. (C and D)Gb3-enriched, filopodia-containing cells invading the bottom surface of filter (note the initial appearance of filopodia, arrows). (E) Caco-2 cells infected with Gb3 synthase siRNA plus GFP (green) do not have Gb3 and do not bind Stx1B-568 (red) 72 h after infection. Note cells are either red or green. (F) x-z section through the filter shows that these GFP-positive, Gb3-free cells do not invade the bottom surface of the filter because of siGb3. (G) HPTLC detected a decrease in the relative amount of Gb3 in siGb3-infected Caco-2 cells (lane 1) vs. scRNA-infected cells (lane 2). Lane 3 shows the standard, which contains a mixture of 30 μg of purified LacCer and 30 μg of Gb3.
Filopodia-containing cells that migrated through the filter created a confluent monolayer of cells. Attempts to clone Gb3-enriched Caco-2 cells resulted in formation of cell monolayer with only ≈50% Gb3-containing cells, similar to that in wild-type Caco-2 monolayers. Analysis of the expression of the Gb3 synthase mRNA, an enzyme responsible for Gb3 synthesis (22, 25), in the subpopulation of Gb3-negative vs. Gb3-up-regulating Caco-2 cells (Fig. 6 A and B, which is published as supporting information on the PNAS web site) showed the presence of Gb3 synthase mRNA in both cell types, with only a nonsignificant 1.4-fold elevation in Gb3-enriched cells (Supporting Materials and Methods). This result indicates that the invasive subpopulation of cells does not occur because of the differential expression of the Gb3 synthase gene but, rather, because of the regulation of protein and/or lipid expression by uncharacterized signal transduction mechanisms.
Gb3 Is Necessary for Colon Cancer Cell Invasiveness. One way to further explore the role of Gb3 in invasiveness is to inhibit Gb3 synthesis in colon cancer cells and determine the impact on cell invasiveness. There are no specific inhibitors of Gb3 synthesis (26). Thus, to address this question, we used siRNA (17) to knock down the Gb3 synthase gene (siGb3). For these experiments, Caco-2 cells were infected with adenovirus containing siGb3 to silence the Gb3 synthase gene and thus prevent Gb3 synthesis (Fig. 3E). To ensure the specificity of siGb3 knockdown, Caco-2 cells were also infected with scRNA-containing viral vector. The effect of siGb3 and scRNA on the relative amount of Gb3 was assessed 48 h later by using HPTLC (Fig. 3G). The amount of Gb3 in siGb3 infected cells was 57 ± 18%, significantly (P < 0.05, n = 3) lower than that in scRNA infected cells (100 ± 21%). In contrast, the amount of β-catenin, adenomatous polyposis coli protein, epithelial sodium-proton exchanger 2, and GAPDH (Fig. 7, which is published as supporting information on the PNAS web site) did not differ in the two types of infection.
For the chemoinvasive assay, confluent Caco-2 cells grown on Transwell membranes were infected with adenovirus containing siGb3 and GFP. All cells that migrated through the pores in the membrane before the infection were scraped off 24 h after infection. The numbers of Gb3-positive cells vs. the GFP-positive, Gb3-free cells that migrated through the filter were counted 72 and 120 h after infection. Analysis of 15 fields of view in three membranes showed that from 3 to 10 cells per field migrated through the filter 72 h after infection and all of them were Gb3-positive (Fig. 3F). In contrast, all GFP-positive, Gb3-free cells remained on top of the membrane and did not migrate, even 120 h after infection (Fig. 3F). We conclude that Gb3 expression is necessary for Caco-2 cells to invade and that down-regulation of Gb3 by inhibition of Gb3 synthase through siGb3 prevents cell migration.
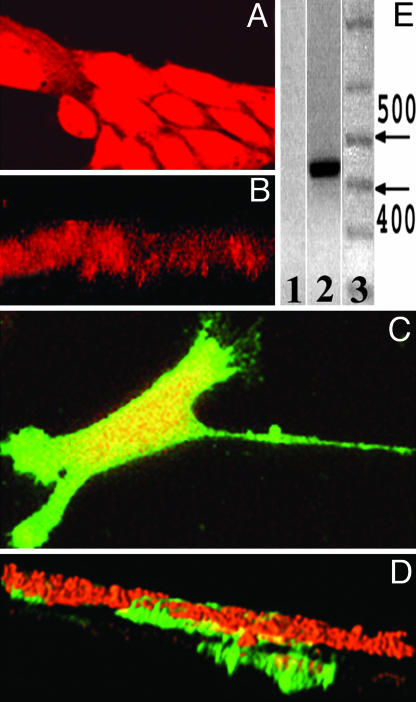
Gb3 Is Sufficient for Epithelial Cell Invasiveness. To test whether Gb3 expression is sufficient for epithelial cells to invade, we studied OK cells, which do not endogenously express Gb3, do not bind Stx1B (Fig. 4A), and are not invasive based on the fact they do not migrate through the membrane pores (Fig. 4B). These cells were transiently transfected with a Gb3 synthase cDNA (Supporting Materials and Methods) (22), shown to express Gb3 synthase (Fig. 4E), and then tested by chemoinvasive assay. Gb3-expressing OK cells formed Gb3-positive filopodia on the leading edge of growing monolayers (Fig. 4C) similar to that in T84, Caco-2, and HT29 cells. Moreover, as shown by the chemoinvasive assay (Fig. 4D), OK cells now penetrated through the filter. Importantly, only Gb3-positive OK cells penetrated through the filter, whereas cells without Gb3 from the same monolayer (Fig. 4D) and control cells transfected only with empty vector did not migrate through the filter at all and behaved similarly to the nontransfected cells (Fig. 4B).
Fig. 4.
Gb3 is sufficient for the development of the invasive cell phenotype: chemoinvasive assay. (A) Subconfluent OK cells labeled by Cyto7 dye (red) do not express Gb3, do not bind Stx1B-488, and do not form filopodia on the leading edge. (B) OK cells do not invade the bottom surface of the membrane but form only a monolayer on the top surface of the filter. (C) OK cells transfected with Gb3 synthase bind Stx1B-488 and form Gb3-enriched filopodia (green). (D) Only Gb3-expressing OK cells (green) invaded the bottom surface of the filter. (E) RT-PCR detects the presence of 428 bp of Gb3 synthase mRNA in OK cells transfected with human Gb3 synthase cDNA; Lanes: 1, control, nontransfected OK cells do not express Gb3 synthase; 2, Gb3 synthase mRNA is present in transfected OK cells, which express Gb3; 3, a base pair standard, with arrows indicating 400- and 500-bp markers.
B Subunit of Shiga Toxin Selectively Kills Gb3-Positive Colon Cancer Cells in Cell Culture Models and Mouse Xenographs. It has been shown that the noncatalytic Stx1B triggers apoptosis of Gb3-expressing Burkitt's lymphoma cells (14). To test whether Stx1B selectively causes apoptosis in Gb3-positive colonic cells, T84 cells were exposed to Stx1B for up to 168 h. Mitochondria were monitored as a viability control by using tetramethylrodamine (7, 27). After 24 h of exposure to Stx1B, virtually all T84 cells that lacked Gb3 and, thus, did not internalize Stx1B had active mitochondria (Fig. 8A, which is published as supporting information on the PNAS web site). However, cells that accumulated Stx1B had inactive mitochondria. Incubation of T84 monolayers with Stx1B for longer times led to elimination of Gb3-positive cells (Fig. 8B). In contrast, in the same monolayer, cells that did not take up Stx1B had active mitochondria and survived. In addition, OK cells, which do not express Gb3, were resistant to Stx1B-mediated apoptosis (Fig. 8 C and D). Stx1B-mediated apoptosis of Caco-2 cells was also confirmed by the DNA-laddering assay (Fig. 8E) (28). We conclude that Stx1B selectively kills Gb3-expressing cells.
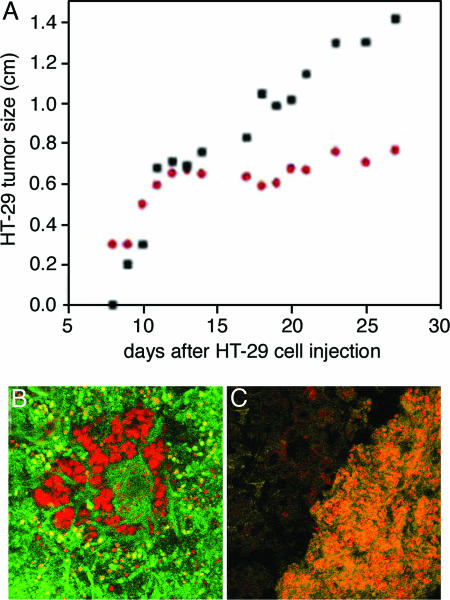
To test whether Stx1B affects growth of colonic tumors that contain Gb3, we used the nude mouse xenograph model (29). The nontoxic dose of Stx1B was first determined in mice (two each) injected s.c. with Stx1B 10 μg/kg, 50 μg/kg, 100 μg/kg, and 200 μg/kg. By day 20 after the Stx1B injection, none of the animals died or appeared ill. These mice were euthanized by CO2 inhalation. The liver, small intestine, and kidney were shown to be histologically normal (data not shown). Then nude mice (n = 10 animals) were injected s.c. in their flanks with 1 × 107 HT29 human colon cancer cells. When tumors reached 0.3 cm in diameter (≈7 days after injection), mice were divided into two groups: the control group (n = 5 mice), in which tumors were injected with PBS only, and a test group, in which tumors on one flank were injected every other day with a nontoxic dose of Stx1B (50 μg/kg, n = 6 mice) and tumors on the other flank were injected with an equal volume of PBS. Animals were monitored over 7 weeks, by which time some tumors reached ≈1.5 cm size, and the animals had to be euthanized according to our animal protocol. As shown in Fig. 5A, Stx1B injections significantly inhibited tumor growth in nude mice. Examination of tumors for the presence of apoptotic cells using the TUNEL assay (28) showed that there were many apoptotic cells in Stx1B-injected tissue (Fig. 5B), whereas only a few apoptotic cells per tumor were detected in tumors injected with PBS (Fig. 5C). These data show that the B subunit of Stx1 alone causes apoptotic death of Gb3-positive human colon cancer cells.
Fig. 5.
An example of inhibition of HT29 colon cancer cell tumor growth in nude mice by Stx1B. (A) HT29 tumor on one flank was injected with Stx1B, whereas the other flank was injected with PBS. Nontreated tumor (black squares) substantially outgrew Stx1B-treated tumor (red dots), which stopped increasing in size ≈7 days after the beginning of Stx1B injections. (B) TUNEL (green) staining of HT29 tumor injected with Stx1B shows that Stx1B caused apoptotic death of tumor cells; cell nuclei stained by propidium iodide are red. (C) HT29 tumor not treated with Stx1B does not have TUNEL-positive green cells. Orange is an overlay color caused by the yellow autofluorescence by tumor cells and their labeling by propidium iodide (red). The dark area is mouse tissue that surrounds the tumor.
Discussion
We have identified a subpopulation of invasive colon cancer cells based on molecular and phenotypic characteristics. Analysis of metastatic human colon cancer tissue samples showed that significant up-regulation of the GSL Gb3 was associated with development of metastasis. A similar subpopulation of cells with high levels of Gb3 expression and a migratory phenotype was found in three colon cancer cell lines established from metastatic human colon cancers. These cell lines may serve as a model for invasiveness development in a cancer cell population. It has been shown that the epithelial cells in human colon lack detectable Gb3 (23, 30). This finding is in good agreement with our data that Gb3 is virtually absent from normal colonic epithelial cells but is present in colon cancer in its metastatic stage.
Gb3 has been associated with other human cancers and was identified as the Burkitt's lymphoma antigen (31). Elevated expression of Gb3 was also detected in astrocytoma cell lines (32) and in several highly metastatic types of human tumors, including ovarian and breast cancers (33). Significant Gb3 accumulation was also previously reported in testicular seminoma, which correlated with the metastatic potential (34, 35). These data support our finding that Gb3 expression is a marker for colonic epithelial tumor cell transformation from primary cancer into the metastatic stage and indicate that several types of invasive tumors may have common mechanisms for metastasis.
Mechanistic studies of the role of Gb3 in metastases were performed on cell culture models. Our data demonstrate that Gb3 overexpression is necessary and sufficient for the appearance of cells with an invasive phenotype. In colon carcinoma epithelial cells, the presence of Gb3-containing filopodia correlates with cell invasiveness. Filopodia are used to penetrate through the permeable support in a chemoinvasive assay. Importantly, elimination of Gb3 from colon cancer cells by Gb3-synthase siRNA knockdown, which was verified by the Stx1B binding assay, completely inhibited cell migration. This result shows that Gb3 is necessary for cell invasiveness. Introduction of human Gb3-synthase into nontransformed, noninvasive OK cells that do not synthesize endogenous Gb3 induced Gb3 and converted noninvasive epithelial cells into invasive cells. Thus, the presence of Gb3 was also sufficient to induce the invasive phenotype in nontransformed cells.
Failure of Caco-2 cells to maintain the migratory phenotype when Gb3-enriched cells separated by FACS were cultured shows that Gb3-enriched invasive cells do not represent separate clones. This result indicates that changes in signal transduction pathways, which lead to Gb3 expression due to some as yet unknown mechanisms, rather than genetic alterations alone, are responsible for appearance of the invasive pool among the population of colon cancer cells.
Gb3 expression can be driven by several molecular mechanisms that do not exclude but rather complement each other. One possibility is that the enzyme Gb3 synthase (α1,4-galactosyltransferase), which catalyzes the transfer of galactose to LacCer, and produces Gb3, is significantly up-regulated during cancer progression from the noninvasive stage into the invasive stage. Human Gb3 synthase has recently been cloned (22, 25). Although expression of Gb3 synthase has not yet been reported in metastatic vs. normal colonic epithelial tissue using gene arrays (Oncomine Cancer array database), the absence of a significant difference in the amount of Gb3 synthase mRNA between Caco-2 with up-regulated Gb3 and Caco-2 without a detectable amount of Gb3 make this mechanism very unlikely to be responsible for the appearance of Gb3-positive cancer epithelial cells. Other mechanisms, such as differences in the amounts of Gb3 synthase enzyme or its activity, may be responsible for the transition of noninvasive colon cancer cells into invasive and/or metastatic cells.
Gb3 is best known as the receptor for the B subunit of Shiga toxin 1. Our data show that, similarly to that in Gb3-positive Burkitt's lymphoma cells (14), Stx1B selectively kills the subpopulation of Gb3-up-regulating colonic epithelial cells, which possess invasive potential. Next, we tested whether the ability of Stx1B to cause apoptosis might be used against colon cancer tumors grown in nude mice. Treatment of HT29 cell tumors in nude mice with Stx1B substantially inhibited tumor growth compared with nontreated control tumors, and, after ≈10 days, Stx1B treatment stabilized the tumor size, whereas nontreated tumors continued to increase in size. Thus, application of Stx1B or Stx1B linked to other more potent antitumor agents might be an approach to target the specific Gb3-expresing subpopulation of cells in human metastatic colon cancers.
In summary, the expression of the GSL Gb3 strongly correlates with the metastatic potential of human colon cancer. Gb3-enriched colon cancer cells represent an invasive subpopulation, and Gb3 expression is necessary and sufficient for this invasiveness in cell culture models. The overexpression of Gb3 makes it a possible marker for detection of a potentially invasive cell subpopulation in colon cancer.
Supplementary Material
Acknowledgments
We thank Drs. Raben and Schnaar for assistance with HPTLC experiments; Dr. Clausen (University of Copenhagen, Copenhagen) for Gb3 synthase pcDNA3.1 vector; Drs. Caterina, Nealen, and Agami for providing the pSUPER adenoviral construct with scRNA; and Oksana Gutsal for LCM cell preparations. This work was supported by National Institutes of Health Grants RO1DK58928, R24DK064388 (to the Hopkins Basic Research Digestive Disease Development Core Center), and P30DK 34928 (to the Center for Gastroenterology Research on Absorptive and Sectretory Processes).
Author contributions: O.K. and M.D. designed research; O.K., R.M., B.B., T.W., C.E., and M.D. performed research; M.A.C. and A.K. contributed new reagents/analytic tools; O.K., R.M., B.B., C.E., M.A.C., and M.D. analyzed data; and O.K. and M.D. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GSL, glycosphingolipid; Gb3, globotriaosylceramide; Stx1B, B subunit of Shiga toxin 1; siRNA, small inhibitory RNA; scRNA, scrambled RNA; HPTLC, high-performance thin-layer chromatography; LCM, laser capture microdissection; LacCer, lactoceramide.
References
- 1.Gorlick, R. & Bertino, J. (1999) Semin. Oncol. 26, 606-611. [PubMed] [Google Scholar]
- 2.Yokota, J. (2000) Carcinogenesis 121, 497-503. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava, S., Verma, M. & Henson, D. E. (2001) Clin. Cancer Res. 7, 1118-1126. [PubMed] [Google Scholar]
- 4.Ridley, A. (2000) Nature 406, 466-467. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori, S. (1989) Adv. Cancer Res. 52, 257-331. [DOI] [PubMed] [Google Scholar]
- 6.Hakomori, S. (1996) Cancer Res. 56, 5309-5318. [PubMed] [Google Scholar]
- 7.Hakomori, S. (2001) Adv. Exp. Med. Biol. 491, 369-402. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura, S., Ohyama, C., Watanabe, R., Satoh, M., Saito, S., Hoshi, S., Gasa, S. & Orikasa, S. (2001) Int. J. Cancer. 94, 343-347. [DOI] [PubMed] [Google Scholar]
- 9.Mirkin, B. L., Clark, S. H. & Zhang, C. (2002) Cell Prolif. 35, 105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li., R, Manella, J., Kong., Y. & Ladisch S. (2000) J. Biol. Chem. 275, 34213-34223 [DOI] [PubMed] [Google Scholar]
- 11.Hakomori, S. (2002) Proc. Natl. Acad. Sci. USA 99, 10231-10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacewicz, M., Clausen, H., Nudelman, E., Donohue-Rolfe, A. & Keusch, G. T. (1986) J. Exp. Med. 163, 1391-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindberg, C. A., Brown, J. E., Stromberg, N., Westling-Ryd, M., Schultz, J. E. & Karlson, K. (1987). J. Biol. Chem. 262, 1779-1785. [PubMed] [Google Scholar]
- 14.Gordon, J., Challa, A., Levens, J. M., Gregory, C. D., Williams, J. M., Armitage, R. J., Cook, J. P., Roberts, L. M. & Lord, J. M. (2000) Cell Death Differ. 7, 785-794. [DOI] [PubMed] [Google Scholar]
- 15.Kovbasnjuk, O., Edidin, M. & Donowitz, M. (2001) J. Cell Sci. 114, 4025-4031. [DOI] [PubMed] [Google Scholar]
- 16.Saiki, I., Murata, J., Nakajima, M., Tokura, S. & Azuma, I. (1990) Cancer Res. 50, 3631-3637. [PubMed] [Google Scholar]
- 17.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- 18.Hoey, D. E., Sharp, L., Currie, C., Lingwood, C. A., Gally, D. L. & Smith, D. G. (2003) Cell. Microbiol. 5, 85-97. [DOI] [PubMed] [Google Scholar]
- 19.Schnaar, R. L. (1994) Methods Enzymol. 230, 348-370. [DOI] [PubMed] [Google Scholar]
- 20.Schnaar, R. L. & Needham, L. K. (1994) Methods Enzymol. 230, 371-389. [DOI] [PubMed] [Google Scholar]
- 21.Ling, H., Boodhoo, A., Hazes, B., Cummings, M. D., Armstrong, G. D., Brunton, J. L & Read, R. J. (1998) Biochemistry 37, 1777-1788. [DOI] [PubMed] [Google Scholar]
- 22.Steffensen, R., Carlier, K., Wiels, J., Levery, S. B., Stroud, M., Cedergren, B., Nilsson Sojka, B., Bennett, E. P, Jersild, C. & Clausen, H. (2000) J. Biol. Chem. 275, 16723-16729. [DOI] [PubMed] [Google Scholar]
- 23.Schüller, S., Frankel, G. & Phillip, A. D. (2004) Cell Microbiol. 6, 289-301. [DOI] [PubMed] [Google Scholar]
- 24.Muller, A., Home, B., Sot, H, Ge, N. F. Catron, D., Buchanan, M. E, McClanahan, T., Murphy, E., Yuan, W., Wagner, S. N., et al. (2001) Nature 410, 50-56. [DOI] [PubMed] [Google Scholar]
- 25.Kojima, Y., Fukumoto, S., Furukawa, K., Okajima, T., Wiels, J., Yokoyama, K., Suzuki, Y., Urano, T., Ohta, M. & Furukawa, K. (2000) J. Biol. Chem. 275, 15152-15156. [DOI] [PubMed] [Google Scholar]
- 26.Lavie, Y., Cao, H., Volne, A., Lucci, A., Han, T. Y., Geffen, V., Giuliano, A. E. & Cabot, M. C. (1997) J. Biol. Chem. 272, 1682-1687. [DOI] [PubMed] [Google Scholar]
- 27.Susin, S. A., Lorenzo, H. K., Zamzami, N., Marzo, I., Brenner, C., Larochette, N., Prevost, M. C., Alzari, P. M. & Kroemer, G. (1999) J. Exp. Med. 189, 381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashkenazi, A. & Dixit, V. M. (1998) Science 281, 1305-1308. [DOI] [PubMed] [Google Scholar]
- 29.Park, B. H., Vogelstein, B. & Kinzler, K. W. (2001) Proc. Natl. Acad. Sci. USA 98, 2598-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holgersson, J., Jovell, P. A. & Breimer, M. E. (1991) J. Biochem. (Tokyo) 110, 120-131. [DOI] [PubMed] [Google Scholar]
- 31.LaCasse, E. C., Bray, M. R., Patterson, B., Lim, W. M., Perampalam, S., Radvanyi, L. G., Keating, A., Stewart, A. K., Buckstein, R., Sandhu, J. S., et al. (1999) Blood 94, 2901-2910. [PubMed] [Google Scholar]
- 32.Arab, S., Rutka, J. & Lingwood, C. (1999) Oncol. Res. 11, 33-39. [PubMed] [Google Scholar]
- 33.Arab, S., Russel, E. & Chapman, W. B. (1997) Oncol. Res. 9, 553-563. [PubMed] [Google Scholar]
- 34.Ohyama, C., Fukushi, Y., Satoh, M., Saitoh, S., Orikasa, S., Nudelman, E., Straud, M. & Hakomori, S. (1990) Int. J. Cancer 45, 1040-1044. [DOI] [PubMed] [Google Scholar]
- 35.Ohyama, C., Orikasa, S., Kawamura, S., Satoh, M., Saito, S., Fukushi, Y., Levery, S. B. & Hakomori, S. (1995) Cancer 76, 1043-1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.