Abstract
Hypocretin-1 and -2 (Hcrt-1 and Hcrt-2), also referred to as orexin-A and -B, are neuropeptides synthesized by a few thousand neurons in the lateral hypothalamus. Hypocretin-containing neurons project throughout the brain, with a prominent input to basal forebrain structures involved in motivation, reward, and stress. However, the role of hypocretins in addiction-related behaviors remains largely unexplored. Here we show that intracerebroventricular infusions of Hcrt-1 lead to a dose-related reinstatement of cocaine seeking without altering cocaine intake in rats. Hcrt-1 also dramatically elevates intracranial self-stimulation thresholds, indicating that, unlike treatments with reinforcing properties such as cocaine, Hcrt-1 negatively regulates the activity of brain reward circuitries. Hypocretin-induced reinstatement of cocaine seeking was prevented by blockade of noradrenergic and corticotropin-releasing factor systems, suggesting that Hcrt-1 reinstated drug seeking through induction of a stress-like state. Consistent with this interpretation, the selective Hcrt-1 receptor antagonist SB-334867 blocked footshock-induced reinstatement of previously extinguished cocaine-seeking behavior. These findings reveal a previously unidentified role for hypocretins in driving drug seeking through activation of stress pathways in the brain.
Keywords: addiction, orexin, relapse, reward, intracranial self-stimulation
Drug addiction is characterized by relapse to drug-taking behavior during periods of abstinence. Identification of brain mechanisms responsible for vulnerability to relapse is crucial for the development of effective treatments for drug addiction (1). Hypocretin-1 and -2 (Hcrt-1 and Hcrt-2), recently discovered lateral hypothalamic (LH) neuropeptides (2, 3), regulate a wide variety of physiological processes such as feeding, energy metabolism (4), and the maintenance of arousal (5, 6). Compelling evidence also indicates that Hcrt neurons in the LH receive inputs from diverse sensory and limbic systems and drive hyperarousal through modulation of stress responses (7, 8) and adaptive behavior associated with energy metabolism (9). Mutant mice deficient in Hcrt fail to respond to fasting with increased activity and wakefulness (10) and display diminished signs of precipitated opiate withdrawal (11). Further, leptin, which hyperpolarizes Hcrt neurons in mice (10), attenuates fasting-induced heroin-seeking behavior in rats (12). These observations suggest a role for LH Hcrt neurons in reward seeking (13-15). Consistent with this hypothesis, c-Fos activation of LH Hcrt neurons was recently correlated with preference, in rats, for an environment repeatedly paired with food and drug rewards (16). Importantly, however, the mechanisms by which Hcrt systems may reinstate drug-seeking behaviors remain largely unexplored. Here, we show that the Hcrt-1 peptide reinstates previously extinguished cocaine-seeking behavior and induces a long-lasting brain reward deficit. Further, we demonstrate that antagonism of Hcrt-1 receptors prevents footshock-induced reinstatement of cocaine-seeking behavior in rats. Overall, these data highlight a dynamic relationship between Hcrt and stress pathways in regulating the reinstatement of previously extinguished drug-seeking behaviors.
Materials and Methods
Animal Housing. We used male Wistar rats (Charles River Laboratories), weighing 250-350 g at the start of each experiment, maintained in a temperature-controlled vivarium under a 12-h light/dark cycle (lights off at 10:00 a.m.), with food and water available ad libitum. Animals were tested during the dark (active) period of the light/dark cycle, except during intracranial self-stimulation (ICSS) testing (see ICSS Procedures). All animals were treated in accordance with the guidelines of the National Institutes of Health regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care.
Apparatus. See Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Surgery. We anesthetized rats by inhalation of 1-3% isoflurane in oxygen. For i.v. surgery, we prepared rats with catheters inserted into the right jugular vein as described in ref. 17. Catheters were flushed daily with 0.2 ml of sterile antibiotic-containing physiological saline. For intracerebroventricular (i.c.v.) administration, rats were implanted with unilateral stainless steel guide cannulas (23 gauge, 7 mm in length) into the lateral ventricle [anterior-posterior (AP): -0.6; mediolateral (ML): ±1.9; dorsoventral (DV): -3.2 from dura, with the incisor bar at +5 mm]. Cannulas were kept patent by using 7.5-mm-long stainless steel stylets (30 gauge). For the ICSS procedure, stainless steel bipolar ICSS electrodes (11 mm in length; Plastics One, Roanoke, VA) were implanted in the medial forebrain bundle at the level of the posterior lateral hypothalamus (AP: -0.5 mm from bregma; ML: ±1.7 mm; DV: 8.3 mm from dura, incisor bar adjusted to 5 mm above the interaural line) (18).
Self-Administration, Extinction, and Reinstatement Procedures. Rats (n = 54) were trained to self-administer i.v. cocaine infusions (a single response on the active lever delivered 0.25 mg of cocaine dissolved in 0.1 ml of sterile 0.9% NaCl over 4 s) under a fixed ratio 1, timeout 20-s (FR1 TO20-s) schedule of reinforcement during seven 1-h daily sessions. Responses on the active lever during the TO period and responses on the inactive lever were recorded but were without scheduled consequence. Access to cocaine self-administration was then increased to 2 h per session for 5-7 consecutive days. After establishment of stable cocaine intake (≤20% variation in cocaine intake for three consecutive sessions), rats underwent a minimum of 14 consecutive 2-h daily extinction sessions, during which cocaine was no longer available, but the light cue associated with cocaine delivery was activated upon completion of the schedule requirements. For the last 3 days of the extinction period, rats received saline infusions into the lateral ventricle immediately before the session. The next day, rats were challenged with various treatments (see Drugs), and the response on the active/inactive levers was assessed during a 2-h session.
For those rats exposed to footshock stress (n = 27 of 54), another extinction period followed the Hcrt-1 challenge test until achievement of extinction criteria (5-7 consecutive sessions were needed before rats display the same stable level of responses as seen before Hcrt infusions). On the day after criteria-levels of extinction were obtained, rats were exposed to intermittent electric footshock in the self-administration chamber for 15 min. Footshock (current intensity, 0.5 mA; train duration, 0.5 s) was administered by means of the grid floor of the chamber under a variable-interval 40-s schedule (interval range, 10-70 s) (19). After termination of footshock, the levers were extended into the chambers, and responses were recorded for 120 min. Reinstatement sessions were conducted under conditions identical to those in effect during extinction, as described above. All rats underwent only a single session of footshock-induced reinstatement.
To examine the effects of Hcrt-1 on reinstatement of responding for a food reinforcer, a new cohort of rats (n = 6) was first food-restricted (14 g of chow pellets per rat per day) and trained to press an active lever to obtain a 45-mg food pellet for 50 min. Training started under a FR1 TO1-s schedule of reinforcement. TO duration was gradually increased, and training stopped after achievement of a stable pellet intake under a FR1 TO20-s schedule of reinforcement (≤20% variation in pellet intake for two consecutive sessions, which represented a total of 5-8 sessions of training). Initiation of the TO period was again indicated by a cue light located above the lever. After a postfood-restriction recovery period in the animal facility (7 days, fed ad libitum), baseline food responding was recorded in rats (non-food-restricted) for two consecutive sessions. Rats then underwent eight consecutive 50-min daily extinction sessions, during which pellets were no longer available, but the light cue paired with pellet delivery remained active. Rats received a saline i.c.v. infusion before the eighth session and were injected with Hcrt-1 before the ninth session. Responding on the active lever after saline and Hcrt-1 infusions was assessed during 50-min reinstatement sessions. No inactive lever was available in this experiment.
To examine whether Hcrt-1 nonspecifically increased lever-pressing behavior, the effect of Hcrt-1 on lever pressing behavior in food-trained and non-food-trained animals was next examined. One group (n = 4, food-trained group) was food-restricted and trained to press the active lever to obtain a 45-mg food pellet during 50-min sessions (see Fig. 1 D and E). A second group (n = 5, non-food-trained group) was placed into the operant chambers during the training sessions (Fig. 1F). Responses on the active lever activated the cue light above the lever but did not result in delivery of food rewards. TO period duration (light illumination) was increased in parallel in both groups of rats. For both groups, pressing the inactive lever had no scheduled consequence. All rats were handled in parallel for eight consecutive sessions (time necessary for food-trained rats to achieve a stable food intake, see Fig. 1 D-F) and then returned to the animal facility for 2 days, where they were fed ad libitum. Both groups of rats then underwent an extinction phase (responses on the active lever activated the cue light but did not result in the delivery of food rewards). All rats received a saline i.c.v. infusion before the eighth extinction session and were injected with Hcrt-1 before the ninth session. Responses on the active/inactive levers were assessed during 50-min sessions.
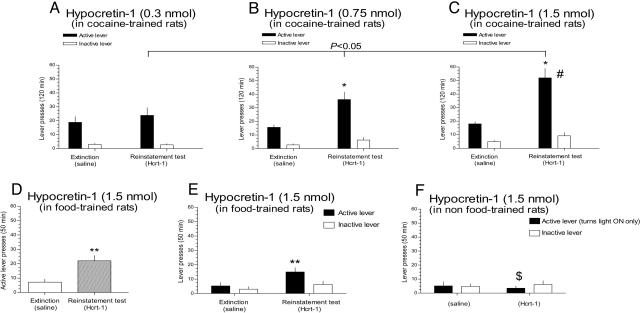
Fig. 1.
Icv infusions of Hcrt-1 reinstate previously extinguished rewarding behaviors. (A-C) Hcrt-1 dose-dependently reinstates cocaine-seeking behavior. Data are expressed as mean (±SEM) number of active or inactive lever responses during different phases of the experiment (n = 7 in each group), during extinction and after i.c.v. Hcrt-1 infusion (reinstatement). The dose-response effect of Hcrt-1 on relapse to cocaine seeking was determined by using a linear regression (P < 0.05). Hcrt-1 infusion into the lateral ventricle also reinstated previously extinguished food-seeking behavior. (D) Mean (±SEM) responses on the active lever in food-trained rats (n = 6) during different phases of the experiment, after a saline infusion before the last extinction session (extinction), and after i.c.v. infusion of Hcrt-1 (1.5 nmol) (reinstatement). (E) Mean (±SEM) responses on the active/inactive levers in food-trained rats (n = 4) for which pressing the active lever was previously paired with the delivery of food pellets. (F) Mean (±SEM) responses on both levers in non-food-trained rats for which pressing the active lever was not previously paired with the delivery of food pellets (n = 5). #, Significant difference (P < 0.05) between Hcrt-1 doses (0.3 vs. 1.5 nmol); $, significant difference (P < 0.05) between food-paired and non-food-paired lever; asterisks, significant differences (*, P < 0.05; **, P < 0.01) between experimental phases (extinction and reinstatement). See Statistical Analyses.
ICSS Procedure. The procedure used for measuring brain reward thresholds was a rate-free, discrete-trial current-threshold procedure described in ref. 20. Briefly, a set of three trials was presented for each current intensity. A noncontingent stimulus that systematically varied in current intensity was presented (current levels were varied in 5-μA steps, in four alternating descending and ascending series), and the rat had 7.5 s to respond on a wheel manipulandum to receive an electrical stimulus identical in all parameters to the noncontingent stimulus. The threshold for each series was defined as the midpoint between two consecutive current intensities that yielded “positive scores” (animals responded for at least two of the three trials) and two consecutive current intensities that yielded “negative scores” (animals did not respond for two or more of the three trials). The overall threshold of the session was defined as the mean of the thresholds for the four individual series. Each testing session was ≈30 min in duration. Rats received i.c.v. injections of sterile saline (n = 5) or Hcrt-1 (n = 9, 1.5 nmol), and ICSS thresholds were assessed 0, 6, 12, 18, 24, 36, and 48 h after injections.
Drugs. Cocaine·HCl was obtained from the National Institute on Drug Abuse and was dissolved in sterile physiological saline (0.9%). Clonidine·HCl was purchased from Sigma-Aldrich, dissolved in physiological saline, and injected i.p. (20 μg/kg) (21) and administered i.p. 15-30 min before the i.c.v. infusion of Hcrt-1. The corticotropin-releasing factor 1/corticotropin-releasing factor 2 (CRF1/CRF2) antagonist d-Phe-CRF12-41, generously provided by Jean Rivier (The Salk Institute, San Diego), was dissolved in saline and injected i.c.v. (1.3 nmol, 5 μg) and administered i.c.v. 10-15 min before the i.c.v. infusion of Hcrt-1. Hcrt-1 peptide (>95% pure by HPLC) was custom synthesized by a commercial supplier (Advanced ChemTech). Peptide identity was confirmed by MALDI spectrometry and amino acid analysis. Hcrt-1 was dissolved in sterile physiological saline and administered i.c.v. 10-15 min before the reinstatement test.
SB-334867 (SmithKline Beecham), purchased from Tocris Cookson (Ellisville, MO), was dissolved in 10% (vol/vol) DMSO/1% (wt/vol) Encapsin (Cyclodextran, Sigma) sterile water and administered i.p. in a volume of 5 ml/kg at the doses of 15 and 30 mg/kg 30 min before footshock (22).
i.c.v. Injection Procedure. Unilateral i.c.v. injections (saline, Hcrt-1, or d-Phe-CRF12-41 dissolved in saline; 2-μl total volume, by using a Harvard microinfusion pump, model 975, Harvard Apparatus) were administered over 62 s through 8.5-mm injectors (30 gauge). After infusion, the injectors were kept in place for an additional 60 s.
Statistical Analyses. For each experiment phase (cocaine baseline, extinction, and reinstatement), the total number of responses on both the active and the inactive levers were recorded over 120 min. Total responses for cocaine baseline, extinction, and reinstatement were analyzed by using a two-way mixed-design ANOVA (treatment × experiment phase) with treatment (Hcrt-1, 0.3, 0.75, or 1.5 nmol, n = 21; Hcrt-1, clonidine, d-Phe-CRF12-41, or clonidine + d-Phe-CRF12-41, n = 33; SB-334867, 0, 15, or 30 mg/kg, n = 27) as the between-subjects factor and the experiment phase as the within-subjects factor. Similarly, a two-way ANOVA (condition × experiment phase) was used to compare the effect of Hcrt-1 (1.5 nmol) in rats trained to respond for food (n = 4), and those that responded for cue light alone (n = 5). For the ICSS experiment, percentage change from baseline reward threshold was calculated by expressing the Hcrt-1 (1.5 nmol) or saline-induced threshold scores as a percentage of the preinjection baseline thresholds. The preinjection baseline thresholds were the thresholds obtained on the day before injection. Percentage changes from baseline scores for the first 12 h were subjected to two-factor repeated-measures ANOVA with treatment (saline or Hcrt-1, n = 14) as the between-subjects factor and ICSS thresholds as the within-subjects factor. A one-way ANOVA was used to analyze the effect of Hcrt-1 (1.5 nmol, n = 6) on rats trained with one lever only. Statistically significant effects in the ANOVAs were followed by planned comparisons among means adjusted by using the False Discovery Rate procedure (23, 24). We used an α level of 0.05 for all analyses.
Results
First, we assessed the effects of i.c.v.-infused Hcrt-1 on reinstatement of drug-seeking behavior. Two-way ANOVA demonstrated that Hcrt-1, at doses below typical wake-promoting doses (25), significantly increased responding on the previously cocaine-paired active lever in extinguished rats [F4,36(treatment × phase) = 2.7344, P < 0.05]. Preplanned comparisons demonstrated that the 0.75-nmol (Fig. 1B) and 1.5-nmol (Fig. 1C) but not 0.3-nmol (Fig. 1 A) doses of Hcrt-1 significantly increased responding on the active lever. Responding on the inactive lever was not significantly altered in the same conditions [F4,32(treatment × phase) = 1.9462, not significant] (Fig. 1 A-C), suggesting that Hcrt-1-induced reinstatement was not secondary to nonspecific locomotor-activating effects. Similar doses of Hcrt-1 did not alter responding during active i.v. cocaine self-administration (see Fig. 5, which is published as supporting information on the PNAS web site). Overall, these data suggest that Hcrt-1 preferentially reinstated previously extinguished cocaine-seeking behavior at doses that did not alter the primary reinforcing effects of cocaine.
The Hcrt system may regulate feeding and mediate the increased arousal usually observed after periods of fasting (10). Thus, we next tested whether Hcrt-1 reinstated extinguished responding for a natural reinforcer. i.c.v. infusion of Hcrt-1 (1.5 nmol, n = 6) significantly increased lever responses in extinguished, non-food-restricted rats previously trained to respond for food reinforcers [F2,10(phase) = 22.346, P < 0.001] (Fig. 1D). Importantly, Hcrt-1 (1.5 nmol) reinstated active lever presses only in rats in which the active lever was previously paired with food rewards and not in rats in which the active lever had activated a cue light (see Materials and Methods) [F2,14(condition × phase) = 350.47, P < 0.001] (Fig. 1 E and F). Further, Hcrt-1 did not alter responses on the inactive lever under any experimental condition (food-paired or non-food-paired to the lever) and the experiment phase (food available, extinction, and reinstatement) [F2,14(condition × phase) = 1.4289, not significant]. Overall, these data support the conclusion that Hcrt-1 selectively reinstated drug- and food-seeking behaviors, and that this action is not secondary to nonspecific locomotor activation effects of Hcrt-1.
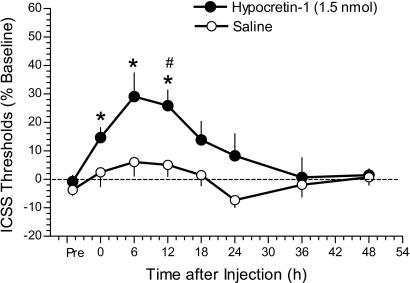
One possible mechanism by which Hcrt-1 reinstated cocaine seeking may have been through induction of a priming effect (e.g., a cocaine-like rewarding effect). Indeed, exposure to cocaine or other rewarding substances (e.g., amphetamine or morphine) during extinction has been shown to robustly reinstate previously extinguished cocaine-seeking behavior in rats (26, 27). To test this hypothesis, the effect of the Hcrt-1 peptide on ICSS thresholds was explored. Mean absolute thresholds before drug treatment were 104.5 ± 11.4 and 129.9 ± 13.6 μA in Hcrt-1- and saline-treated rats, respectively. Hcrt-1 (1.5 nmol, i.c.v.), but not saline, induced persistent, long-lasting elevations in ICSS thresholds (decrease in reward) up to 12 h after injection [F1,12(treatment) = 8.4482, P < 0.05; and F3,36(time) = 9.0904, P < 0.001] (Fig. 2). Importantly, the ICSS procedure used here was a rate-independent measure of brain reward sensitivity. Thus, the effects of Hcrt-1 were not secondary to alterations in rates of responding (20, 28). Consistent with this conclusion, no changes in ICSS response latencies were observed in Hcrt-1- or saline-treated rats (data not shown). These data demonstrate that Hcrt-1 negatively regulates the activity of brain reward systems and induces a long-lasting reward deficit similar to that observed after i.c.v. administration of CRF (29). Thus, it is unlikely that Hcrt-1 reinstates drug-seeking behavior by inducing a cocaine-like positive affective state (30).
Fig. 2.
Elevated ICSS reward thresholds in rats (n = 9) measured up to 48 h after Hcrt-1 administration into the lateral ventricle. *, statistically significant differences (P < 0.05) compared with Pre thresholds (baseline); #, statistically significant differences (P < 0.05) compared with thresholds in saline-treated rats (n = 5) at the same time points, as determined by planned comparisons among means after statistically significant main effect in the ANOVA. See Statistical Analyses.
Activation of brain stress pathways is a major factor that precipitates reinstatement of drug-seeking behaviors in rodents (31, 32). Recent data suggest that Hcrt and CRF systems interact closely in regulating responsiveness to stress (8), and that Hcrt-1 increases noradrenergic transmission throughout the brain (33, 34), also known to contribute to stress responses (35). Thus, we next examined the role of CRF and noradrenergic systems in Hcrt-induced reinstatement.
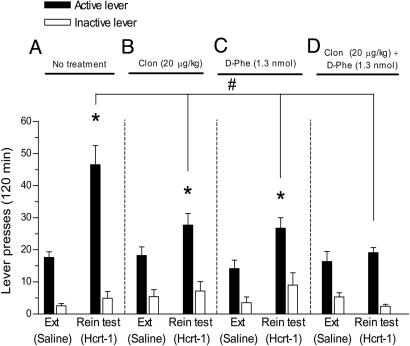
Clonidine (n = 8; 20 μg/kg, i.p.) or d-Phe-CRF12-41 (n = 10; 1.3 nmol, i.c.v.) attenuated the effect of Hcrt-1 (n = 8; 1.5 nmol, i.c.v.), and coadministration of both clonidine and d-Phe-CRF12-41 (n = 7; 20 μg/kg, i.p. and 1.3 nmol, i.c.v., respectively) abolished Hcrt-1-induced reinstatement for cocaine seeking [F6,58(treatment × phase) = 3.4595, P < 0.01] (Fig. 3). Responding on the inactive lever was not altered [F6,52(treatment × phase) = 1.7355, not significant]. These data suggest that simultaneous activation of CRF and noradrenergic systems by Hcrt-1 regulates the reinstatement of cocaine-seeking behavior induced by Hcrt-1.
Fig. 3.
Noradrenergic and CRF systems regulate Hcrt-1-induced reinstatement of cocaine seeking. Data are expressed as mean (±SEM) number of active or inactive lever responses during different phases of the experiment, during extinction (Ext), and after i.c.v. Hcrt-1 infusion (reinstatement, Rein). (A) In absence of pretreatment, Hcrt-1 (n = 8; 1.5 nmol, i.c.v.) induced a robust reinstatement of lever pressing (P < 0.05). Pretreatment with the α2 agonist clonidine (Clon; n = 8; 20 μg/kg, i.p.) (B) or the CRF1/CRF2 antagonist d-Phe-CRF12-41 (n = 10; 1.3 nmol, i.c.v.) (C) attenuated the effect of Hcrt-1 (1.5 nmol, i.c.v.) on reinstatement (P < 0.05), but responding on the previously cocaine-paired active lever remained significantly increased compared with extinction responses (P < 0.05). (D) Coadministration of clonidine (20 μg/kg, i.p.) and d-Phe-CRF12-41(1.3 nmol, i.c.v.) completely blocked the Hcrt-induced reinstatement of cocaine seeking (n = 7, P < 0.05). #, significant difference (P < 0.05) compared with the Hcrt-1; *, statistically significant differences (P < 0.05) between experimental phases (extinction and reinstatement). See Statistical Analyses.
Next, we examined the role of endogenous Hcrt systems in regulating stress-induced reinstatement of cocaine seeking.
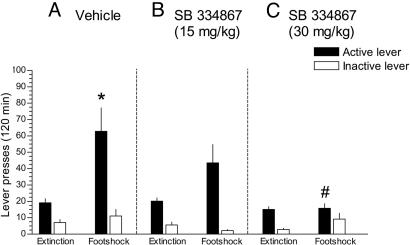
Extinguished rats (n = 14) treated with vehicle and exposed to footshock exhibited a robust reinstatement of responding reflected by a significant increase in the number of responses on the previously cocaine-paired active lever (Fig. 4A), whereas rats treated with the Hcrt-1 receptor antagonist SB-334867 (15 mg/kg, n = 8; 30 mg/kg, n = 5) and exposed to footshock did not exhibit a significant increase in the number of responses on the previously cocaine-paired lever (Fig. 4 B and C) [F4,48(treatment × phase) = 3.1226, P < 0.05]. Responding on the inactive lever was not significantly altered across the experimental phases in both groups of rats [F4,42(treatment × phase) = 1.1955, not significant]. These data suggest a role for endogenous Hcrt-1 in stress-induced reinstatement of cocaine seeking.
Fig. 4.
Involvement of the Hcrt system in stress-induced relapse of cocaine seeking. Footshock induced a significant increase in the number of responses on the previously cocaine-paired active lever in vehicle-treated rats (n = 14) (A), whereas pretreatment with the Hcrt-1 receptor antagonist SB-334867 (15 mg/kg i.p., n = 8; 30 mg/kg i.p., n = 5) did not induce a significant increase in responding on the active lever (B and C). Data are expressed as mean (±SEM) number of active or inactive lever responses during extinction and footshock-induced reinstatement for cocaine seeking. *, Significant difference (P < 0.05) between experimental phases (extinction and footshock); #, significant difference (P < 0.05) compared with vehicle-treated rats. See Statistical Analyses.
Discussion
The present results demonstrate that i.c.v. infusions of the Hcrt-1 peptide reinstated extinguished cocaine-seeking, and to a lesser extent, food-seeking behaviors. In addition, antagonism of Hcrt-1 receptors blocked footshock-induced reinstatement of previously extinguished cocaine-seeking. Importantly, Hcrt-1 significantly elevated ICSS thresholds in rats, reflecting a decrease in the activity of brain reward systems. This action of Hcrt-1 on ICSS thresholds is opposite to the well known threshold-lowering effects of cocaine, an index of cocaine-induced excitation of brain reward system (30). Overall, these data provide strong evidence suggesting that Hcrt-1 reinstates cocaine seeking by mechanisms different from increased dopamine release, and the blockade of Hcrt-1 induced reinstatement by CRF/noradrenergic antagonism rather suggests that Hcrt and stress systems may closely interact to regulate cocaine-seeking behaviors.
Maintenance of energy homeostasis requires the coordination of systems that regulate feeding, body temperature, and autonomic and endocrine functions, with those that modulate an appropriate state of arousal and motivation. The Hcrt system relays inputs from diverse sensory and limbic systems to forebrain and brainstem nuclei involved in motivation, reward, and stress (7, 36). Accumulating evidence suggests that Hcrt systems coordinate appropriate behavioral responses by means of changes in the activity of arousal centers to maintain physiological homeostasis and alertness (6, 9). Loss of Hcrt systems results in narcolepsy-like phenotypes in mice (37) and dogs (38), and human narcoleptic patients exhibit a drastic reduction in Hcrt-1 in cerebrospinal fluid (39) and in the number of Hcrt neurons (40, 41). Based on the data discussed in detail below, we propose that Hcrt neurons may also play an important role in maintaining homeostasis in stress and reward systems in the brain, and that dysregulation of Hcrt systems may contribute to reinstatement of drug-seeking behaviors.
Recently, it was reported that activation of LH Hcrt neurons [or infusion of Hcrt-1 directly into the ventral tegmental area (VTA)] reinstated an extinguished preference for an environment repeatedly paired with natural and nonnatural reward in rats, and that a morphine priming injection activated LH Hcrt neurons of extinguished rats (16). Based on these observations, Harris and colleagues (16) speculated that Hcrt may regulate reward processing, notably through activation of the mesocorticolimbic dopaminergic system. However, morphine potently activates hypothalamic CRF and noradrenergic systems (42). Thus, it is possible that morphine-induced c-Fos activation of Hcrt neurons was secondary to activation of brain stress pathways and not related to morphine's rewarding effects. Further, stress-activation of the mesocorticolimbic dopamine system has been well documented; notably, CRF induces glutamate release in the VTA of cocaine-experienced, but not cocaine-naïve, rats (43). Strikingly, only experienced rats responded to Hcrt-1 infusions. Because hypocretins have been shown to act synergistically with glutamatergic afferents to depolarize cholinergic neurons in the laterodorsal tegmental area (44) and dopaminergic neurons in the VTA (45), it is tempting to speculate that Hcrt may not only directly activate stress systems but also indirectly activate dopaminergic stress responses in the frontal cortex, which could explain the results obtained by Harris and colleagues (16).
Importantly, we demonstrate here that a dose of Hcrt-1 that reinstated cocaine seeking also elevated ICSS thresholds, indicating a decrease in excitability of brain reward systems. This action of Hcrt-1 is in sharp contrast to the well known cocaine-induced lowering of ICSS thresholds that is considered to reflect an increased reward sensitivity that underlies, or at least contributes to, the positive affective state associated with drug consumption (30). Although functional heterogeneity of Hcrt neurons in different hypothalamic areas, as suggested by Harris and colleagues (16), cannot be excluded, it is unlikely that Hcrt-1 reinstated cocaine seeking through an increased dopamine-release mechanism, according to our observations. In contrast, blockade of CRF and noradrenergic systems, important components of brain stress pathways known to play a role in stress-induced reinstatement, abolished Hcrt-induced reinstatement of cocaine-seeking behavior. Further, antagonism of Hcrt-1 receptors blocked footshock-induced reinstatement of previously extinguished cocaine-seeking behavior. Therefore, the present results rather suggest a role for hypocretins in coordinating motivated behaviors in response to stress factors. Consistent with previous observations in which Hcrt-deficient mice failed to increase alertness and locomotor activity in response to fasting (10), we suggest that Hcrt drives hyperarousal not only by stabilizing the firing of brainstem neurons that control wakefulness and rapid eye movement sleep (6), but also through modulation of motivated behavior, possibly by coordinating the stress responsivity in conjunction with brain stress systems in the extended amygdala of the basal forebrain (8). Supporting this hypothesis, anatomical observations showed that Hcrt neurons in the LH project with a prominent input to the central amygdala, ventral bed nucleus of the stria terminalis, nucleus accumbens shell, ventral pallidum, and ventral tegmental area (7, 36), brain structures considered to play a crucial role in the limbic and motor circuitry underlying footshock-induced reinstatement for cocaine-seeking behavior (46). Also consistent with this hypothesis, recent recordings of electrophysiologically identified neurons indicate that firing of Hcrt neurons correlates with active, motivated, and exploratory behavior (47, 48), suggesting that hypocretins are involved in adapting motor activity and cortical arousal according to motivational and/or emotional state. Finally, further support for this hypothesis is the observation of pronounced attenuation of morphine withdrawal symptoms in Hcrt-deficient mice (11), which can be interpreted as a deficit in coordinating behavioral responses to interoceptive cues (15).
Overall, the present finding that stress activation of Hcrt release leads to reinstatement of previously extinguished cocaine-seeking behavior identifies a mechanism by which stress can influence relapse for drug seeking. We conclude that the Hcrt system may have a role in drug craving and vulnerability to relapse, possibly by driving drug seeking through coordination of stress pathways in the brain. The Hcrt system may therefore represent a target for preventing relapse for drug seeking during protracted abstinence.
Supplementary Material
Acknowledgments
We thank Dr. Jean Rivier for his generous gift of D-Phe-CRF12-41, Drs. Stéphanie Caille and Christopher V. Dayas for their relevant comments on the experimental design, and Dr. Harinder Aujla for statistical analyses. We thank Tony Kerr and Cory Wright for technical assistance and Mike Arends for assistance with manuscript preparation. We also acknowledge Profs. Olivier Halfon (Service Universitaire de Psychiatrie de l'Enfant et de l'Adolescent, University of Lausanne) and Pierre J. Magistretti (Center for Psychiatric Neuroscience, University of Lausanne) for their financial support of B.B. This work was supported by La Fondation pour la Recherche Médicale (to B.B.); National Alliance for Research on Schizophrenia and Depression (to P.J.K.); and National Institutes of Health Grants DA04398 (to G.F.K.), DA11946 (to A.M.), AA13241 and MH58543 (to L.d.L.), and DA07348 (to Dr. Friedbert Weiss, supporting R.M.-F). This article is publication no. 17418-MB from The Scripps Research Institute.
Author contributions: B.B., A.M., G.F.K., and L.d.L. designed research; B.B., P.J.K., and S.E.S. performed research; R.M.-F. and A.M. contributed new reagents/analytic tools; B.B., P.J.K., G.F.K., and L.d.L. analyzed data; and B.B., P.J.K., G.F.K., and L.d.L. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CRF, corticotropin-releasing factor; FR1 TOn, fixed ratio 1, timeout n-s; Hcrt, hypocretin; ICSS, intracranial self-stimulation; i.c.v., intracerebroventricular; LH, lateral hypothalamic.
References
- 1.O'Brien, C. P. (1997) Science 278, 66-70. [DOI] [PubMed] [Google Scholar]
- 2.de Lecea, L., Kilduff, T. S., Peyron, C., Gao, X., Foye, P. E., Danielson, P. E., Fukuhara, C., Battenberg, E. L., Gautvik, V. T., Bartlett, F. S., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 322-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., Williams, S. C., Richardson, J. A., Kozlowski, G. P., Wilson, S., et al. (1998) Cell 92, 573-585. [DOI] [PubMed] [Google Scholar]
- 4.Willie, J. T., Chemelli, R. M., Sinton, C. M. & Yanagisawa, M. (2001) Annu. Rev. Neurosci. 24, 429-458. [DOI] [PubMed] [Google Scholar]
- 5.Taheri, S., Zeitzer, J. M. & Mignot, E. (2002) Annu. Rev. Neurosci. 25, 283-313. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe, J. G. & de Lecea, L. (2002) Nat. Rev. Neurosci. 3, 339-349. [DOI] [PubMed] [Google Scholar]
- 7.Baldo, B. A., Daniel, R. A., Berridge, C. W. & Kelley, A. E. (2003) J. Comp. Neurol. 464, 220-237. [DOI] [PubMed] [Google Scholar]
- 8.Winsky-Sommerer, R., Yamanaka, A., Diano, S., Borok, E., Roberts, A. J., Sakurai, T., Kilduff, T. S., Horvath, T. L. & de Lecea, L. (2004) J. Neurosci. 24, 11439-11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai, T. (2003) Curr. Opin. Clin. Nutr. Metab. Care 6, 353-360. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka, A., Beuckmann, C. T., Willie, J. T., Hara, J., Tsujino, N., Mieda, M., Tominaga, M., Yagami, K., Sugiyama, F., Goto, K., et al. (2003) Neuron 38, 701-713. [DOI] [PubMed] [Google Scholar]
- 11.Georgescu, D., Zachariou, V., Barrot, M., Mieda, M., Willie, J. T., Eisch, A. J., Yanagisawa, M., Nestler, E. J. & DiLeone, R. J. (2003) J. Neurosci. 23, 3106-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalev, U., Yap, J. & Shaham, Y. (2001) J. Neurosci. 21, RC129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorpe, A. J., Cleary, J. P., Levine, A. S. & Kotz, C. M. (2005) Psychopharmacology 182, 75-83. [DOI] [PubMed] [Google Scholar]
- 14.Winsky-Sommerer, R., Boutrel, B. & de Lecea, L. (2005) Mol. Neurobiol. 32, 285-294. [DOI] [PubMed] [Google Scholar]
- 15.Boutrel, B., Kenny, P. J., Markou, A. & Koob, G. F. (2005) in Hypocretins: Integrators of Physiological Functions, eds. de Lecea, L. & Sutcliffe, J. G. (Springer, New York), pp. 315-324.
- 16.Harris, G. C., Wimmer, M. & Aston-Jones, G. (2005) Nature 437, 556-559. [DOI] [PubMed] [Google Scholar]
- 17.Caine, S. B., Lintz, R. & Koob, G. F. (1993) in Behavioural Neuroscience: A Practical Approach, ed. Sahgal, A. (IRL, Oxford), Vol. 2, pp. 117-143. [Google Scholar]
- 18.Pellegrino, L. J, Pellegrino, A. S. & Cushman, A. J. (1979) A Stereotaxic Atlas of the Rat Brain (Plenum, New York).
- 19.Martin-Fardon, R., Ciccocioppo, R., Massi, M. & Weiss, F. (2000) NeuroReport 11, 1939-1943. [DOI] [PubMed] [Google Scholar]
- 20.Kornetsky, C. & Esposito, R. U. (1979) Fed. Proc. 38, 2473-2476. [PubMed] [Google Scholar]
- 21.Erb, S., Hitchcott, P. K., Rajabi, H., Mueller, D., Shaham, Y. & Stewart, J. (2000) Neuropsychopharmacology 23, 138-150. [DOI] [PubMed] [Google Scholar]
- 22.Rodgers, R. J., Halford, J. C., Nunes de Souza, R. L., Canto de Souza, A. L., Piper, D. C., Arch, J. R., Upton, N., Porter, R. A., Johns, A. & Blundell, J. E. (2001) Eur. J. Neurosci. 13, 1444-1452. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini, Y. & Hochberg, Y. (1995) J. R. Stat. Soc. B 57, 289-300. [Google Scholar]
- 24.Shaffer, J. P. (1995) Annu. Rev. Psychol. 46, 561-584. [Google Scholar]
- 25.Hagan, J. J., Leslie, R. A., Patel, S., Evans, M. L., Wattam, T. A., Holmes, S., Benham, C. D., Taylor, S. G., Routledge, C., Hemmati, P., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 10911-10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Wit, H. & Stewart, J. (1981) Psychopharmacology 75, 134-143. [DOI] [PubMed] [Google Scholar]
- 27.Lynch, W. J., Heaser, W. A. & Carroll, M. E. (1998) Exp. Clin. Psychopharmacol. 6, 255-263. [DOI] [PubMed] [Google Scholar]
- 28.Markou, A. & Koob, G. F. (1992) Physiol. Behav. 51, 111-119. [DOI] [PubMed] [Google Scholar]
- 29.Macey, D. J., Koob, G. F. & Markou, A. (2000) Brain Res. 866, 82-91. [DOI] [PubMed] [Google Scholar]
- 30.Kenny, P. J., Koob, G. F. & Markou, A. (2003) Behav. Neurosci. 117, 1103-1107. [DOI] [PubMed] [Google Scholar]
- 31.Erb, S., Shaham, Y. & Stewart, J. (1996) Psychopharmacology 128, 408-412. [DOI] [PubMed] [Google Scholar]
- 32.Shaham, Y., Shalev, U., Lu, L., De Wit, H. & Stewart, J. (2003) Psychopharmacology 168, 3-20. [DOI] [PubMed] [Google Scholar]
- 33.Horvath, T. L., Peyron, C., Diano, S., Ivanov, A., Aston-Jones, G., Kilduff, T. S. & van den Pol, A. N. (1999) J. Comp. Neurol. 415, 145-159. [PubMed] [Google Scholar]
- 34.Walling, S. G., Nutt, D. J., Lalies, M. D. & Harley, C. W. (2004) J. Neurosci. 24, 7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koob, G. F. (1999) Biol. Psychiatry 46, 1167-1180. [DOI] [PubMed] [Google Scholar]
- 36.Peyron, C., Tighe, D. K., van den Pol, A. N., de Lecea, L., Heller, H. C., Sutcliffe, J. G. & Kilduff, T. S. (1998) J. Neurosci. 18, 9996-10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chemelli, R. M., Willie, J. T., Sinton, C. M., Elmquist, J. K., Scammell, T., Lee, C., Richardson, J. A., Williams, S. C., Xiong, Y., Kisanuki, Y., et al. (1999) Cell 98, 437-451. [DOI] [PubMed] [Google Scholar]
- 38.Lin, L., Faraco, J., Li, R., Kadotani, H., Rogers, W., Lin, X., Qiu, X., de Jong, P. J., Nishino, S. & Mignot, E. (1999) Cell 98, 365-376. [DOI] [PubMed] [Google Scholar]
- 39.Nishino, S., Ripley, B., Overeem, S., Lammers, G. J. & Mignot, E. (2000) Lancet 355, 39-40. [DOI] [PubMed] [Google Scholar]
- 40.Thannickal, T. C., Moore, R. Y., Nienhuis, R., Ramanathan, L., Gulyani, S., Aldrich, M., Cornford, M. & Siegel, J. M. (2000) Neuron 27, 469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peyron, C., Faraco, J., Rogers, W., Ripley, B., Overeem, S., Charnay, Y., Nevsimalova, S., Aldrich, M., Reynolds, D., Albin, R., et al. (2000) Nat. Med. 6, 991-997. [DOI] [PubMed] [Google Scholar]
- 42.Xu, G. P., Van Bockstaele, E., Reyes, B., Bethea, T. & Valentino, R. J. (2004) J. Neurosci. 24, 8193-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, B., Shaham, Y., Zitzman, D., Azari, S., Wise, R. A. & You, Z. B. (2005) J. Neurosci. 25, 5389-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burlet, S., Tyler, C. J. & Leonard, C. S. (2002) J. Neurosci. 22, 2862-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korotkova, T. M., Sergeeva, O. A., Eriksson, K. S., Haas, H. L. & Brown, R. E. (2003) J. Neurosci. 23, 7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFarland, K., Davidge, S. B., Lapish, C. C. & Kalivas, P. W. (2004) J. Neurosci. 24, 1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mileykovskiy, B. Y., Kiyashchenko, L. I. & Siegel, J. M. (2005) Neuron 46, 787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee, M. G., Hassani, O. K. & Jones, B. E. (2005) J. Neurosci. 25, 6716-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.