Abstract
Neurotrophins (NTs) such as BDNF, NT-3, and NT-4 are important modulators of neuronal function and survival. Their expression in the CNS after various insults is thus of major therapeutic consequence. Glatiramer acetate [(GA) Copaxone], an approved drug for the treatment of multiple sclerosis, has been shown to induce Th2/3 cells that accumulate in the CNS, expressing in situ antiinflammatory cytokines and BDNF. In the present study, we investigated whether s.c. injections of GA, applied at various stages of experimental autoimmune encephalomyelitis, affect the expression of NTs, particularly BDNF, in the brain. In untreated experimental autoimmune encephalomyelitis mice, the expression of NTs was elevated shortly after disease appearance but subsequently declined below that of naive mice. In contrast, GA treatment led to sustained augmentation in the expression of BDNF, NT-3, and NT-4 in various brain regions as demonstrated by histological analysis of immunostained brain sections. GA treatment, even when started 45 days after disease induction, restored the impaired level of NTs to that of healthy mice. BDNF elevation after GA treatment was demonstrated on both protein and mRNA levels. Prominent staining was manifested not only by infiltrating GA-induced T cells, but also by CNS resident cells (neurons and astrocytes), indicative of a bystander therapeutic effect. Of importance, in GA-treated mice, intense BDNF expression was manifested by neuronal progenitors that migrated into lesions in injured regions. These results indicate that the immunomodulator GA exerts not only an antiinflammatory effect, but also enhances neuroprotection and regeneration of neural elements in the diseased brain.
Keywords: immunomodulation, neurotrophins, BDNF, NT-3, NT-4
In multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), the immune system provokes the detrimental process via autoimmune inflammatory mechanisms (1, 2). Still, axonal injury and neuronal loss, which are initiated at an early phase of the disease, have a crucial role in determining the extent of the permanent neurological disability (3). It is therefore essential to assess current MS treatments not only by their antiinflammatory activity, but also by their ability to enhance repair mechanisms and effective neuroprotection in the CNS. Members of the neurotrophin (NT) family such as BDNF, NT-3, and NT-4 are important regulators of neuronal function and survival (4, 5). Besides their well established role in neuronal development, process growth, and regulation of synaptic plasticity, they have the capacity to protect neurons against various pathological insults. BDNF, in particular, was shown to rescue degenerating neurons and promote axonal outgrowth, remyelination, and regeneration (5, 6), hence its modulation in the CNS is of major therapeutic consequence. Increased NT levels at early phases of EAE (7, 8) and MS (9) may reflect self-repair mechanisms similar to the enhanced neurogenesis triggered by EAE and other brain insults (10, 11). However, it is important to investigate the levels of NTs at extended periods after disease onset, during the chronic phase, when exhausted self-compensating neuroprotection fails.
Current studies indicate that immune cells in the CNS constitute a potent source of BDNF and are thus candidates for mediating neuroprotective effects in MS (12). Yet these studies argue that BDNF production correlates with inflammatory and demyelinateing activity, involving both damaging and protective roles for infiltrating T cells (7, 9, 12). Glatiramer acetate [(GA) Copaxone], an approved drug for MS treatment (13), has been shown to be effective in several animal models with EAE (13), immune rejection (14), optic neuropathies (15), inflammatory bowel disease (16), and Parkinson's disease (17). GA is a potent inducer of Th2/3 cells that secrete high levels of antiinflammatory cytokines (18), cross the blood-brain barrier, accumulate in the CNS (19), and express in situ IL-10, TGF-β, and BDNF (20). Furthermore, the GA-specific cells induced the bystander effects on neighboring CNS cells to express these beneficial factors and reduce the expression of the inflammatory cytokine IFN-γ, suggesting that immune cells can mediate in situ both antiinflammation and neuroprotection (11, 20).
In the present study, we investigated whether the expression of NTs, particularly BDNF, is induced not only by adoptive transfer of GA-specific T cells, but also by s.c. daily injections of GA as such, similar to the practice used to treat MS patients. We focused on expression of NTs resulting from GA treatment administered at extended time intervals after disease induction, when disease is already overt. We now report that in untreated EAE mice expression of NTs was elevated after disease appearance, but subsequently declined below that of naive mice. In contrast, GA treatment led to sustained augmentation of the expression of BDNF, NT-3, and NT-4 in various brain regions, endorsing a direct linkage between immunomodulation, neuroprotection, and therapeutic consequences in the CNS.
Materials and Methods
Mice. C57BL/6 and (SJL/JxBALB/c)F1 female mice, 8-12 wk of age, were purchased from Harlan Laboratories (Jerusalem) and handled according to the regulations of the Institutional Animal Care and Use Committee.
GA (Copaxone and Copolymer 1). GA consists of acetate salts of synthetic polypeptides containing four amino acids: l-alanine, l-glutamate, l-lysine, and l-tyrosine (13). GA from batch 242990599, with an average Mr of 7,300 kDa, was obtained from Teva Pharmaceutical Industries (Petah Tiqva, Israel).
EAE and GA Treatment. Disease was induced by immunization with peptide 35-55 of myelin oligodendrocyte glycoprotein, as described (11). GA treatment was applied by 8 to 10 daily s.c. injections (2 mg per mouse) at different stages of the disease: (i) starting immediately after induction (prevention); (ii) starting at the appearance of clinical manifestations, 11 days after induction (suppression); or (iii) during the chronic phase from day 45 (delayed suppression).
T Cells. GA-induced T cells, whole lymphocyte populations, and T cell lines, were obtained from brains and spleens of GA-immunized mice (2 mg per mouse, 10 daily injections), as described (19).
BDNF Secretion. To measure BDNF protein secretion, we applied a split system, in which irradiated antigen-presenting cells were preincubated with GA, washed, and then added to the tested cells. The amounts of BDNF in the culture supernatants were measured by ELISA (21).
RT-PCR. Total mRNA was isolated from GA-specific T cells at various time points after exposure to GA. The following sets of oligonucleotides were used: BDNF, (sense) 5′-GGTATCCAAAGGCCAACTGA-3′ and (antisense) 5′-CTTATGAATCGCCAGCCAAT-3′ at 63°C for 30 cycles; actin, (sense) 5′-AGCCATGTACGTAGCCATCC-3′ and (antisense) 5′-TTTGATGTCACGCACGATTT-3′ at 63°C for 19 cycles.
Immunocytochemistry. Mice were perfused transcardially and fixed (11). Free-floating sections (16 μm thick) were coronally cut, preincubated with 20% horse serum and 0.05% Saponin, or 0.5% Triton X-100, and incubated overnight with primary Abs (1-10 μg/ml): chicken anti-BDNF (Promega), goat anti-NT3 (R & D Systems), goat anti-NT4 (R & D Systems), goat anti-Doublecortin (DCX) C-18 (Santa Cruz Biotechnology), mouse antineuronal nuclear antigen (Chemicon), mouse antiglial fibrillary acidic protein (Pharmingen), goat anti-IL-10 (Santa Cruz Biotechnology), rabbit anti-TGF-β (Santa Cruz Biotechnology), and rat anti-IFN-γ (BioSource International, Camarillo, CA). The second Ab step was performed by labeling with highly cross-absorbed cy2- or cy3-conjugated species-specific Abs (Jackson ImmunoResearch), 1:200 for 20-40 min.
In Situ Hybridization. BDNF in situ hybridization on frozen sections was performed by using antisense and sense digoxigenin-labeled BDNF cRNA probes, as described (22).
Quantification. BDNF, NT3, or NT4 protein expression was quantified by counting immuno-positive cells in areas of 0.13 mm2 in the cortex (layers 2-3 and 6 in both the anterior and the posterior motor cortex), in the dorsal striatum, and the accumbens nucleus. Results were averaged from two to four mice for each treatment group and eight sections for each brain structure. Expression of BDNF mRNA was quantified in corresponding regions by multiplying the positively stained area with the averaged density (integrated optical density), using image pro+ 4.5 software (Media Cybernetics, Silver Spring, MD), averaged from two mice for treatment group and six sections for each region. The significance compared with naive control or with EAE-untreated mice was assayed by Student's t test, (P < 0.05).
Results
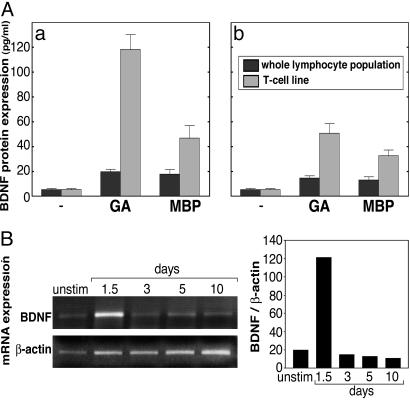
BDNF Production by GA-Specific T Cells. The secretion of BDNF by GA-induced T cells was followed by measuring the BDNF concentration in culture supernatants of whole lymphocyte populations (ex vivo) and GA-specific T cell lines (in vitro). To avoid the interference in BDNF ELISA reported before (21), we applied a split system in which GA-preincubated antigen-presenting cells were added to the test cells. The results demonstrated in Fig. 1A indicate that lymphocyte populations isolated from brains and spleens of mice that had been injected with GA, secreted BDNF in response to the immunizing antigen (GA) and to the myelin antigen [myelin basic protein (MBP)] but in small amounts (12-20 pg/ml). The highly reactive GA-specific T cell lines established from these lymphocyte populations, after three stimulation cycles with GA (19), secreted much higher levels, e.g., 112 pg/ml and 45 pg/ml, in response to antigen-presenting cells pulsed with GA and MBP, respectively. Of note, BDNF secretion by the T cells originating in the CNS (Fig. 1Aa) was higher than by the peripheral T cells originating from the spleens of the same mice (Fig. 1Ab). Consistent with the protein secretion data, mRNA levels as followed by RT-PCR (Fig. 1B) revealed a weak band in the absence of GA (2 wk after the last stimulation) and a stronger band after exposure to GA, a 6.1-fold elevation (relative to β-actin expression). After stimulation (3 d), mRNA expression returned to the basal level, although GA was still present in vitro and remained unchanged at days 5 and 10, when GA was already removed from the cultures.
Fig. 1.
Production of BDNF by GA-specific T cells. (A) BDNF protein secretion by whole lymphocyte populations and T cell lines isolated from brains (a) and spleens (b) of GA-immunized mice, in response to medium alone, GA (50 μg/ml), or MBP (100 μg/ml). BDNF concentrations were measured by ELISA. (B) RT-PCR analysis of BDNF and housekeeping β-actin mRNA from GA-specific T cell line, at various time points after exposure to GA.
BDNF Expression in Brains of EAE-Induced Mice and the Effect of GA Treatment. Induction of EAE by the myelin oligodendrocyte glycoprotein 35-55 peptide resulted in chronic (nonremitting) disease, that started 10-14 days after induction, reached an average score of three by days 20-24, and remained in a chronic phase (grade 2-2.5) for an extended period. GA treatment was applied by 8 to 10 daily injections at different stages of the disease: (i) starting immediately after induction (prevention); (ii) starting at the appearance of clinical manifestations, 11 days after induction (suppression); or (iii) during the chronic phase from day 45 (delayed suppression). GA ameliorated the clinical manifestations of EAE, when applied at each of these time points, and its beneficial effect was sustained until the mice were killed.
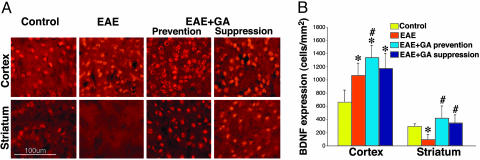
In all of the treatment groups, the presence of BDNF was evidenced by its immunoreactivity over a wide distribution in various brain regions such as the cortex, accumbens, hippocampus, septum, thalamus, and to a lesser extent in the striatum. Immunohistochemcal analysis at the peak of clinical symptoms (22 days after disease induction) (Fig. 2) revealed increased BDNF expression in the cortex of EAE mice, as manifested in layers 2 and 3 by a 1.7-fold elevation in the number of BDNF-expressing cells in comparison to the naive controls. In contrast, in the striatum of EAE mice, the number of BDNF-positive cells was decreased by two-thirds relative to the controls. GA-treated mice tested at the same time point manifested elevated BDNF expression. In the cortex, significant elevation was manifested above controls, 2.1- and 1.8-fold by prevention and suppression, respectively (significance over EAE only by the prevention treatment). In the striatum, GA-induced substantial elevation over EAE-untreated mice for both treatment regimes (4.2- and 3.5-fold) and restored BDNF expression to the normal level.
Fig. 2.
BDNF expression. (Left) BDNF expression by immunostaining (red) in brains of EAE-induced mice at the peak of clinical symptoms, 22 days after induction, and the effect of GA treatment, starting either immediately after disease induction (prevention) or at the appearance of clinical manifestations at day 11 (suppression). (Right) Quantitative analysis of BDNF staining was performed by counting positively stained elements, three mice per treatment group, eight sections for each region. *, Significant effect over naive control; #, significant effect over EAE-untreated mice.
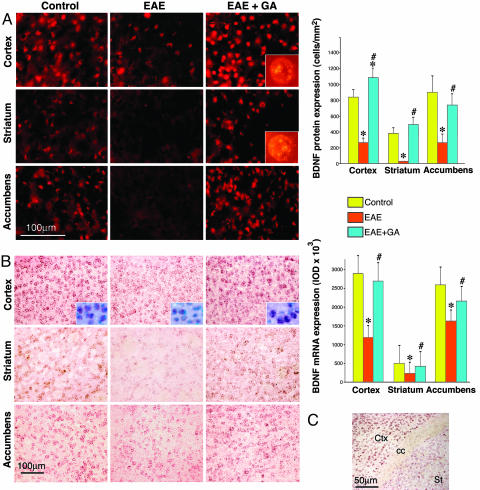
We then studied the effect of GA on BDNF expression when treatment was initiated in the chronic phase of the disease (delayed suppression). Immunohistochemical analysis (Fig. 3A) revealed that BDNF expression in EAE mice in this phase (60 days after induction) was drastically decreased, as manifested by a 3-fold reduction in both the cortex layer 2-3 and the accumbens, in comparison to naive controls. Moreover, in the striatum of EAE mice, BDNF-expressing cells practically disappeared (only 7 ± 2 positive cells per mm2 were detected in comparison to 403 ± 65 cells per mm2 in naive mice). In contrast, in EAE mice treated with GA during this late disease phase, the number of BDNF-positive cells was much higher. Hence, in the accumbens it was 3-fold higher than in EAE mice, but somewhat lower than the normal level, and in the cortex it was 4-fold higher than in EAE, higher than in naive controls. Even in the striatum, in which BDNF expression was extremely low in untreated chronically diseased mice, GA-treatment-induced elevated BDNF levels that somewhat surpassed the naive controls (507 ± 84 cells per mm2). The changes in BDNF protein expression were manifested mainly in the number of the BDNF-immunopositive cells, and to a lesser extent in the mean density of expression per single cell, suggesting that GA augments predominantly the number of BDNF-expressing cells. BDNF staining was observed in the nucleus and the cytoplasm of the cells (Fig. 3A Insets).
Fig. 3.
BDNF expression in brains of EAE-induced mice at the chronic disease phase, 60 days after induction, and the effect of GA treatment, starting at day 45 (delayed suppression). (A) Immunohistochemical analysis of BDNF protein level (red). (B) In situ hybridization of BDNF mRNA (pink) and nuclear staining (blue, Inset). Quantitative analysis of protein expression was performed by counting positively stained cells, and analysis of mRNA was performed by measuring integrated optical density. *, Significant effect over naive control; #, significant effect over EAE-untreated mice. (C) BDNF mRNA in coronal section depicting part of the cortex (Ctx), the striatum (st), and the corpus callosum (cc). Considerably less mRNA expression is observed in the striatum than in the cortex.
These patterns of BDNF expression detected immunohistochemically on the protein level were further corroborated on the mRNA level by using in situ hybridization (Fig. 3B). Thus, in EAE mice, an extensive decline in mRNA expression was found at the chronic phase, e.g., integrated optical density of mRNA expression was reduced by 2.4-fold in the cortex and 1.6-fold in the accumbens at day 60 after induction. GA treatment induced significant elevation in BDNF mRNA expression, 2.3- and 1.4-fold higher than in EAE mice in the cortex and the accumbens, respectively, resulting in mRNA levels similar to that of naive mice. The expression of BDNF mRNA in the striatum in all treatment groups was drastically lower than in other brain regions (Fig. 3C), still a lower integrated optical density in EAE mice in comparison to naive controls; restoration to a normal level by GA treatment was observed in this region as well.
Decreased BDNF expression in the chronic phase of EAE and increased expression induced by GA were observed in other brain regions, such as the septum and the thalamus. The results demonstrated in Figs. 2 and 3, relating to the cortex, depict layer 2-3 of the anterior motor cortex. Similar ratios were obtained in this layer in the posterior cortex and in layer 6 of both the anterior and posterior cortex.
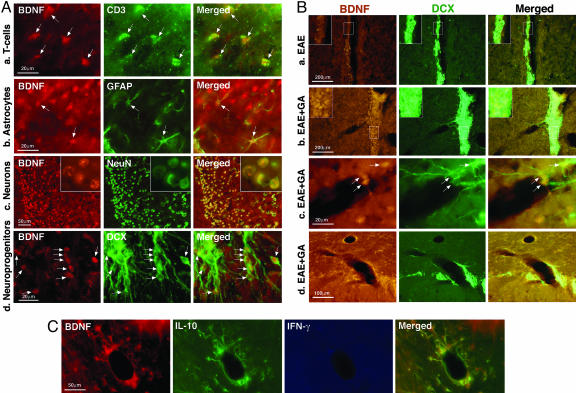
Characterization of BDNF-Expressing Cells in Brains of GA-Treated Mice. Identification of the cell types that express BDNF after GA treatment was performed by double staining of BDNF-immuoreactive cells with specific subset markers, i.e., CD3, which depicts T cells, glial fibrillary acidic protein for astrocytes, and neuronal nuclear antigen for neurons (Fig. 4A). As confirmed by colocalization of corresponding fields, BDNF was expressed by T cells (Fig. 4Aa) and astrocytes (Fig. 4Ab). Yet most of the BDNF-expressing cells were neurons (Fig. 4Ac). BDNF was found not only on mature neurons but also on neuronal progenitor cells, identified by the immature neuronal marker DCX (Fig. 4Ad). Thus, BDNF expression after GA treatment involves not only infiltrating T cells, but also the resident CNS cells.
Fig. 4.
Immunohistochemical analysis of BDNF-expressing cells. (A) Characterization of BDNF+ cells (red) in GA-treated mice by double staining with specific subset markers (green): CD3, which depicts T cells shown in the talamus (a), glial fibrillary acidic protein (GFAP) for astrocytes in the corpus callosum (b), NeoN for neurons in the singulate cortex (c), and DCX for neuronal progenitors in the accumbens (d). (B) DCX+ neuronal progenitors (green) expressing BDNF (orange) in the SVZ of EAE (a) and EAE + GA (b) mice. (Insets) A larger proportion of cells in the GA-treated mice coexpressed BDNF. Neuroprogenitors expressing BDNF migrated from the SVZ toward lesions in the striatum (c) and the corpus callosum (d). Note the DCX-expressing fibers extending around the lesions. (C) Cytokine expression by BDNF-expressing cells; triple staining in the optic tract of a GA-treated mouse. Coexpression of BDNF (red), IL-10 (green), and lack of IFN-γ (blue). Arrows indicate double positive cells. Representative figures from four EAE and five EAE + GA mice are shown.
Fig. 4B depicts the subventricular zone [(SVZ) the site in which DCX+ neuronal progenitors usually proliferate] and its BDNF expression in EAE (Fig. 4Ba) and in EAE + GA (Fig. 4Bb) mice. The amount of DCX+ neuroprogenitor found in the SVZ of the GA-treated mice was much higher than in EAE-untreated mice, and a larger proportion from these cells coexpressed BDNF (see Fig. 4B Insets). Of note, neuroprogenitors expressing BDNF migrated from the SVZ toward lesions in various brain regions as shown in Fig. 4B in lesions in the striatum (Fig. 4 Bb and Bc) and the corpus callosum (Fig. 4Bd). The DCX-expressing fibers extended around and into lesions, suggesting axonal regeneration or sprouting in the growth-promoting environment induced by BDNF.
The BDNF-expressing CNS cells in GA-treated mice were also characterized by their cytokine profile. As shown in Fig. 4C in a region of the optic tract, BDNF+ astrocytes manifested intense staining for the antiinflammatory Th2 cytokine IL-10 but no trace of the prototypic Th1 inflammatory cytokine IFN-γ.
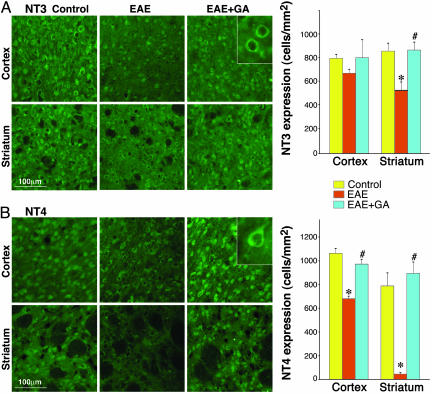
The Effect of GA on the Expression of NT3 and NT4 in Brains of EAE-Induced Mice. To find out whether GA affects additional neurotrophic factors, we studied the expression of NT3 and NT4 (Fig. 5). Both NTs showed cytoplasmatic expression in glia and neurons, NT3 somatic (Fig. 5A Inset) and NT4 somatodendrite (Fig. 5B Inset) neuronal localization. The consequence of EAE induction on these NTs was similar to that found for BDNF expression. Thus, shortly after disease onset, at the peak of clinical symptoms (day 22), a moderate increase of both factors in the cortex and a decrease in the striatum were observed in EAE mice (data not shown). At the chronic phase (60 days after disease induction), the expression of both NTs, NT3 (Fig. 5A) and NT4 (Fig. 5B), was decreased in the cortex and even more in the striatum. GA treatment applied at various disease stages augmented the expression of NT3 and NT4. Even when treatment started at the chronic disease phase, it restored their level to that of the naive control (Fig. 5 A and B). Hence, the effect of GA on the neurotrophic factors NT3 and NT4 is similar to its effect on BDNF.
Fig. 5.
Expression of NTs by immunostaining (green) in the brains of EAE-induced mice at the chronic disease phase, 60 days after induction, and the effect of GA treatment, starting at day 45 (delayed suppression). NT3 (A) and NT4 (B) expression in the motor cortex (layers 2 and 3) and the striatum. Quantitative analysis was performed by counting positively stained cells, two to three mice per treatment group and eight sections for each region. *, Significant effect over naive control; #, significant effect over EAE-untreated mice.
Discussion
The major finding reported here is that peripheral immunomodulatory treatment of EAE by GA augments the expression of BDNF and additional members of the NT family by T cells as well as by the resident CNS cells. At the peak of clinical symptoms, the expression of NTs in EAE mice was slightly elevated in various brain regions (represented by BDNF in cortex layer 2-3 in Fig. 2) in comparison to naive controls, corroborating other studies that found increased levels of NTs during the acute phase of EAE (7, 8) and MS (9). This elevation reflects self-repair mechanisms, similar to the enhanced neurogenesis triggered by EAE and other brain insults (10, 11). Yet, as the disease progresses to the chronic phase, BDNF (Fig. 3) and NT3 and NT4 (Fig. 5) drastically declined, much below that of naive control, indicative of the impairment inflicted by the disease. GA administration to EAE mice at various stages of the disease (prevention, suppression, or delayed suppression treatments) augmented BDNF, NT-3, and NT-4 expression over that of untreated mice. Of special significance is the restoration of NTs to a normal level by GA treatment in the chronic phase of the disease because this phase in EAE/MS is regarded as the stage in which exhausted self-compensating neuroprotection fails and extensive neurodegeneration takes place (3).
The modulation of three distinct NTs implies that GA treatment induces a genuine neuroprotective effect in the CNS. The quantitative differences in NT-3 expression among the various treatment groups (Fig. 5A) were indeed less prominent than for NT-4 (Fig. 5B) and BDNF (Fig. 3A). This result is possibly because of the method of counting the positive cells because the changes in NT-4 and BDNF were manifested mainly in the number of the immunoreactive cells, whereas for NT-3 the extent of expression, namely the density and area of staining per single cell, was mostly affected.
The elevated immunoreactivity could result from augmentation of synthesis of NTs and the expansion of new producing cells. Alternatively it could indicate higher uptake and redistribution of NTs, which could have been originally released from neighboring cells (4, 6). To distinguish between these possibilities, the effect of EAE and GA treatment on BDNF mRNA was tested by using in situ hybridization. mRNA expression revealed similar patterns to those detected on the protein level, i.e., moderate elevation in EAE and small further augmentation by GA, shortly after disease exacerbation (data not shown), as well as extensive decline at the chronic phase, which was restored by GA treatment (Fig. 3B). Still, the changes detected on the mRNA level were smaller than on the protein level, suggesting that BDNF production and the amounts of receptor-bound BDNF, both of considerable therapeutic significance, were affected.
The changes in BDNF expression were observed mainly in the cortex, accumbens, and the striatum. BDNF in the striatum is regarded as distinctly low. It was even claimed that the striatum lacks BDNF mRNA, and BDNF is just transported and released into it (4). The results presented in this study show that, in all treatment groups (Figs. 2 and 3) striatal BDNF, especially on the mRNA level (Fig. 3C), was drastically lower than in other regions, but a certain degree of expression was clearly evident. Of interest, the striatum was the most responsive region for BDNF modulation, manifesting a reduction in the number of positive cells early after disease appearance, their almost disappearance in the chronic phase, and complete restoration by GA treatment. These intense changes in contrast to the invariable expression in other systems (6) may suggest further involvement of the striatum in EAE.
These findings establish that peripheral GA treatment results in the augmentation of neurotrophic factors in the CNS and restores their impaired expression induced by the disease process. Reduced levels of BDNF in the serum and cerebrospinal fluid of MS patients, and its reversal by GA therapy were recently reported (23), indicating that this effect of GA is relevant to human therapy as well. The plausible candidates to mediate this effect are the Th2/3 GA-specific T cells that were reported in spleens and lymph nodes of experimental animals and in peripheral mononuclear cells of humans as a result of GA treatment (13, 18). GA-specific T cells can actually produce BDNF as demonstrated in vitro for peripheral mouse (15) and human (21, 24) T cell lines. In this study, too, the production of BDNF by GA-induced cells, whole lymphocyte populations, and T cell lines from both the CNS and the periphery, was evident at the protein and the mRNA levels (Fig. 1). GA-specific T cells were already shown to cross the blood-brain barrier, accumulate in the brain (19), and express there BDNF as well as antiinflammatory cytokines (20). The persistence of GA-specific T cells in the CNS was attributed to their cross-reactivity with the natural autoantigen MBP and hence to their ability to be stimulated in situ (18). This cross-reactivity demonstrated earlier by Th2/3 cytokine secretion was confirmed here for BDNF, suggesting that the production of NTs can occur in response to the CNS myelin antigen. Hence, BDNF expression by GA cells in the brain, 7 days after their injection to the periphery (20), despite their inability to express BDNF mRNA 3 days after stimulation (Fig. 1C), can be explained by their in situ restimulation.
Although T cells expressing NTs were frequently found in brains of mice treated by GA (Fig. 4Aa), their numbers could not account for this substantial elevation, as most of the positive cells of NTs were CNS resident cells: neurons and to a lesser extent astrocytes (Fig. 4 Ab and Ac). Diverse CNS cell types, glial, as well as neurons, can produce BDNF, NT-3, and NT-4 (4, 5). NTs were shown to be stored in vesicles and subject to regulated release (6). Indeed in brains of mice adoptively transferred with labeled GA-specific T cells, BDNF as well as IL-10 and TGF-β were expressed not only by the infiltrating T cells but also by the CNS resident cells in their vicinity (20). The bystander effect of GA was further corroborated in this study by the finding that BDNF-positive astrocytes in the GA-treated mice coexpressed IL-10 but not IFN-γ (Fig. 4C). This finding is in contrast to the reported association between NT production and inflammatory activity in EAE/MS (7, 9, 12). Hence, the beneficial effect of GA treatment is manifested not only in the suppression of the detrimental inflammatory response but also in the secretion of neurotrophic factors that confer neuroprotection, by GA-induced infiltrating T cells and bystander CNS resident cells in their propinquity.
In this context it is important that BDNF was expressed not only by mature neurons but also by neuronal-progenitor cells (Fig. 4Ad). We recently demonstrated that GA treatment induces neurogenesis (11), so the number of neuronal-progenitors in brains of GA-treated mice is significantly augmented in comparison to EAE-untreated mice and naive controls. Indeed, the amount of neuroprogenitor found in the SVZ of GA-treated mice was much higher than in EAE untreated mice, and a larger proportion of these cells showed intense staining for BDNF (Fig. 4 Ba and Bb). Furthermore, the newly generated neurons expressing BDNF migrated from the neuro-proliferative zones toward lesions in various brain regions (Fig. 4 Bc and Bd). Hence, in addition to their availability for the replacement of dead or dysfunctional cells, they could induce a growth-promoting environment that supports neuroprotection and regeneration at the injury site. The full-length BDNF receptor, tyrosine kinase receptor B, has been found in neurons in the vicinity of MS plaques and in reactive astrocytes in MS lesions (12). Thus, the elevated production of NTs induced by GA treatment could actually be of functional relevance and counteract the neurodegenerative disease course. The cumulative results presented in this study suggest that the immunomodulator GA exerts not only antiinflammatory effects, but also neuroprotection and regeneration of neural elements in the brain.
Acknowledgments
We thank Prof. Yaron Cohen for critical reading of the manuscript. This study was supported in part by a grant from Terry Oster and Dr. Claude Oster, a special fund of the Eugene Applebaum Family Foundation (Bloomfield Hills, MI), and a grant from Teva Pharmaceutical Industries (Israel). M.S. is on the board of directors of the Teva Pharmaceutical Industries.
Author contributions: R. Aharoni designed research; R. Aharoni, R.E., H.D., and G.L. performed research; R. Aharoni, R.E., M.S., and R. Arnon analyzed data; and R. Aharoni, M.S., and R. Arnon wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: GA, glatiramer acetate; NT, neurotrophin; MS, multiple sclerosis; EAE, experimental autoimmune encephalomyelitis; DCX, Doublecortin; SVZ, subventricular zone; MBP, myelin basic protein.
References
- 1.Behi, M. E., Dubucquoi, S., Lefranc, D., Zephir, H., De Seze, J., Vermersch, P. & Prin, L. (2005) Immunol. Lett. 96, 11-26. [DOI] [PubMed] [Google Scholar]
- 2.Hellings, N., Raus, J. & Stinissen, P. (2002) Immunol. Res. 25, 27-51. [DOI] [PubMed] [Google Scholar]
- 3.Bjartmar, C., Wujek, J. R. & Trapp, B. D. (2003) J. Neurol. Sci. 15, 165-171. [DOI] [PubMed] [Google Scholar]
- 4.Lassmann, V., Gottmann, K. & Malcangio, M. (2003) Prog. Neurobiol. 69, 341-374. [DOI] [PubMed] [Google Scholar]
- 5.Riley, C. P., Cope, T. C. & Buck, C. R. (2004) J. Mol. Histol. 35, 771-783. [DOI] [PubMed] [Google Scholar]
- 6.Murer, M. G., Yan, Q. & Raisman-Vozari, R. (2001) Prog. Neurobiol. 63, 7-124. [DOI] [PubMed] [Google Scholar]
- 7.Hammarberg, H., Lidman, O., Lundberg, C., Eltayeb, S. Y., Gielen, A. W., Muhallab, S., Svenningsson, A., Linda, H., Van der Meide, P. H., Cullheim, S., et al. (2000) J. Neurosci. 20, 5283-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhang, J., Li, Y., Chen, J., Cui, Y., Lu, M., Elias, S. B., Mitchell, J. B., Hammill, L., Vanguri, P. & Chopp, M. (2005) Exp. Neurol. 195, 16-26. [DOI] [PubMed] [Google Scholar]
- 9.Caggiula, M., Batocchi, A. P., Frisullo, G., Angelucci, F., Patanella, A. K., Sancricca, C., Nociti, V., Tonali, P. A. & Mirabella, M. (2005) Scand. J. Immunol. 62, 176-182. [DOI] [PubMed] [Google Scholar]
- 10.Magavi, S. S., Leavitt, B. R. & Macklls, J. (2000) Nature 405, 951-955. [DOI] [PubMed] [Google Scholar]
- 11.Aharoni, R., Arnon, R. & Eilam, R. (2005) J. Neurosci. 25, 8217-8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadelmann, C., Kerscensteiner, M., Misgeld, T., Bruck, W., Hohlfeld, R. & Lassmann, H. (2002) Brain 125, 75-85. [DOI] [PubMed] [Google Scholar]
- 13.Arnon, R. & Sela, M. (2003) J. Mol. Recognit. 16, 412-421. [DOI] [PubMed] [Google Scholar]
- 14.Aharoni, R., Yussim, A., Sela, M. & Arnon, R. (2005) Int. Immunopharmacol. 5, 23-32. [DOI] [PubMed] [Google Scholar]
- 15.Kipnis, J., Yoles, E., Porat, Z., Cohen, A., Mor, F., Sela, M., Cohen, I. R. & Schwartz, M. (2000) Proc. Natl. Acad. Sci. USA 97, 7446-7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aharoni, R., Kayhan, B. & Arnon, R. (2005) Inflamm. Bowel Dis. 11, 106-115. [DOI] [PubMed] [Google Scholar]
- 17.Boska, M. D., Lewis, T. B., Destache, C. J., Benner, E. J., Nelson, J. A., Uberti, M., Mosley, R. L. & Gendelmann, H. E. (2005) J. Neurosci. 16, 1691-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1997) Proc. Natl. Acad. Sci. USA 94, 10821-10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aharoni, R., Teitelbaum, D., Leitner, O., Meshorer, A., Sela, M. & Arnon, R. (2000) Proc. Natl. Acad. Sci. USA 97, 11472-11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aharoni, R., Kayhan, B., Eilam, R., Sela, M. & Arnon, R. (2003) Proc. Natl. Acad. Sci. USA 100, 14157-14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziemssen, T., Kumpfel, T., Klinkert, W. E. F., Neuhaus, O. & Hohlfeld, R. (2002) Brain 125, 2381-2391. [DOI] [PubMed] [Google Scholar]
- 22.Yakubov, E., Gottlieb, M., Gil, S., Dinerman, P., Fuchs, P. & Yavin, E. (2004) Mol. Brain Res. 127, 10-26. [DOI] [PubMed] [Google Scholar]
- 23.Azoulay, D., Vachapova, V., Shihman, B., Miler, A. & Karni, A. (2005) J. Neuroimmunol. 167, 215-218. [DOI] [PubMed] [Google Scholar]
- 24.Chen, M., Valenzuela, R. M. & Dhib-Jalbut, S. (2003) J. Neurol. Sci. 215, 37-44. [DOI] [PubMed] [Google Scholar]