Abstract
Dysfunction of mitochondrial complex I is a feature of human neurodegenerative diseases such as Leber hereditary optic neuropathy and Parkinson's disease. This mitochondrial defect is associated with a recruitment of the mitochondrial-dependent apoptotic pathway in vivo. However, in isolated brain mitochondria, complex I dysfunction caused by either pharmacological or genetic means fails to directly activate this cell death pathway. Instead, deficits of complex I stimulate intramitochondrial oxidative stress, which, in turn, increase the releasable soluble pool of cytochrome c within the mitochondrial intermembrane space. Upon mitochondrial permeabilization by the cell death agonist Bax, more cytochrome c is released to the cytosol from brain mitochondria with impaired complex I activity. Given these results, we propose a model in which defects of complex I lower the threshold for activation of mitochondrial-dependent apoptosis by Bax, thereby rendering compromised neurons more prone to degenerate. This molecular scenario may have far-reaching implications for the development of effective neuroprotective therapies for these incurable illnesses.
Keywords: mitochondria, neurodegeneration, Parkinson's disease
Reduced activity in mitochondrial complex I (NADH/ubiquinone oxidoreductase) is associated with a wide spectrum of neurodegenerative diseases (1). Low complex I activity due to mitochondrial DNA point mutations is found in many cases of Leber hereditary optic neuropathy, which is characterized by a massive retinal ganglion cell degeneration resulting in a rapid loss of central vision (2). Reduced complex I activity has also been reported in both autopsy brain tissues and platelets of patients affected with sporadic Parkinson's disease (PD) (3, 4). The pathogenic role of this mitochondrial dysfunction is supported by demonstrations that natural and synthetic complex I antagonists provoke neuronal death in animals (5–7). The molecular basis of neuronal death mediated by defective complex I activity is just beginning to be deciphered, in part by the utilization of the mitochondrial poisons 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone. For instance, it is now established that inhibition of complex I in rodents leads to degeneration of dopaminergic neurons of the substantia nigra pars compacta, as seen in PD (5), through activation of apoptotic molecular pathways (8–10). Moreover, it is believed that complex I dysfunction and the subsequent impairment of mitochondrial respiration provoke the activation of the mitochondrial-dependent apoptotic machinery by directly triggering the release of the apoptogenic molecule cytochrome c from the defective mitochondria (11–14).
Here we show that, contrary to the proposed direct effect of complex I deficit on cytochrome c release and consequent cell death, complex I defects do not autonomously recruit the apoptotic machinery. Instead, we show that complex I deficiency sensitizes neurons to mitochondrial-dependent apoptosis in response to the cell death agonist Bax through mitochondrial oxidative damage, by increasing the releasable soluble pool of cytochrome c within the mitochondrial intermembrane space. This molecular scenario sheds light into the mechanisms of cell death in chronic diseases linked to complex I deficiency and may have far-reaching implications for the development of new neuroprotective therapies for these incurable illnesses.
Materials and Methods
Animals and Treatment. Eight-week-old wild-type or Bax-deficient male mice received one i.p. injection of MPTP-HCl per day (30 mg/kg per day of free base; Sigma-Aldrich) for 5 consecutive days and were killed at 0, 2, 4, 7, 21, and 42 days after the last injection; control mice received saline injections only (n = 3–10 mice per time point, treatment, and genotype).
Subcellular Fractionation. Protein extraction of mitochondrial and cytosolic fractions was performed on fresh ventral midbrain tissue from saline- and MPTP-injected mice, as described (15).
Antibodies. The following primary antibodies were used for Western blot analysis: mouse monoclonal anti-cytochrome c (PharMingen); mouse monoclonal anti-cytochrome c oxidase-IV (Molecular Probes); rabbit polyclonal anti-cleaved caspase-3 (CM1; Idun Pharmaceuticals, La Jolla, CA); rabbit polyclonal anti-cleaved caspase-9 (Asp-353; Cell Signaling Technology, Beverly, MA); mouse monoclonal anti-β-actin (clone AC15; Sigma); mouse monoclonal anti-Bax (B-9; Santa Cruz Biotechnology); rabbit polyclonal anti-sulfite oxidase (gift from J. L. Johnson, Duke University Medical Center, Durham, NC); and goat polyclonal anti-HSP60 (Santa Cruz Biotechnology).
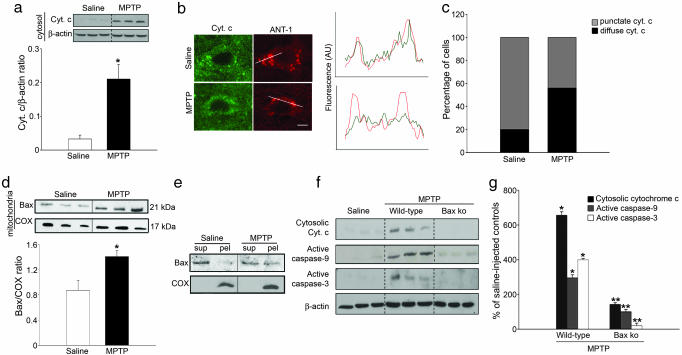
Immunofluorescence. For double immunofluorescence and confocal microscopy, a mouse monoclonal anti-cytochrome c (catalog no. 556432; PharMingen) and a rabbit polyclonal anti-adenine nucleotide translocase-1 (ANT-1; Oncogene, Boston) were used. Distribution of the fluorescent signal for both ANT-1 and cytochrome c stainings was analyzed by using the imagej 1.28u software (National Institutes of Health), similarly as described (16, 17). Briefly, the analysis is based on a comparison of the intensity profile of pixels generated by the two fluorochromes. A typical pixel profile generated along a straight line transecting a cell immunostained for ANT-1 will show highintensity pixels over mitochondria alternating with low-intensity pixels over the cytosol devoid of mitochondria. Thus, in a healthy cell, cytochrome c is confined to mitochondria, giving rise to a pixel profile for cytochrome c that overlaps with that of the mitochondrial marker (see Fig. 1b). Conversely, in a sick cell with cytochrome c translocation to the cytosol, the pixel profile for cytochrome c is more diffuse and diverges from that of the mitochondrial marker (see Fig. 1b).
Fig. 1.
Bax-dependent recruitment of mitochondrial apoptotic pathway following complex I inhibition in mice. (a) Cytochrome c levels are increased in ventral midbrain cytosolic fractions of MPTP-intoxicated mice at day 4 after the last MPTP injection, as determined by immunoblot. (b and c) Double immunofluorescence of substantia nigra pars compacta (SNpc) sections with cytochrome c (green) and the mitochondrial marker ANT-1 (red) show that in saline injected animals, 80% of SNpc neurons (n = 77) exhibit cytochrome c immunostaining colocalized with ANT-1, indicative of its mitochondrial localization. After complex I inhibition by MPTP, ≈60% of SNpc neurons (n = 206) show a diffuse cytochrome c staining no longer colocalized with ANT-1, indicative of its cytosolic redistribution. The fluorescence intensity profiles reported in the diagram correspond to the lines drawn in the confocal images (see Materials and Methods for details) (AU, fluorescence arbitrary units). (d) Bax levels are increased in ventral midbrain mitochondrial fractions at day 4 after the last MPTP injection, indicating Bax mitochondrial translocation. (e) In saline-injected animals, most of mitochondrial Bax appears in the supernatant fraction (Sup) after alkaline extraction, indicating its loose association with mitochondrial membranes. In contrast, after complex I blockade by MPTP, a significant fraction of mitochondrial Bax remains in the mitochondrial pellet (Pel), indicating its insertion into mitochondrial membranes. The inner mitochondrial membrane protein cytochrome oxidase was not extracted by alkaline treatment. (f and g) Genetic ablation of Bax in mutant mice (Bax ko) attenuates MPTP-induced cytochrome c release and caspase activation. *, P < 0.05, compared with saline-injected mice; **, P < 0.05, compared with MPTP-injected wild-type mice.
Alkaline Extraction. Ventral midbrain mitochondrial fractions from saline-injected and MPTP-intoxicated mice were resuspended in 0.1 M Na2CO3 (pH 11.5) and incubated for 30 min on ice. The membranes were then centrifuged (75,000 × g, 10 min), and both the pellet and the supernatant were analyzed by Western blot for Bax or cytochrome c oxidase.
Isolation of Brain Mitochondria, Polarography, and Cytochrome c Release Studies. Isolation of nonsynaptosomal brain mitochondria and monitoring of mitochondrial oxygen consumption were performed as described (18). For cytochrome c release experiments, 250 μg of isolated brain mitochondria were incubated for different lengths of time (from 15 to 60 min) with different amounts of recombinant oligomeric Bax (kindly provided by S. J. Korsmeyer, Dana–Farber Cancer Institute, Harvard Medical School, Boston), complex I inhibitors 1-methyl-4-phenylpyridinum ion (MPP+) or rotenone (Sigma-Aldrich) and/or the antioxidant M40401 or its inactive homologue M40404 (kindly provided by Metaphore Pharmaceuticals, Fort Lee, NJ). The percentage of cytochrome c release was estimated by assessing the intensities of the immunoblot bands for the soluble fractions versus total fractions (soluble + particulate). Two different types of buffers were used: low (225 mM mannitol/75 mM sucrose/10 mM KCl/5 mM Hepes/2 mM K2HPO4) and high (125 mM KCl/2 mM K2HPO4/1 mM MgCl2/5 mM Hepes) ionic strength.
Measurements of Mitochondrial H2O2 Production. Samples were prepared as for the polarographical study. H2O2, converted from superoxide by manganese-superoxide dismutase, was measured by using 5 μM Amplex red and 5 units/ml horseradish peroxidase, both in presence or absence of ADP, as described (19).
Cybrid Cells. Cybrid cell lines were constructed by using enucleated fibroblasts from Leber hereditary optic neuropathy (LHON) probands as mitochondria donors carrying 3460 LHON primary mutations and the osteosarcoma (143B.TK-)-derived 206 cell line as acceptor rho0 cell line. Parental and cybrid cell lines were grown in DMEM supplemented with 15% FBS/100 units/ml penicillin/100 μg/ml streptomycin at 37°C in an incubator with a humidified atmosphere of 5% CO2. Mitochondrial extraction, polarography, H2O2 production and cytochrome c release studies were performed as described above.
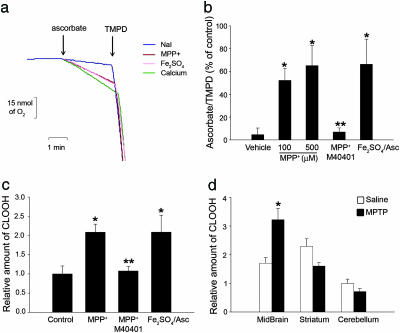
Ascorbate/N,N,N′,N′-Tetramethyl-1,4-Benzenediamine (TMPD) Assay. These experiments were performed as described (20). Briefly, 1 mg/ml mitochondria was incubated in sucrose buffer (0.2 M sucrose/10 mM Trisphosphate-4-morpholinepropanesulfonic acid, pH 7.4/1 mM Pi/5 mM glutamate/2.5 mM malate/10 μM EGTA-phosphate Tris, pH 7.4) and treated as indicated in Fig. 4. After the indicated time, 400 pmol carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and 1 nmol antimycin A per mg of protein-1 were added, and the reaction was transferred to a Clark-type oxygen electrode chamber. Final volume was 1 ml at 25°C. After 2 min, 6 mM ascorbate was added, followed by 300 μM TMPD 3 min thereafter. The ascorbate-driven oxygen consumption rate over the total TMPD-driven rate is plotted as a percentage of the ratio in the untreated mitochondria.
Fig. 4.
Complex I inhibition increases the soluble pool of cytochrome c in the mitochondrial intermembrane space by oxidizing cardiolipin. (a and b) Complex I inhibition by MPP+ induced a dose-dependent increase of mitochondrial ascorbate/TMPD-driven respiration ratio, consistent with an increased intermembrane soluble pool of cytochrome c. This effect was prevented by 50 μM M40401 and could be reproduced by the ROS-generating compound, Fe2SO4 (60 μM)/ascorbate (500 μM). Sodium iodide (NaI) was used as vehicle, because MPP+ was used in the form of MPP+-I. Ca2+-mediated mitochondrial swelling, which increases the soluble pool of cytochrome c (20), was used as a positive control. (c) Complex I inhibition by MPP+ induced oxidation of inner mitochondrial membrane cardiolipin in isolated brain mitochondria, as assessed by determining cardiolipin hydroperoxide (CLOOH) content by HPLC. Oxidation of cardiolipin was also produced by Fe2SO4/ascorbate and was attenuated by M40401. (d) Oxidized cardiolipin was detected in ventral midbrain samples from MPTP-intoxicated mice but not in regions devoided of MPTP-induced cell loss, such as striatum and cerebellum. *, P < 0.05, compared with controls; **, P < 0.05, compared with MPP+-treated mitochondria.
MPP+–Cardiolipin Interaction Assay. Different amounts of [3H]-MPP+ were incubated in a cardiolipin-coated ELISA plate (Alpha Diagnostics, San Antonio, TX) for 15 min. Residual radioactivity was measured after washing out radioactive MPP+.
Lipid Extraction and HPLC. Lipids from isolated brain mitochondria were extracted as described (21). Lipid extraction from midbrain, striatal, and cerebellar brain mitochondria was performed by pooling the above-mentioned anatomical regions from five different saline- or MPTP-intoxicated mice. The HPLC measurements were carried out by using an adaptation of a previously described method (21).
Statistical Analysis. All values are expressed as the mean ± SEM. Differences among means were analyzed by using one- or two-way ANOVA with time, treatment, or genotype as the independent factors. When ANOVA showed significant differences, pair-wise comparisons between means were tested by Student–Newman–Keuls post hoc testing. In all analyses, the null hypothesis was rejected at the 0.05 level.
Results
Complex I Inhibition Relies on Bax to Engage Mitochondrial-Dependent Apoptosis. Bax, a proapoptotic member of the Bcl-2 family, plays a critical role in the demise of dopaminergic neurons provoked by complex I inhibition (8). In most circumstances, including the pharmacological blockade of complex I (22), Bax-mediated cell death is accompanied with a mitochondrial release of cytochrome c and activation of caspase-9 and -3. We confirmed that mitochondrial release of cytochrome c does occur in MPTP-intoxicated mice (Fig. 1). This molecular event is time-dependent and coincides with the induction (8) and relocation of Bax from the cytosol to the mitochondria (Fig. 1). We also show that Bax mitochondrial translocation and cytochrome c release parallel the activation of downstream caspases (Fig. 1) and the previously reported time course of neuronal apoptosis caused by MPTP (8). Supporting the pivotal role of Bax in this molecular cascade is the demonstration that the release of cytochrome c and the activation of caspases are absent in Bax-deficient mutant mice treated with MPTP (Fig. 1 f and g); these mice were previously found resistant to MPTP-induced neurodegeneration (8). However, abrogation of Bax in mutant mice did not impair the potency of MPTP to inhibit complex I (Fig. 6, which is published as supporting information on the PNAS web site). Our results thus indicate that complex I deficiency operates together with Bax to engage the mitochondrial-dependent apoptotic pathway in vivo.
Inhibition of Complex I Potentiates Bax-Induced Cytochrome c Release. We next ascertained in isolated brain mitochondria the respective roles of complex I deficiency and Bax activation in the recruitment of the mitochondrial-dependent neuron death program, as well as the molecular basis for their interaction. First, we incubated purified brain mitochondria with different concentrations of MPTP's active metabolite, MPP+, or rotenone. These experiments confirmed that both MPP+ and rotenone caused, in a dose-dependent manner: (i) a reduction of ADP-stimulated oxygen consumption (state 3 respiration) supported by the NADH-linked substrates glutamate/malate (Fig. 2a and Fig. 7, which is published as supporting information on the PNAS web site); and (ii) an increased production of reactive oxygen species (ROS) (Figs. 2b and 7), likely generated by a higher rate of molecular oxygen reduction into superoxide radical in response to the hampered terminal step of electron transfer from the highest potential iron–sulfur cluster of complex I to ubiquinone (23). Contrary to the effect of complex I deficiency on cytochrome c release in intact cells in vivo, we found no evidence that MPP+- or rotenone-induced complex I inhibition elicited a release of cytochrome c from purified brain mitochondria, even when oxygen consumption was reduced by nearly 100% (Figs. 2c and 7). Recombinant oligomeric Bax protein, at concentrations as high as ≈100 nM and in the absence of complex I inhibitors, elicited only a minimal, not statistically significant, cytochrome c release (≈18%) from brain mitochondria (Fig. 2c). Unexpectedly, when brain mitochondria were incubated with both recombinant Bax and complex I inhibitors, up to 65% of the mitochondrial cytochrome c was released (Figs. 2c and 7). Thus, neither complex I inhibition nor permeabilization of the outer membrane with Bax, alone, triggers overt release of cytochrome c, whereas their combination results in a marked release (>60%) of this proapoptotic molecule.
Fig. 2.
Complex I inhibition stimulates ROS production and promotes Bax-dependent cytochrome c release in isolated brain mitochondria. (a) MPP+ induces a dose-dependent inhibition of complex I-driven mitochondrial respiration, as assessed by monitoring oxygen consumption after addition of ADP, which in normal mitochondria induces a transient mitochondrial depolarization with a subsequent burst of oxygen consumption (state 3 respiration) until the added ADP is converted to ATP (state 4). (b) Complex I inhibition by MPP+ induces dose-dependent ROS production in brain mitochondria, as assessed by measuring H2O2 using the fluorescent dye Amplex Red. (c) Complex I inhibition with 100 μM MPP+ or incubation with ≈100 nM recombinant Bax, alone did not trigger significant release of cytochrome c from isolated brain mitochondria. However, combining complex I inhibition with recombinant Bax resulted in a marked release (>60%) of cytochrome c. This effect was abolished by 50 μM of the superoxide dismutase mimetic M40401. Matrix mitochondrial protein HSP60 was not mobilized by any of the tested conditions. *, P < 0.05, compared with untreated mitochondria; **, P < 0.05, compared with mitochondria treated with MPP+ and recombinant Bax.
ROS Are Mandatory for the Interaction Between Complex I Inhibition and Bax. We next explored the molecular basis for the observed mobilization of cytochrome c release induced by the combination of complex I inhibition and Bax. None of the tested conditions (complex I inhibition, recombinant Bax, or both) caused a release of the matrix mitochondrial heat-shock protein HSP-60 (Fig. 2c), ruling out the possibility that the mobilization of cytochrome c observed by combining complex I inhibition and Bax resulted from a mitochondrial structural damage. Because the ionic strength of the mitochondrial buffer may modify the electrostatic attachment of cytochrome c to the inner membrane and, therefore, the ability of the mitochondria to release cytochrome c (21), it is noteworthy that comparable results were obtained with low (mannitol/sucrose/Hepes) and high (KCl) ionic strength buffers (see Materials and Methods). Furthermore, sulfite oxidase, a soluble mitochondrial intermembrane protein, was not released by complex I inhibition alone. Sulfite oxidase was released by recombinant Bax but, in contrast to cytochrome c, no enhancement of its release was observed by combining Bax with complex I inhibition (not shown). These results indicate that complex I dysfunction alone is unable to engage the mitochondrial-dependent apoptotic pathway. They also demonstrate that activated oligomeric Bax, while able to permeabilize the outer mitochondrial membrane, stimulates only significant release of cytochrome c in conjunction with complex I defect.
Remarkably, the enhanced release of cytochrome c obtained by combining Bax and complex I inhibition was abolished by the lipophilic superoxide dismutase mimetic M40401 (24), but not by its inactive homologue M40404 (Figs. 2c and 7), indicating that this interaction relies on the mitochondrial production of ROS. Supporting this assertion is the fact that oxidative stress generated by Fe2SO4/ascorbate also enhances the release of cytochrome c induced by recombinant Bax in isolated rat liver mitochondria (21).
A Genetic Defect in Complex I Also Potentiates Bax-Induced Cytochrome c Release. Because inhibitors of complex I cause an acute loss of mitochondrial respiration, we sought to confirm the above findings in a chronic genetic model of complex I deficiency. Accordingly, we used an osteosarcoma-derived cytoplasmic hybrid (cybrid) cell line harboring the pathogenic point mutation of the mitochondrial DNA 3460G>A in complex I's ND1 subunit gene, which causes a deficit of complex I activity (25). In humans, the 3460/ND1 mutation is associated with massive retinal ganglion cell degeneration and loss of central vision in Leber hereditary optic neuropathy (26). Upon incubation in galactose-enriched medium, 3460/ND1 cybrids die by activating the mitochondrial-dependent apoptotic pathway, as shown by the release of cytochrome c (27).
We found that isolated mitochondria from 3460/ND1 cybrids, but not from wild-type cybrids or parental osteosarcoma cells, exhibited reduced complex I-driven mitochondrial respiration (Fig. 3a) associated with an increased production of ROS that could be quenched by M40401 (Fig. 3b). Similar to what we observed with pharmacological inhibition of complex I, the genetic disruption of mitochondrial respiration in 3460/ND1 cybrids was not associated with any detectable release of cytochrome c from isolated mitochondria in the absence of Bax, whereas ≈50% of cytochrome c was released in these mutant cybrids in the presence of recombinant oligomeric Bax (Fig. 3c). The latter effect was markedly attenuated in mitochondria isolated from 3460/ND1 cybrids grown in the presence of M40401 and was not observed in mitochondria isolated from wild-type cybrids or parental osteosarcoma cells (Fig. 3c).
Fig. 3.
Genetic disruption of complex I in cybrid cells stimulates ROS production and promotes Bax-dependent cytochrome c release. (a) Isolated mitochondria from mutant 3460/ND1 cybrids (cybrid mt) exhibit reduced complex I-driven mitochondrial respiration, as assessed by monitoring oxygen consumption supported by NADH-linked substrates glutamate/malate. (b) Impairment of mitochondrial respiration in mutant 3460/ND1 cybrids is associated with an increased production of ROS that was quenched by 50 μM of M40401. (c) Recombinant Bax (≈100 nM) induced a marked release (≈50%) of cytochrome c in mitochondria isolated from mutant 3460/ND1 cybrids but not from wild-type cybrids (cybrid wt) or parental osteosarcoma cells. This effect was attenuated by 50 μM M40401. *, P < 0.05, compared with noncybrid and cybrid wild-type mitochondria; **, P < 0.05, compared with mutant cybrid mitochondria.
Inhibition of Complex I Increases the Soluble Pool of Cytochrome c in the Mitochondrial Intermembrane Space in a ROS-Dependent Manner. Under physiological conditions, most cytochrome c is bound to the inner mitochondrial membrane by anionic phospholipids, primarily cardiolipin, whereas only a fraction is soluble in the intermembrane space and thus releasable upon outer membrane permeabilization (20). Therefore, we asked whether oxidative damage linked to complex I inhibition could increase the soluble pool of cytochrome c in the intermembrane space. Because ascorbate is capable of reducing only soluble cytochrome c, whereas the uncharged reductant TMPD is membrane-permeant and reaches all cytochrome c, the ratio of ascorbate-driven respiration over the total TMPD-driven respiration provides an index of cytochrome c soluble pool (20). Inhibition of complex I by MPP+ resulted in a dose-dependent increase of mitochondrial ascorbate/TMPD-driven respiration ratio (Fig. 4 a and b), consistent with an increased intermembrane space soluble pool of cytochrome c. This effect was also prevented by M40401 (Fig. 4b) and could be reproduced by Fe2SO4/ascorbate (Fig. 4 a and b), indicating its dependency on ROS production.
ROS Generated by Complex I Inhibition Oxidize the Inner Mitochondrial Lipid Cardiolipin both in Vitro and in Vivo. It has been shown in isolated rat liver mitochondria that cytochrome c can be freed from the inner mitochondrial membrane by oxidative modifications of cardiolipin (21). Therefore, we tested whether cardiolipin peroxidation occurred after complex I blockade. After having excluded the possibility that MPP+ displaces cytochrome c from the negatively charged cardiolipin by simple electrostatic interaction (see Materials and Methods), we assessed by HPLC the contents of oxidized cardiolipin in isolated brain mitochondria. Complex I blockade by MPP+ resulted in a marked increase of oxidized cardiolipin that was attenuated by M40401, indicating its dependency on ROS production (Fig. 4c). Furthermore, incubation of isolated brain mitochondria with the ROS-generating system Fe2SO4/ascorbate also increased cardiolipin oxidation (Fig. 4c). Supporting a role in neurodegeneration induced in vivo by complex I inhibition, cardiolipin oxidation was also detected in mitochondria isolated from ventral midbrain of mice intoxicated with MPTP (Fig. 4d) in a time-dependent manner that preceded activation of Bax and apoptotic neuron death in this model of complex I deficiency (8).
Discussion
The mitochondrial-dependent apoptotic pathway has been shown to be instrumental in the neuronal degeneration associated with disruption of mitochondrial respiration caused by complex I deficiency, as demonstrated by targeting molecules of this pathway such as Bax, caspase-9, or Apaf-1 (8, 22, 28). Our study, while confirming that complex I defects lead to a recruitment of the mitochondrial-dependent apoptotic pathway in vivo, sheds light onto the molecular mechanisms linking these two events. For instance, after MPTP administration, there is indeed a time-dependent and region-specific mitochondrial release of cytochrome c that occurs in association with activation of both caspase-9 and -3. All of these molecular alterations appear to be regulated by the death agonist Bax, because they coincide with Bax up-regulation and translocation to the mitochondria and are prevented by genetic ablation of Bax. Although Bax induction was previously shown to rely on p53 activation after complex I inhibition (29), the mechanism driving Bax mitochondrial translocation after complex I inhibition remains to be determined (10). Both Bid and Bak are known for cooperating with Bax to initiate mitochondrial-dependent apoptosis in response to the ligation of cell-surface death receptors. However, in contrast to the pivotal role of Bax, both Bid and Bak have been shown to be dispensable in complex I deficiency-mediated neuronal death (22, 30).
Our study also clarifies the process by which complex I defects contribute to the actual recruitment of the mitochondrial-dependent cell death pathway. It has been widely assumed that complex I inhibition and the subsequent impairment of mitochondrial respiration directly trigger the release of cytochrome c from the affected mitochondria (11–14). Contrary to this view, our data demonstrate that complex I inhibition does not directly induce cytochrome c release from isolated brain mitochondria but, instead, it increases the “releasable” soluble pool of cytochrome c in the mitochondrial intermembrane space that can be subsequently released to the cytosol by some cell death agonists, such as Bax.
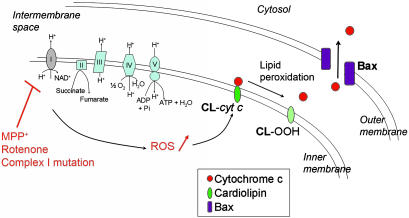
In both the pharmacologic and genetic models used here, the initiating event is complex I dysfunction, which, in turn, stimulates the mitochondrial production of ROS. Because the latter can be abated by M40401, it can be asserted that the endogenous antioxidant arsenal of the mitochondria is not sufficient to scavenge the excess of ROS generated by the complex I defect. We can also state that, because M40401 is a superoxide dismutase mimetic devoid of catalase activity (24), superoxide is the main reactive species produced. Yet, superoxide poorly penetrates membranes, hence its damaging effects are likely to be confined to the mitochondrial matrix and inner membrane. Although a broad range of molecules is likely modified by ROS in response to complex I dysfunction, our HPLC data indicate that cardiolipin is among the targets. Therefore, our data argue that complex I inhibition produces a mitochondrial oxidative damage that includes cardiolipin peroxidation. The latter alteration affects the binding of cytochrome c to the mitochondrial inner membrane, leading to an increased soluble pool of cytochrome c in the intermembrane space. Consequently, upon permeabilization of the outer mitochondrial membrane by activated Bax, a larger amount of mitochondrial cytochrome c can be released, making compromised neurons more likely to undergo apoptosis (Fig. 5).
Fig. 5.
Proposed pathogenic scenario induced by complex I deficiency. Pharmacological or genetic inhibition of complex I disrupts mitochondrial respiration and stimulates the mitochondrial production of ROS. As a consequence, an array of molecules is likely oxidatively modified in response to complex I defect, including the inner mitochondrial membrane lipid cardiolipin. Cardiolipin peroxidation, in turn, affects the binding of cytochrome c to the mitochondrial inner membrane, leading to an increased soluble pool of cytochrome c in the intermembrane space. Consequently, upon permeabilization of the outer mitochondrial membrane by activated Bax, a larger amount of mitochondrial cytochrome c can be released, making it more likely for a compromised neuron to undergo apoptosis (see Discussion for more details).
Mobilization of the cytochrome c stores has been previously associated to a remodeling of mitochondrial cristae structure by Bid (20). Because Bid is not required for cytochrome c release in the context of complex I inhibition (22), and rotenone does not induce ultrastructural changes in mitochondrial morphology (11), it is unlikely that such a remodeling accounts for the observed ROS-related mobilization of cytochrome c associated with complex I inhibition. Instead, our results support the concept that oxidative modifications of cardiolipin may be responsible for the increased intermembrane stores of soluble cytochrome c following complex I deficiency.
Conclusion
Our study supports a pathogenic scenario in which complex I deficiency, which occurs in numerous neurodegenerative situations, does not autonomously kill cells, but rather sensitizes neurons to the action of death agonists such as Bax, through mitochondrial oxidative damage. Our results also provide a molecular basis to the observation of strong allometric correlations between mitochondrial ROS production and rates of brain and retinal neurodegeneration (31) and support the concept that steady-state ROS production, acting on the mitochondrial stress integration machinery, may be pivotal in setting the threshold for the occurrence of apoptosis for a given species and cell type in response to a cellular stress (31). Finally, our study shows that membrane-permeant antioxidants block the neuronal death-promoting interaction between mitochondrial oxidative damage and Bax-induced permeabilization of the outer mitochondrial membrane, suggesting that these compounds, of which a few are near approval for human use, may prove effective in mitigating neurodegeneration in complex I cytopathies.
Supplementary Material
Acknowledgments
We thank Drs. Gary Fiskum (University of Maryland School of Medicine, Baltimore), Paolo Bernardi (University of Padova, Padova, Italy), Luca Scorrano (Venetian Institute of Molecular Medicine, Padova), Ian Reynolds (University of Pittsburgh, Pittsburgh), and Suzanne L. Iverson and Sten Orrenius (Karolinska Institute, Stockholm) for their thoughtful comments and/or technical advice, as well as Mr. Matthew Lucas for assistance in preparing this manuscript. We also thank Metaphore Pharmaceuticals (Fort Lee, NJ) for providing the antioxidant M40401 and its inactive homologue M40404 and Stanley J. Korsmeyer (Dana–Farber Cancer Institute) for providing recombinant oligomeric Bax. This study was supported by National Institutes of Health National Institute on Aging Grant AG21617 (to M.V. and S.P.); National Institute of Neurological Disorders and Stroke Grants NS38586, NS42269, NS38370, and NS11766 (to S.P.); U.S. Department of Defense Grants DAMD 17-03-1-0482 (to M.V.) and DAMD 17-99-1-9474, 17-03-1-0002 (to S.P.); Morris K. Udall Parkinson's Disease Research Center of Excellence Grant NS38370; the Lowenstein Foundation; the Lillian Goldman Charitable Trust; the Parkinson's Disease Foundation; the National Parkinson Foundation; the American Parkinson Disease Association; the Muscular Dystrophy Association/Wings Over Wall Street; and the Telethon Fondazione Onlus (Italy).
Author contributions: C.P., M.H., S.P., and M.V. designed research; C.P., K.T., C.G., C.C., V.J.-L., and M.V. performed research; V.C., A.M., and M.H. contributed new reagents/analytic tools; C.P. and M.V. analyzed data; and S.P. and M.V. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PD, Parkinson's disease; MPTP, 1-methyl-4-pheny-1,2,3,6-tetrahydropyridine; ROS, reactive oxygen species; ANT-1, adenine nucleotide translocase-1; MPP+, 1-methyl-4-phenylpyridinum ion; TMPD, N,N,N′,N′-tetramethyl-1,4-benzenediamine.
References
- 1.Schon, E. A. & Manfredi, G. (2003) J. Clin. Invest. 111, 303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace, D. C., Singh, G., Lott, M. T., Hodge, J. A., Schurr, T. G., Lezza, A. M. S., Elsas, L. J. & Nikoskelainen, E. K. (1988) Science 242, 1427-1430. [DOI] [PubMed] [Google Scholar]
- 3.Parker, W. D., Jr., Boyson, S. J. & Parks, J. K. (1989) Ann. Neurol. 26, 719-723. [DOI] [PubMed] [Google Scholar]
- 4.Schapira, A. H., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P. & Marsden, C. D. (1990) J. Neurochem. 54, 823-827. [DOI] [PubMed] [Google Scholar]
- 5.Dauer, W. & Przedborski, S. (2003) Neuron 39, 889-909. [DOI] [PubMed] [Google Scholar]
- 6.Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V. & Greenamyre, J. T. (2000) Nat. Neurosci. 3, 1301-1306. [DOI] [PubMed] [Google Scholar]
- 7.Qi, X. P., Lewin, A. S., Hauswirth, W. W. & Guy, J. (2003) Ann. Neurol. 53, 198-205. [DOI] [PubMed] [Google Scholar]
- 8.Vila, M., Jackson-Lewis, V., Vukosavic, S., Djaldetti, R., Liberatore, G., Offen, D., Korsmeyer, S. J. & Przedborski, S. (2001) Proc. Natl. Acad. Sci. USA 98, 2837-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatton, N. A. & Kish, S. J. (1997) Neuroscience 77, 1037-1048. [DOI] [PubMed] [Google Scholar]
- 10.Vila, M. & Przedborski, S. (2003) Nat. Rev. Neurosci. 4, 365-375. [DOI] [PubMed] [Google Scholar]
- 11.Clayton, R., Clark, J. B. & Sharpe, M. (2005) J. Neurochem. 92, 840-849. [DOI] [PubMed] [Google Scholar]
- 12.Green, D. R. & Kroemer, G. (2004) Science 305, 626-629. [DOI] [PubMed] [Google Scholar]
- 13.Kroemer, G. & Reed, J. C. (2000) Nat. Med. 6, 513-519. [DOI] [PubMed] [Google Scholar]
- 14.Cassarino, D. S., Parks, J. K., Parker, W. D., Jr., & Bennett, J. P., Jr. (1999) Biochim. Biophys. Acta 1453, 49-62. [DOI] [PubMed] [Google Scholar]
- 15.Guegan, C., Vila, M., Rosoklija, G., Hays, A. P. & Przedborski, S. (2001) J. Neurosci. 21, 6569-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petronilli, V., Penzo, D., Scorrano, L., Bernardi, P. & Di, L. F. (2001) J. Biol. Chem. 276, 12030-12034. [DOI] [PubMed] [Google Scholar]
- 17.Daugas, E., Susin, S. A., Zamzami, N., Ferri, K. F., Irinopoulou, T., Larochette, N., Prevost, M. C., Leber, B., Andrews, D., Penninger, J., et al. (2000) FASEB J. 14, 729-739. [PubMed] [Google Scholar]
- 18.Tieu, K., Perier, C., Caspersen, C., Teismann, P., Wu, D. C., Yan, S. D., Naini, A., Vila, M., Jackson-Lewis, V., Ramasamy, R., et al. (2003) J. Clin. Invest. 112, 892-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starkov, A. A., Polster, B. M. & Fiskum, G. (2002) J. Neurochem. 83, 220-228. [DOI] [PubMed] [Google Scholar]
- 20.Scorrano, L., Ashiya, M., Buttle, K., Weiler, S., Oakes, S. A., Mannella, C. A. & Korsmeyer, S. J. (2002) Dev. Cell 2, 55-67. [DOI] [PubMed] [Google Scholar]
- 21.Ott, M., Robertson, J. D., Gogvadze, V., Zhivotovsky, B. & Orrenius, S. (2002) Proc. Natl. Acad. Sci. USA 99, 1259-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viswanath, V., Wu, Y., Boonplueang, R., Chen, S., Stevenson, F. F., Yantiri, F., Yang, L., Beal, M. F. & Andersen, J. K. (2001) J. Neurosci. 21, 9519-9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsay, R. R., Kowal, A. T., Johnson, M. K., Salach, J. I. & Singer, T. P. (1987) Arch. Biochem. Biophys. 259, 645-649. [DOI] [PubMed] [Google Scholar]
- 24.Salvemini, D., Wang, Z. Q., Zweier, J. L., Samouilov, A., Macarthur, H., Misko, T. P., Currie, M. G., Cuzzocrea, S., Sikorski, J. A. & Riley, D. P. (1999) Science 286, 304-306. [DOI] [PubMed] [Google Scholar]
- 25.Howell, N., Bindoff, L. A., McCullough, D. A., Kubacka, I., Poulton, J., Mackey, D., Taylor, L. & Turnbull, D. M. (1991) Am. J. Hum. Genet. 49, 939-950. [PMC free article] [PubMed] [Google Scholar]
- 26.Carelli, V., Ross-Cisneros, F. N. & Sadun, A. A. (2004) Prog. Retin. Eye Res. 23, 53-89. [DOI] [PubMed] [Google Scholar]
- 27.Ghelli, A., Zanna, C., Porcelli, A. M., Schapira, A. H. V., Martinuzzi, A., Carelli, V. & Rugolo, M. (2003) J. Biol. Chem. 278, 4145-4150. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki, H., Hayakawa, H., Migita, M., Shibata, M., Tanaka, R., Suzuki, A., Shimo-Nakanishi, Y., Urabe, T., Yamada, M., Tamayose, K., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10918-10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan, W., Zhu, X., Ladenheim, B., Yu, Q. S., Guo, Z., Oyler, J., Cutler, R. G., Cadet, J. L., Greig, N. H. & Mattson, M. P. (2002) Ann. Neurol. 52, 597-606. [DOI] [PubMed] [Google Scholar]
- 30.Fannjiang, Y., Kim, C. H., Huganir, R. L., Zou, S. F., Lindsten, T., Thompson, C. B., Mito, T., Traystman, R. J., Larsen, T., Griffin, D. E., et al. (2003) Dev. Cell 4, 575-585. [DOI] [PubMed] [Google Scholar]
- 31.Wright, A. F., Jacobson, S. G., Cideciyan, A. V., Roman, A. J., Shu, X. H., Vlachantoni, D., McInnes, R. R. & Riemersma, R. A. (2004) Nat. Genet. 36, 1153-1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.