Abstract
This, our Inaugural Article as Academy Members, is ironically our swan song from the field of the actin cytoskeleton. By reviewing what we have learned and what we think is going on during development, we hope to lure you, the reader, into applying your skills to the bristle cell. The processes of the assembly and disassembly of actin bundles is laid out in time and space in an organism that lends itself to genetic manipulation. The cell provides every process you could want: filament nucleation, growth of microvilli, joining of microvillar bundles into modules, assembly of modules into bundles, time-dependent use of at least two crossbridging proteins, filament turnover, treadmilling, disassembly, and filament translocation.
Keywords: cytoskeleton, development
Many of the morphological features of animals are driven, albeit indirectly, by changes in cell shape. Cell shape is a consequence of cytoskeletal structures within the cell, but, because there are so many players that can act as cytoskeletal agents, we need some way of sorting out what each can do. We have long been advocates for specialized cells as useful in selecting subsets of these agents. The idea is that specialized cells build specialized cytoskeletal structures from some subset of the cytoskeletal proteins. Often, they build these structures in a particular location and at a particular developmental time and thus expose cytoskeletal pathways. In our earlier work, we examined the sperm cells of marine animals such as the horseshoe crab, and, in our later work, we peered into hair cells of the inner ear of the alligator lizard and chicken. More recently, we studied nurse cells in Drosophila, an organism with the potential for genetic manipulation. Over the years we have collected insights into the processes that generate form, but more information is needed before the mechanisms can be understood. We write this review as our final contribution to the field and as an encouragement to others to step in with the more elegant tools now available.
Why Bother with Drosophila Bristles?
Most recently, we selected the Drosophila bristle as a window into the role of the actin cytoskeleton, in this case actin bundles, in defining cell shape. Our rationale for this choice is that mutants affecting bristle morphology have been collected since 1912, that the locations of the affected genes on the four Drosophila chromosomes have been established, and that most of these mutants, which now number well over 100, are readily available. Why were bristles so popular in genetic studies? The answer is simply that alterations in bristle morphology are easy to detect in living flies, that mutants in bristle genes tend not to be lethal, and that small changes in the actin cytoskeleton induced by drugs or mutations often result in an easily detectable phenotype. Accordingly, Morgan and Bridges (1) and subsequent investigators used many of these mutant genes as chromosomal markers relative to which other genes could be mapped. Within the past 10 years, more bristle mutants have been isolated, and currently a bristle-specific promoter has been identified. Because the actin bundles in bristles are derived developmentally from microvilli (2), these bundles are assembled differently from the well studied lamellopodia in moving cells. Drosophila bristles are an attractive, tractable model system, and we encourage new investigators to dig in.
The Bristle, Nerve, Sheath, and Supporting Cells Arise from a Single Precursor Cell
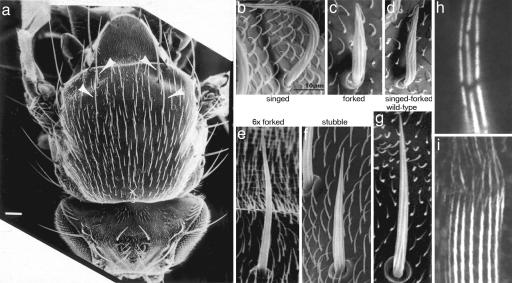
Although bristles are present on the head, thorax, abdomen, wings, and legs, we concentrated on those on the dorsal thorax because they occur here in the greatest number. Decorating the thorax are 22 precisely positioned bristles called macrochaetes, each 250–300 μm long, and 200 or more 70-μm-long bristles termed microchaetes (Fig. 1a). Moreover, there are scads of 10- to 20-μm-long hairs, or trichomes, which are related in some key aspects to the bristle yet differ in others. The hairs provide a developmental structure against which to compare results from the bristles. Each bristle cell arises from the sensory organ precursor cell (SOP), which divides two times to form four cells. The first division of the SOP gives rise to two cells, one of which is always anterior to the other. Both cells divide a second time. The daughters of the anterior cell form a sheath cell, which corresponds in function to a glial or Schwann cell in vertebrates, and a neuronal cell. The posterior cell divides to produce a trichogen or bristle cell and a tormogen or socket cell. The socket cell surrounds the base of the bristle cell body and shaft. The bristles in adult flies are mechanoreceptors and in some cases chemoreceptors, and thus the dendritic extension of the nerve cell innervates the bristle shaft. This dendritic extension is attached to the upper surface of the bristle from the earliest stages in bristle elongation, which begins in 32-h-old pupae. What is important here is that the cytoplasm of the bristle is an extension of a single cell whose giant polyploid nucleus and centrosome reside in the thorax proper.
Fig. 1.
Scanning electron and light micrographs of fly bristles and hairs. (a) The thorax of an adult, wild-type fly. The arrows point to four macrochaete bristles. The shorter bristles are microchaetes. (Scale bar, 100 μm.) [Reprinted with permission from ref. 6 (Copyright 1995, The Rockefeller University Press).] (b–g) Representative microchaetes from mutant flies. Surrounding the microchaetes are the much shorter hairs. [Reprinted with permission from ref. 3 (Copyright 2004, American Society for Cell Biology).] (h) Light micrograph of fluorescent bundles from wild-type, 48-h-old pupae. Note that the bundles are made of modules, which tend to be in transverse register. [Reprinted with permission from ref. 4 (Copyright 1996, The Rockefeller University Press).] (i) Light micrograph from bundles in a bristle from a 33-h-old pupa. The bundles are stained with fluorescent antibody against the forked protein. Note how the gaps in the modules fill in as one moves from the tip to the base. (Scale bar, 5 μm.) [Reprinted with permission from ref. 11 (Copyright 2003, The Rockefeller University Press).]
Flies Are Particular About Their Bristle Geometry
Both macrochaetes and microchaetes curve posteriorly over the thorax like the slicked back, ducktail hairstyle of the fifties, not like the spiked hair of a punk rocker or the unkempt hair of Einstein. Mutants (Fig. 1 b–g) rearrange the geometry so that the bristles appear singed or suffer from split ends (forked) or stick up (javelin). In the well groomed fly, what determines or fixes the bristle's geometric parameters (i.e., the direction each bristle lies along the fly's body, the take-off angle each bristle makes with the thorax surface, the curvature of each bristle, and the diameter and length each bristle attains)? Are all of the parameters set by some single, diabolically clever developmental pathway, or might they be set by parallel developmental pathways? A bit is known, and a bit more is hinted at, but here is the stuff of the puzzle we posed at the outset.
The direction of each bristle relative to the axis of the fly's body is fixed but depends on location on the fly's body. The dendrite associated with the bristle is always on the side away from the direction the bristle falls, suggesting that the dendrite is the determinant of the direction of the bristle relative to the body axis (3). The length and diameter of the bristle correlate with the size of the cell and its oversized polyploid nucleus, and one imagines that bigger bristles require and perhaps are determined by the degree of polyploidy, which determines the cell's ability to deliver the components in a timely fashion. What is unknown is what causes the curvature of the bristle; it may well be a consequence of the cell's cytoskeleton, and we will return to the topic anon.
Actin Filaments, Not Microtubules, Are Essential for Bristle Growth
Because the bristle cytoplasm contains actin filament bundles as well as microtubules, we first had to establish which filament type was responsible for bristle elongation. Inhibitors of actin assembly, such as latrunculin or cytochalasin, rapidly arrest bristle elongation. On the other hand, nocodazole, colchicine, and vinblastine, inhibitors of microtubule assembly, do not affect bristle elongation (2).
The Actin Bundles in Bristles Are Composed of a Series of Short Units or Modules Attached End to End
In microchaetes there are 7–11 actin bundles, which are evenly spaced around the outer perimeter of the bristle just inside the membrane and which extend from the base of the bristle to its tip 65–70 μm away. In macrochaetes, the situation is the same except that there are 12–18 bundles that cover the bigger girth and that extend out 350 μm in length. By decorating the actin bundle with subfragment 1 of myosin to determine filament polarity, we know that (i) all of the filaments within each module have identical polarity and (ii) the barbed ends of the filaments in every module are located at the bundle end nearest the bristle tip (4). We wondered, how long are the actin filaments in these bundles? When we used fluorescent phalloidin to examine the actin bundles in bristles that had elongated to their mature lengths, we were surprised to discover that there were breaks or small gaps in each bundle. This and subsequent studies showed that each bundle was composed of units or modules of crosslinked actin filaments 1–5 μm in length (4). What was particularly surprising was that the gaps between adjacent modules were often in transverse register, an observation we will return to later (Fig. 1h).
Why Are the Long Actin Bundles Composed of Modular Units Attached End to End?
We presume that the answer lies in the need to form a curved bristle. The bristle and its scaffolding of bundles are curved, and a modular design for actin bundles “makes sense” because bundles made by continuous elongation from a short bundle base are ramrod straight, as is the case in the 60-μm-long, stiff stereocilia of the alligator lizard. To accommodate or perhaps to generate curvature, the bundles over the upper, outer surface must be longer than those on the lower, inner surface while those along the lateral margins must be intermediate in length. An obvious way of achieving this pattern is to form each bundle from units or modules and join the modular units together with a glue that fills in the intermodule gaps of different lengths.
How Are Successive Modules Attached to Each Other to Produce Rigid Bundles?
Examination of fluorescent actin filaments by confocal microscopy reveals that, near the tip of an elongating bristle, each of the 7–11 bundles around the bristle periphery is composed of discrete modules separated by weakly fluorescent or nonfluorescent gaps. In bristles that have developed to two-thirds their mature length, the gaps disappear as one moves away from the tip toward the base, and one finds instead a solid and continuous fluorescent bundle that extends to the base of the bristle shaft (Fig. 1i). At the same time, the fluorescence intensity within the modules just basal to the bristle tip increases to the point at which the modules disappear into a continuous bundle. From these images it seems clear that gaps are filled in by a glue of filamentous actin, which accounts for the disappearance of the modules as discrete units, and that the modules are increasing in width by the lateral addition of actin filaments, which accounts for the increase in fluorescent intensity. These observations were confirmed by the examination of the bundles in electron micrographs.
Crosslinking Proteins Join Actin Filaments into Bundles
From the many publications on actin bundles, as well as those specifically on Drosophila bristles, we now recognize that, to form a rigid actin bundle, a cell must crosslink adjacent actin filaments. That said, crosslinking of actin filaments in a bundle is determined by the geometry of the sites on actin to which the crosslinking proteins bind and the disposition of the corresponding actin-binding sites on the crosslinkers. Thus, a particular crosslinker requires a particular arrangement of actin filaments. In most bundles, actin filaments are in transverse register. The greater the number of crosslinks between filaments, the more rigid the bundle. Because each actin filament is a helix composed of monomers, each with binding sites for crosslinking proteins, crosslinking is limited to positions along the filament where the binding sites have the geometry dictated by the corresponding sites on the crosslinker. Accordingly, the geometry of the helix, given a specific crosslinker, specifies the maximum number and position of the crosslinks (5).
Moreover, we found that, besides fascin, there are additional crosslinkers (2, 6), albeit in reduced copy number relative to fascin; a major one is the forked protein (6). Other kinds of actin bundles, for example, those found in microvilli and stereocilia, also contain two or more crosslinkers per bundle (7), but what is the role of each kind of crosslinker?
Why Are There Two or More Crosslinkers Used in Bristle Bundles?
The main advantage of studying Drosophila bristles is that genetic and/or molecular biological techniques can be readily used. Thus, we were able to increase or reduce the dosage of the crosslinker. From such studies, from the analysis of in vitro extractions of specific crosslinkers, and from our immunofluorescence observations, it became clear that the key to understanding why two or more crosslinkers are used lies in studying bristle development.
In their 1944 classic paper, Lees and Picken (8) demonstrated that bristle elongation, like the erection of a skyscraper, occurs at the tip. Thus, new modules are generated at and push out the bristle tip. These new modules become attached to the modules immediately beneath them, which in turn form the stiff backbone of the bristle.
How does a module form? Examination of the tips of elongating bristles reveal that modules form in three steps (4). In step 1, tiny membrane-associated actin bundlets, each containing ≈10 filaments, appear at the bristle tip. The tip of each bundlet appears to be attached to the plasma membrane by a tiny plaque of electron-dense material similar to that seen at the tips of the microvilli in intestinal epithelial cells (9); the basal ends of the bundlets extend into the bristle cytoplasm. The structures are indeed microvilli, a fact we established by treating elongating bristles with the sponge toxin, jasplakinolide, a compound that stabilizes F-actin (10). With jasplakinolide, the bundlets at the bristle tip elongate so that it is easy by both light and electron microscopy to identify these structures as microvilli, each of which has a core actin bundlet composed of ≈10 actin filaments (2). In step 2, the cortical bundlets aggregate into modules, and the modules, in turn, are joined into bundles. There are 7–11 such bundles, which contain up to 200 filaments and which are spaced at approximately equal intervals around the periphery of the bristle tip. One side of each of these larger bundles is membrane-associated. At this stage, the filaments within these bundles display a loose order. In step 3 the size of each bundle increases, with those near the bristle base having >600 filaments, and the filaments within the 7–11 bundles are now hexagonally packed. In contrast to the two earlier stages, longitudinal sections through each bundle reveal the 12-nm transverse striping attributed to presence of the fascin crosslink (6). In the bristle, these steps in time are presented simultaneously in space; that is, step 1 occurs near the tip of the bristle, and step 2 and then step 3 take place at short but increasing distances from the tip.
Using fluorescent antibodies to the two known crosslinkers, forked and fascin, we find that, during bristle elongation, the forked protein is concentrated at the tips of elongating bristles presumably attached to the numerous microvillar bundlets, none of which is resolved as discrete bundlets in the light microscope because of their small sizes. Forked protein, which is concentrated at the bristle tip, is also prevalent in the knuckles, where actin filaments glue modules into bundles (Fig. 1i) (11). There is no diffuse cytoplasmic staining where bundles are not present. In contrast, antibodies to fascin reveal a diffuse staining of the bristle cytoplasm, staining that is not restricted to the bundles. These observations and others show us that fascin is produced but not bound early in the bristle elongation whereas the forked protein production appears limited, not exceeding what can be bound (12).
Detailed analysis of actin bundles in mutants, in which the amount of each crosslinker is genetically manipulated from none to approximately half the wild-type amount to up to six times the wild-type amount, allowed us to begin to unravel what each crosslinker contributes to the bundles. Briefly, an unknown crosslinker is used to form the microvillar bundlets in step 1, and the forked proteins are used to aggregate these microvillar bundlets into the larger, albeit poorly ordered modules in step 2. In step 3, the fluorescence of the tagged forked protein decreases, suggesting that the forked crosslinkers within the modules are somehow displaced upon the entry of the fascin; fascin then changes the loosely crosslinked bundle of filaments into an extensively crosslinked, hexagonally packed filament bundle, thereby increasing bundle rigidity. The actin filaments that glue the modules together and form the gently bent knuckles of the bundle are held by the forked protein (11). These knuckles remain forked-rich, a fact that will be returned to anon. What is clear is that the sequential binding of these crosslinkers must be orchestrated (13). Sequential binding of crossbridges must be controlled in part by phosphorylation because, if kinases are inhibited using staurosporine, fascin entry into bundles is inhibited (14).
The general scheme seems to be that forked appears early loosely organizing the microvillar bundlets into modules and crosslinking the less well organized filaments that join neighboring modules. Fascin, in contrast, appears later, displacing forked to form the more regular, more tightly packed filament bundles.
The Characteristics of Each Bristle Depend on the Presence and Abundance of a Variety of Actin-Binding Proteins and Other Components
Not only do we want to know precisely what each cytoskeletal protein or modifier of each cytoskeletal protein accomplishes in the assembly, stabilization, function, and disassembly of the cytoskeleton, but we also want to know precisely how this cytoskeleton affects the overall shape of the cell (e.g., cell movement, endocytosis, and exocytosis) and how it affects the cell surface (e.g., secretion and the generation of extracellular coats). The bristle cell lends itself particularly well to relating changes in the cytoskeleton to changes in the bristle morphology and thus gives us information on how and when gene products have their effect on the whole cell.
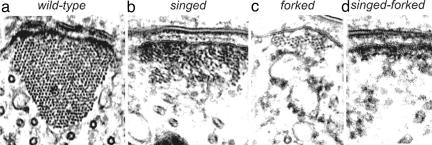
Let us mention a few examples. We have already said that there are two well defined actin filament crosslinkers in bristles. If the fascin crosslinker, which is present in the greatest concentration and which plays a major role in producing and maintaining rigid bundles, is “knocked out,” the bristle looks “singed”; that is, the bristle presents a twisted, almost curled phenotype (Fig. 1b). It no longer looks stiff. However, the bristle elongates to the same length as does the wild-type bristle. This phenotype is understandable because the bristle's bundles are flat and thin, not round, and the filaments in these bundles are poorly ordered and not as extensively crosslinked (Fig. 2b). If, on the other hand, the forked gene is eliminated, the bristle is 40% shorter than normal, and the bristle is not rigid but lies over the surface of the thorax (Fig. 1c). This is due to the actin bundles within being small (Fig. 2c). If both crosslinkers are “knocked out,” bristles still form, although they are shorter than those of wild-type. They appear flaccid and irregularly bent. The loss of rigidity is a consequence of the altered cytoskeleton, which consists only of tiny clusters of actin filaments and small monolayers of actin filaments attached to the plasma membrane (Fig. 2d). In contrast to these null mutants, if we increase the dosage of the forked gene to six times that of the wild type, the number and size of the actin bundles increase dramatically, and the bristle becomes stiffer and less curved (Fig. 1e). Some of the macrochaetes near their tips have a “fish hook” appearance because the additional bundles extend at right angles to the bristle shaft. In contrast to removing one or more crosslinkers or increasing their copy number, we incubated elongating bristles with staurosporine, a kinase inhibitor (14). We found that the elongating tips of the bristle ballooned out and then curved back upon itself. This is due to the failure to form bundles at the elongating tip.
Fig. 2.
Transverse sections through bundles from wild-type and mutant flies. Note that, although the number of filaments in the bundles vary widely, the area of membrane that the bundle occupies is about the same. [Reprinted with permission from ref. 16 (Copyright 2003, American Society for Cell Biology).]
Thus, we can relate changes in specific proteins to changes cell shape. Because in studies of all these mutations flies still form bristles, overall we can conclude that the qualities of the bristle, i.e., its length, its curvature, its rigidity or lack of it, its twist, its bends, and its ballooning are related to the actions of specific proteins.
Turnover Is a Key Process in Assembly
In the assembly of stereocilia on the surface of the hair cells of the inner ear, it is a curious observation that the cell begins by covering its apical surface with microvilli. Some of these microvilli develop into stereocilia while the remainder disappear (15). The cell thus makes more than it needs and takes apart what it doesn't use. This method, which involves the turnover of assembled filaments and bundles, seems to be a characteristic of the assembly process in the bristle.
Turnover of actin filaments occurs continuously throughout bristle elongation. Besides the 7–11 actin bundles, we find snarls of uncrosslinked actin filaments and small internal bundles that form throughout the bristle cytoplasm only to disappear within 4 min. In short, formation and later removal of actin filaments are prominent features of elongating bristles. To demonstrate how significant the formation and subsequent disassembly of internal bundles and snarls are, and accordingly how important turnover is in eliminating unwanted filaments, we stabilized all polymerized actin filaments with jasplakinolide, a membrane-permeable inhibitor of actin filament depolymerization. The result was truly amazing; in the presence of jasplakinolide, the cytoplasm is stuffed with snarls and small bundles. The effect of jasplakinolide's filling the cytoplasm with filaments is also seen in bristles of the fascin–forked double mutant flies.
Turnover of Actin Filaments Is Regulated by Crosslinking
The question then becomes, how does the cell target these snarls and tiny internal bundles for disassembly yet maintain the 7–11 rib-like actin bundles that support the bristle? Examination of mutants (16) and bundles that were recovering their fluorescence after photobleaching or fluorescence recovery after photobleaching provided insights. What we find is that interactions of the actin bundles with the plasma membrane and the extent of crossbridging between adjacent actin filaments together in bundles inhibit depolymerization or turnover. Thus, highly cross-bridged and membrane-bound actin bundles turn over slowly and thus persist, whereas poorly crosslinked filaments turn over more rapidly and disappear. Thus, if the bristle tip is photobleached, recovery of fluorescence occurs in 2 min or less. Yet, if a small segment from the midpoint of a bristle is irradiated, recovery occurs in 40 min, and it takes 4 h or more for recovery of fluorescence of a bundle segment at the bristle base, where the bundles are huge and contain up to 600 filaments per bundle. Recall that only the forked proteins are present at the bristle tip yet both fascin and forked are present in the bundles farther down the bristle. But what is particularly significant is that, if mutant bristles of the same age (e.g., 42-h-old pupae) that lack the fascin gene are irradiated, the time for fluorescence recovery of the actin bundles at the bristle tip is the same as in wild-type flies, but, near the bristle base, recovery occurs in only 30 min versus 4 h for the wild type. Thus, crossbridging, and in particular the fascin crossbridging, which, as mentioned earlier, is extensive in these bundles, stabilizes the bundles by inhibiting turnover.
In short, the bristle cell is able to eliminate selectively snarls and/or poorly crosslinked bundles because the filaments within them are not stabilized against disassembly, but at the same time, bundles that are extensively fascin-crossbridged and are membrane-associated turnover more slowly. Large bundles, by having proportionately more filaments that are well crossbridged to each other, will be more stable than smaller bundles (16). This is due to the greater surface-to-volume ratio of small bundles compared with large bundles. Compared with large bundles, small bundles have proportionally more filaments at the surface of the bundle, where crossbridging cannot occur. Thus, the filaments in larger bundles such as those at the bristle base should turn over more slowly than those in smaller bundles midway down the bristle or at the tip; this is what we find experimentally. Ultimately, this will lead to few large bundles attached to the plasma membrane where they are stabilized, rather than many small bundles, which again is what we see in thin sections. As a result, we now understand that filament turnover plays an important role in regulating the cytoskeleton, which in turn defines cell shape.
Is Turnover a Consequence of Treadmilling?
There are two possibilities to account for turnover, both of which occur in the bristle. First, turnover might be a consequence of treadmilling, in which actin filaments or small bundles simply disappear as a consequence of treadmilling; i.e., for single filaments or small bundles, disassembly at the pointed end eventually leads to their disappearance. This is not unreasonable, because if removal and addition of subunits occur at random, filaments or small bundles will shrink and grow, but, if they shrink to zero, they are gone. The second possibility is that turnover involves the taking off of subunits from the barbed end, a process that appears to occur during disassembly of the bristle cytoskeleton (see below).
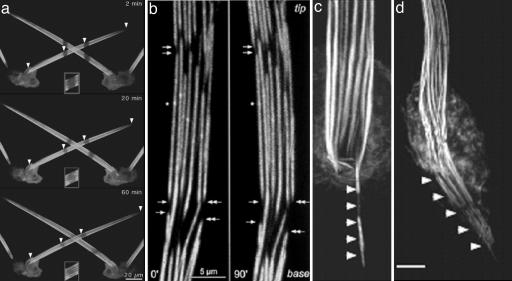
Treadmilling of the filaments in the actin bundle in bristles occurs as was shown originally by Fei et al. (17) in aristae, which are bristle-like structures on the Drosophila head, and more recently by G. M. Guild, P. S. Connelly, L. Ruggiero, K. A. Vranich, and L.G.T. (personal communication) for thoracic bristles (Fig. 3a). Treadmilling, or retrograde flow, seems to be a common feature of apparently stable actin bundles, e.g., bundles of actin filaments at the leading edge of fibroblasts (18), in the nerve growth cones (19), in microvilli (20), and in stereocilia (21). In stereocilia, Rzadzinska et al. (21) demonstrated that the actin bundles treadmill by the removal of actin subunits at the base and addition at the tips. This makes biological sense because, if the actin filaments in the stereocilia are damaged, for example, by loud noise, recovery of damaged filaments by treadmilling will occur within a few days. The purpose for treadmilling in Drosophila bristles is not clear, especially because the rate is very slow, e.g., 4 μm/h, when compared with the time taken to assemble and disassemble the bristle cytoskeleton. It does not make much sense that treadmilling in the case of the bristle is to remove damage because the cytoskeleton is put together and then taken completely apart in the space of a little over 1 day, e.g., after the exoskeleton is formed (see below). What is an interesting possibility is that treadmilling is an integral part of bundle assembly and length determination.
Fig. 3.
Images of bristles taken with fluorescently labeled actin. (a) Images taken at three different times after stripes are bleached in the bristles. Note that the stripes move toward the base. Insets show that there is recovery of fluorescence in the bleached area (courtesy of G. M. Guild). (b) Two time-lapse images taken of the same disassembling bundles. Note that disassembly takes place from the ends of filaments nearest the tip, which are the barbed ends. (Reprinted from ref. 25). (c and d) Images of a bundles treated from cells treated with jasplakinolide. Note that the bundles are translocated in from the bristle into the cytoplasm. [Reprinted with permission from ref. 16 (Copyright 2003, American Society for Cell Biology).]
To explain how a bundle can remain at constant length yet still treadmill, Mitchison and Kirschner (22) hypothesized that the retrograde flow must be regulated by a balance between tip assembly at the barbed ends of the filaments in the bundle and removal of subunits at the pointed end. However, if these rates were not in balance, the filapodium or cell extension would extend or shorten (23, 24). Accordingly, by controlling the rate of actin assembly and disassembly from different ends of the actin filaments—processes that can be accomplished by ancillary proteins such as cappers or severers or membrane attachment proteins such as the myosins or crosslinkers, proteins that in turn can be controlled separately—the cell can control the length and stability of its bundles. This is in fact what we see happening in bristle bundles, as we mentioned in preceding sections. What is unusual about the bundles in bristle, however, is that each bundle is not made up of filaments that run the length of the bundle but rather of short filaments in modules that are grafted together end to end. Even so, the entire bundle if photobleached shows retrograde flow. Does this mean that all of the modules, even though they are differentially stable and of different lengths, are treadmilling at the same rate? If so, how is this controlled? Of course, the same problems exist in cells where the filaments in the bundles are of different lengths (see ref. 7 for examples).
What we have discussed is the appearance of turnover/treadmilling in assembly of the cytoskeleton, but is it also important in the disassembly of the cytoskeleton? As the bristles elongate, they lay down an extracellular surface, a chitin cuticle, which behaves as an external skeleton; after all, Drosophila is an insect with a stiff external skeleton. No longer in need of an internal stiff cytoskeleton, the cell disassembles the internal actin scaffolding. By studying stages in bundle disassembly, we find further insights into the roles of the two crosslinkers, treadmilling and subunit removal.
During Disassembly of the Bundles Are Filaments Severed, and Are Actin Subunits Then Removed from the Pointed or Barbed Ends of Filaments?
Bristles first sprout from the thoracic surface in 32-h-old pupae, and they attain their mature length 16 h later, or in 48-h-old pupae. Surprisingly, many of the bundles in 44-h-old pupae have gaps between modules (Fig. 3b); the cell-proximal parts of the bundle begin to disassemble even though the tips are still growing. These gaps tend to be in transverse register (4), which is expected if they arise from filament disassembly in the weak knuckles that grew between the modules (Fig. 3b). Then the width of the gaps between adjacent modules increases as the modules shrink in length (compare Left and Right in Fig. 3b). At the same time that the gaps are widening, depolymerization appears to slice its way down into the bundle, fracturing it into a set of smaller bundles. Careful examination of modules by confocal microscopy reveals that the basal ends of the fractured modules tend to be flat and regular whereas the apical ends tend to be irregularly pointed. Although this occurs on all bundles in the bristle, the rate of gap widening varies from bundle to bundle (25).
To determine from which end of the module actin subunits are removed, we labeled actin with GFP and allowed the bristle to elongate. We then watched the removal of subunits from the bundles using time-lapse recording. An identifying region, e.g., a tiny bump or bend on an individual module, was selected so one could determine which end of the bundle was shortening, e.g., the end closest to the tip (the barbed ends of the filaments) or the end nearest to the base (the pointed ends of the filaments). We found that subunit loss must be from the barbed ends of the filaments. The average rate of loss was ≈0.02 μm/min.
This result is surprising because in other systems filament breakdown is thought to proceed from the pointed ends of the filaments. Bristles may be a special case for two reasons. First, the filaments in modules are crosslinked; second, antibodies against Drosophila cofilin show intense staining of the nerve attached to the bristle but little or no staining of the bristle. But, more to the point, the breakdown of the actin bundles in bristles is a very slow process, taking >12 h (25).
We can delay or speed up the disassembly of the bundles by the use of specific inhibitors. For example, if we apply inhibitors of protein synthesis (e.g., cyclohexamide) or of actin assembly (e.g., cytochalasin) to 40- to 42-h-old pupae, the gaps between modules appear earlier than they do in wild-type flies and module shrinkage (breakdown) is facilitated. Alternatively, if we apply jasplakinolide, a membrane-permeable stabilizer of F-actin, breakdown (shrinkage) of the modules is inhibited, and not only do the gaps not form, but, if gaps had formed before incubation in this drug, the gaps disappear (25).
What these inhibitor studies seem to be telling us, when coupled with the fact that bundle shortening occurs from the barbed ends of filaments, is that the barbed end of the bundle is dynamic and subunits must be coming on and off the filaments that make up the bundle even before the bristle has reached its mature length. Accordingly, turnover of the actin subunits at the barbed ends of the filaments could account for the disassembly of the bundle provided that the rate of new actin synthesis slows. Such a conclusion is consistent with the fact that it takes 16 h for the actin bundles to disassemble totally.
Thus, the disassembly of the bundles and modules is not exclusively a consequence of treadmilling in which disassembly runs faster than assembly. First, the bundles are turned back into modules by elimination of the knuckles. If the disassembly were purely a consequence of treadmilling, the modules would remain intact and would be removed at the base of the bristle. Second, treadmilling cannot account for the disappearance of the module because the depolymerization is at the barbed end whereas, if it were a process related to treadmilling, the disassembly would be at the pointed end. Here is the challenge we leave to you: Is the turnover of snarls and small bundles at the growing tip of the bristle a consequence of treadmilling, the disassembly pathway in the preceding paragraphs, or as yet some other process?
Some Thoughts on the Roles of the Actin-Binding Proteins Involved in the Assembly and Disassembly of Bundles
Bundles are made of modules, which in turn are assembled from microvilli. In a previous review (7), we discussed the idea that microvilli initiate many of the bundles found in cells and that there must be a microvillus-making factory that the cell can turn on. Given that when neither fascin nor forked is present we still find small bundles positioned around the bristle periphery, we conclude that there is a set of actin-associated proteins in addition to forked and fascin. Microvilli, we think, arise from nucleators other than the Arp2/3 complex used in making pseudopodia. There must also be some other crosslinker to hold together the actin filaments in the microvillar core. As the cores are stitched together, there appears to be a protein complex that targets the assembling bundle to the bristle membrane. This complex, which is seen as a dense patch along the membrane, appears to determine the width of the bundle along the membrane and hence the number of bundles, which form the ribs of the bristle (16). Forked appears somewhere during this process and well before fascin. Forked, we imagine, is the initial crosslink that keeps things in place rather like the basting stitches seamstresses use in making clothes. Fascin then enters to do the final crosslinking, much as the seamstress puts in the tight stitching that replaces the basting stitches to finish a garment. The basting stitches are removed much as the forked is largely displaced. The stitching together by forked does not appear to be as stable as that produced by fascin because it is the forked-linked filaments that disappear first. The parts held by fascin disassemble more slowly.
These differences are reflected in the ordered arrangement of filaments. Fascin produces tightly bundled actin filaments whereas those made when only forked but not fascin is present are more loosely held. We imagine that forked is a more flexible crosslink than fascin and can tolerate a wider range of crosslinking geometries than fascin can. When fascin is activated and invades the modules, it will stitch tightly the filaments more loosely held by forked. Fascin stitches together as best it can but cannot form a module in which all of the filaments are perfectly arranged in a hexagonal array. Disorder is often locked in; indeed, the process of bundle formation by fascin in vitro suggests this to be true (26, 27). Forked may well remain at the boundaries, where the fascin cannot bind, and these interfaces represent the “weak” features that result in the fragmentation of modules during disassembly.
We also surmise that, when the actin glue fills in the gaps between modules, it is constrained to grow from the end of one module and somehow join up as best it can to the end or side of the next module along the bundle. Given the constraints, it is not free to adopt tight packing with its neighboring filaments. Thus, in these knuckles, one expects and finds more forked than fascin, and, again, one expects and finds that this region is disassembled before the more tightly stitched regions where fascin prevails.
If assembly and disassembly were as simple as our description so far, we could have much more confidence in our hypotheses that forked holds filaments loosely in place until fascin comes along and nails them down. The processes are, however, more dynamic, and, although this makes the system more complicated, it also makes it more attractive as a window into the dynamic aspects of the cytoskeleton.
How Does Curvature of the Hairs and Bristles Arise?
We have been fascinated by the manner in which curvature of the hairs and bristles appear during development. What we find is that the curvature is greatest near the base and decreases as one goes toward the tip, which is the portion of the hair or bristle made last (3). There are too many possibilities and too little information to put forward a model, but we can amuse ourselves by thinking about some possible choices. We divided our mechanisms into three broad classes: those mechanisms that arise from the external environment of the hair or bristle cells, those that arise from the internal components of the cell, and those that arise from a combination of external and internal mechanisms.
A Mechanism That Involves External Factors
One possibility, which corresponds to the first class and which we suggested in an earlier publication (3), depends on the cuticle that surrounds the developing fly. As the fly develops, it becomes smaller and thus shrinks away from the cuticle. The essence of the idea is that the developing bristle or hair is bent mechanically by contact with the cuticle as it tries to grow outward. The decrease in curvature might be a consequence of the shallower angle between the growing tip and the cuticle or a consequence of an increase in the rate at which the fly shrinks from the cuticle. Although we proposed it, we now think it is not all that likely a mechanism. A macrochaete bristle might be 400 μm long while a hair is at most 20 μm. It does not seem possible that hairs and bristles, which are found intermingled, could both be in contact with the cuticle during the critical developmental phase. Moreover, such a model ignores the common asymmetric feature in the cytoskeleton, a feature that correlates with the plane of curvature; that is, the axial actin bundles, which lie along the membrane, are larger on the inside of the curve. This is the case for hairs as well as for bristles.
A Mechanism That Derives from the Internal Asymmetry of the Actin Cytoskeleton
The appearance of such a striking and consistent asymmetry in the cytoskeleton begs for a curvature-generating mechanism that is a consequence. What springs to mind is the bimetallic strip, which changes curvature with temperature. In the strip, there are two layers of metals that have different coefficients of expansion. Assume that the strip is straight to begin with and that we increase the temperature causing one layer to become longer than the other. The strip will curve with the longer layer being on the outside track and the shorter layer being on the inside track. If the temperature along the strip varies, so will the curvature. The take-home message is that one needs a mechanism whereby the rates of elongation on opposite sides of the growing bristle or hair differ. Here are two possible mechanisms: one involves a change in the amount of membrane on opposite sides of the bristle or hair, and the other involves a differential rate in the elongation of actin bundles on opposite sides of the bristle or hair.
The first of these two might occur as a consequence of the assembly of the bundles from small microvillar-like bundles that sprout from the bristle's lateral membrane and must take their membrane sheath from the membrane adjacent to them. If there are more such “sprouts” on the side with larger bundles and if the membrane does not flow easily, then the membrane on the side with larger bundles will be in effect contracted, generating a force for curvature. Our information is insufficient to decide whether such a model is likely.
A more attractive model involves the differential rate of bundle elongation. G. M. Guild, P. S. Connelly, L. Ruggiero, K. A. Vranich, and L.G.T. (personal communication) have discovered that bundle formation involves tip elongation and retrograde translation (i.e., axial movement toward the cell body) and depolymerization; that is, bundles are continuously shortening at their cell-proximal ends. In addition, there could also be an internal change in lengths of the bundles such that, even if tip elongation and base shortening ceased, bundles could still change length by a loss or a gain in module length within the bundle. If the rate of change of bundle length depends on bundle diameter, and if there are the appropriate structural links between bundles, then one can imagine a curvature-generating force resulting from differing rates of interbundle elongation. Moreover, the change in curvature might be a simple consequence of a change in the rate of elongation with length, which Tilney et al. (12) observed; thus, if the average rate of elongation increases while the differential rates between bundles remains constant, the curvature will decrease as observed.
Finally, it is possible that myosins might play a role by controlling the elongation; myosin (myosin-XVa) is responsible for differential elongation of hair cell stereocilia (21). Or myosin might supply an axial force that is stronger on the side of the bristle with bigger bundles than on the opposite side with smaller bundles.
There are a variety of interesting structural mutants that might provide insights into the mechanism involved. One that comes to mind is javelin, which generates longer, straighter bristles. The function of the javelin protein is not known, and elucidation of the pathway it affects might provide a key insight.
Why You Should Take Up Work on Bristles
What we have tried to point out are the advantages of bristles in understanding the role of the actin cytoskeleton, including its dynamic aspects, in the generation of form. It shows many of the treadmilling properties of the stereocilium but in a system that is more tractable genetically and that has modules, which can serve as markers. Moreover, the stages of assembly and disassembly are laid out at the same time along the bristle; while assembly is taking place at the tip, disassembly is taking place at the base, and all in the face of subunit turnover and treadmilling that appear to be proceeding along the entire length of the structure. For geneticists, there is work to discover the pathways of morphogenesis; are there parallel pathways, and what bristle characteristics can vary independently? For cell biologists, there is a gold mine of tractable complexity; what are the proteins involved and when and where do they act? In particular, Tilney et al. (16) found that, when depolymerization of actin was inhibited, the bundles were translated into the cytoplasm (Fig. 3 c and d) as if the retrograde motion could occur independent of depolymerization (see figure 10 in ref. 16). For structural biologists and biochemists, there are proteins to be isolated and characterized and protein complexes whose structures we need to know in detail; what are the properties of forked, and how do forked and fascin interact in a single actin bundle? Because a bundle is a structure made of modules joined end to end, it is possible in this system to separate processes that operate on the intact bundle from those that operate on the modules or within the bundle proper. Our goal is not to suggest which are the critical questions or which are the critical components but rather to get you, the reader, involved. Here is a specialized cell, the kind of system we have long advocated studying, that has all of the features you could want: segments bearing bristles can be cut out and development watched “in vitro”; the cells can be manipulated by genetics and molecular biology; there are many interesting mutants that are readily available; and the pathway of assembly and disassembly is laid out in space as well as time.
Acknowledgments
We thank Greg Guild (University of Pennsylvania, Philadelphia) for Fig. 3a, and we thank him and Kelly Vranich for their help with the manuscript. This work was supported by National Institutes of Health Grants GM26357 (to D.J.D.), GM62580 (to D.J.D.), and GM52857 (to L.G.T.).
Author contributions: L.G.T. and D.J.D. wrote the paper.
Conflict of interest statement: No conflicts declared.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 28, 1998, and April 29, 2003.
References
- 1.Morgan, T. H. & Bridges, C. B. (1916) Publ. Carnegie Inst. 237, 1–88. [Google Scholar]
- 2.Tilney, L. G., Connelly, P. S. & Guild, G. M. (2004) J. Cell Sci. 117, 3531–3538. [DOI] [PubMed] [Google Scholar]
- 3.Tilney, L. G., Connelly, P. S., Ruggiero, L., Vranich, K. A., Guild, G. M. & DeRosier, D. (2004) Mol. Biol. Cell 15, 5481–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilney, L. G., Connelly, P., Smith, S. & Guild, G. M. (1996) J. Cell Biol. 135, 1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeRosier, D. J. & Tilney, L. G. (1982) Cold Spring Harbor Symp. Quant. Biol. 46, 525–540. [DOI] [PubMed] [Google Scholar]
- 6.Tilney, L. G., Tilney, M. S. & Guild, G. M. (1995) J. Cell Biol. 130, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeRosier, D. J. & Tilney, L. G. (2000) J. Cell Biol. 148, 1–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Lees, A. D. & Picken, L. E. R. (1944) Proc. R. Soc. London Ser. B 132, 396–423. [Google Scholar]
- 9.Tilney, L. G. & Cardell, R. R., Jr. (1970) J. Cell Biol. 47, 408–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubb, M. R., Spector, I., Beyer, B. B. & Fosen, K. M. (2000) J. Biol. Chem. 275, 5163–5170. [DOI] [PubMed] [Google Scholar]
- 11.Guild, G. M., Connelly, P. S., Vranich, K. A., Shaw, M. K. & Tilney, L. G. (2003) J. Cell Biol. 162, 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilney, L. G., Connelly, P. S., Vranich, K. A., Shaw, M. K. & Guild, G. M. (2000) J. Cell Sci. 113, 1255–1265. [DOI] [PubMed] [Google Scholar]
- 13.Tilney, L. G., Connelly, P. S., Vranich, K. A., Shaw, M. K. & Guild, G. M. (1998) J. Cell Biol. 143, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilney, L. G., Connelly, P. S., Vranich, K. A., Shaw, M. K. & Guild, G., M. (2000) J. Cell Biol. 148, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilney, L. G., Cotanche, D. A. & Tilney, M. S. (1992) Development 116, 213–226. [DOI] [PubMed] [Google Scholar]
- 16.Tilney, L. G., Connelly, P. S., Ruggiero, L., Vranich, K. A. & Guild, G. M. (2003) Mol. Biol. Cell 14, 3953–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei, X., He, B. & Adler, P. N. (2002) J. Cell Sci. 115, 3797–3806. [DOI] [PubMed] [Google Scholar]
- 18.Wang, Y. L. (1985) J. Cell Biol. 101, 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forscher, P. & Smith, S. J. (1988) J. Cell Biol. 107, 1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyska, M. J. & Mooseker, M. S. (2002) Biophys. J. 82, 1869–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rzadzinska, A. K., Schneider, M. E., Davies, C., Riordan, G. P. & Kachar, B. (2004) J. Cell Biol. 164, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchison, T. & Kirschner, M. (1988) Neuron 1, 761–772. [DOI] [PubMed] [Google Scholar]
- 23.Katoh, K., Hammar, K., Smith, P. J. & Oldenbourg, R. (1999) Mol. Biol. Cell 10, 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallavarapu, A. & Mitchison, T. (1999) J. Cell Biol. 146, 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guild, G. M., Connelly, P. S., Vranich, K. A., Shaw, M. K. & Tilney, L. G. (2002) J. Cell Sci. 115, 641–653. [DOI] [PubMed] [Google Scholar]
- 26.Sukow, C. & DeRosier, D. J. (2003) Biophys. J. 85, 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes, D. L. & DeRosier, D. J. (1991) Biophys. J. 59, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]