Abstract
Little is known about the neural bases of the reduced auditory and cortical processing speeds that have been recorded in language-impaired, autistic, schizophrenic, and other disabled human populations. Although there is strong evidence for genetic contributions to etiologies, epigenetic factors such as perinatal anoxia (PA) have been argued to be contributors, or causal, in a significant proportion of cases. In this article, we explored the consequences of PA on this elementary aspect of auditory behavior and on auditory system function in rats that were briefly perinatally anoxic. PA rats had increased acoustic thresholds and reduced processing efficiencies recorded in an auditory behavioral task. These rats had modestly increased interpeak intervals in their auditory brainstem responses, and substantially longer latencies in poststimulus time histogram responses recorded in the primary auditory cortex. The latter were associated with degraded primary auditory cortex receptive fields and a disrupted tonotopy. These processing deficits are consistent with the parallel behavioral and physiological deficits recorded in children and adults with a history of language-learning impairment and autism.
Keywords: auditory behavior, auditory brainstem responses, auditory cortex, autism, language learning impairment
Clinical and epidemiological studies of language impairment and autism have shown a strong genetic component with multiple genes involved (1-3). The distributed complex of brain system abnormalities, however, also indicates that exposure to environmental factors at early stages of brain development may be a contributing or even causal factor in a significant percentage of cases. Epigenetic factors, such as infections, prenatal exposure to drugs and alcohol, obstetric complications, postnatal noise exposure, environmental toxins, and perinatal anoxia (PA), have been argued to be especially important in genetically vulnerable subjects (4, 5, ¶).
There is significant and growing evidence that PA may commonly contribute to the severity of deficits, especially in the auditory and language domains. Anatomical, experimental, and clinical data have demonstrated a special susceptibility of brainstem auditory neurons to oxygen shortage during development (6-10). Auditory processing deficits have been observed in several neurodevelopmental disorders that show a correlation of emergent deficits with epochs and severity of PA (11-14). Sectors of the auditory and aural speech cortex specifically contributing to cognitive processing of auditory and aural speech inputs appear to be especially sensitive to PA (7). Lesions of the auditory nuclei are a prominent and consistent finding in histopathological studies of the brain of children who have died of suffocation (15). Increased hearing thresholds, modality-specific deficits in auditory cortical-evoked potentials, and structural alterations of auditory nuclei have been described in monkeys after experimental PA (7, 16, 17).
Despite the well-documented sensitivity of the auditory system to oxygen shortage, the behavioral and physiological consequences of experimental PA have received little attention. In the present study, auditory behavior and auditory system development were explored in rats that were briefly anoxic at birth and related to auditory processing deficits described in subjects affected by neurodevelopmental disorders.
Methods
Auditory Behavior. All experimental procedures described in this study were carried out in accordance with the Guidelines laid down by the National Institutes of Health, and the Committee on Animal Research at the University of California, San Francisco approved all animal use. Thirty-one newborn rats from four litters were randomly assigned to control or experimental groups. The experimental paradigm has been described in detail in ref. 18. Briefly, pups assigned to the experimental group experienced two epochs of anoxia of 12 min each, on the day of birth (P0) and on the following day (P1). Pups assigned to the two anoxic groups were placed over a thermal blanket (37°C) in a Plexiglas, airtight chamber. Pure nitrogen gas (100% N2) was passed through the inlet. After ≈1 min, the pups became hyperactive, and within the next 1-2 min, hyperactivity was followed by a change in skin color from light pink to bluish, loss of movement, and sporadic gasping. Twelve minutes after the beginning of the hyperactivity in most of the pups, the N2 was turned off, the chamber was opened, and the pups quickly were removed, resuscitated, and left in normal atmospheric conditions until they returned to their original pink color and were breathing regularly. The procedure was repeated 24 h later.
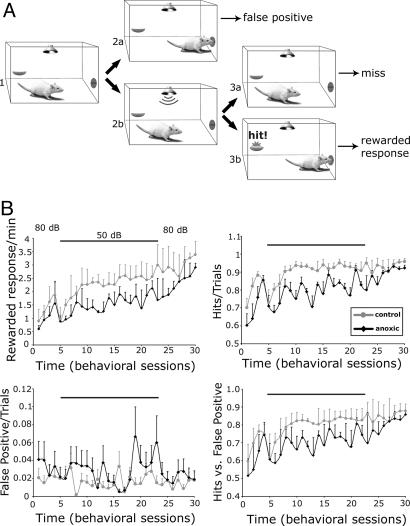
After ≈60 days, a subset of eight rats (four control and four anoxic rats) were evaluated for behavioral deficits by using a timed auditory signal-detection task. Rats were placed in an operant behavior chamber, enclosed within a sound-attenuating box. Their daily task required a nose-poke response to a 300-ms, 9-kHz tone pip presented at 80 or 50 dB sound pressure level (SPL), with correct responses resulting in a food pellet reward (see Fig. 1A). Responses were marked as “hits” if the animal responded within a 7-s reward window, as “false-positive” if rats inserted their noses in the nose-poke before the tone presentation, or as “misses” if rats did not respond within the reward window. Hits were rewarded with a food pellet; false-positives and misses were punished by a 30-s timeout. The corrected hit rate, HR, was defined as the proportion of rewarded responses (RR) over the total number of presentations, given that a tone was played in the previous 7 s, and it was calculated by HR = RR/(RR + M), where M is the misses, and plotted vs. time (Fig. 1B Upper Left). The false-positive rate was defined as the probability of responding, given that no tone was played in the previous 7 s, and is given by the ratio FP/{[TT - (RR + M)·7 - 30·M]/7}, where FP are the false-positives, TT is the total time of the task in seconds, and M is the misses (Fig. 1B Lower Left). The performance index (PI) was defined by PI = HR·S, where HR is the corrected hit rate and S is the safe rate correction factor (S = 1 - false-positive rate; see ref. 19). Control and experimental animals were trained during each session in a randomized daily order.
Fig. 1.
Operant behavior. (A) Schematic of the auditory signal-detection task. Rats placed in an operant behavior chamber enclosed within a sound-attenuating box wait for a 9-kHz, 300-ms tone pip (1). Possible trial outcomes included: (i) a nose poke before the tone was played (2a), scored as a false-positive; (ii) failure to respond after the tone was played within a 7-s reward time-window (2b and 3a), scored as a miss; (iii) response within the reward time-window (2b and 3b), scored as a hit. (B) Plots of the performance over time. The total numbers of hits, misses, and false-positives were collected during each session. Hits and false-positives were plotted as hit rate (Upper Left), corrected hit rate (Upper Right), and false-positive rate (Lower Left) vs. time, respectively. (Lower Right) The performance index (see Methods) was obtained by the corrected hit rate × the safe rate correction factor and plotted vs. time (behavioral sessions).
Electrophysiology. Auditory brainstem responses (ABRs) and primary auditory cortex (A1) organization were determined from a second subset of 20 rats (11 control and 9 anoxic rats) anesthetized with 60 mg/kg pentobarbital i.p. ABRs were obtained from six controls and seven PA rats by placing silver wires subdermally at the scalp midline (negative), posterior to the stimulated ear (positive), and on the midline of the back 1-2 cm posterior to the neck. ABRs were obtained by averaging the electroencephalographically recorded responses evoked by 1,000 clicks of a 100-μs duration at click intensities ranging from 28 to 68 dB SPL, delivered at a rate of 20 clicks per second. Bioelectrical activity was recorded, amplified (×10,000), and filtered (0.3-10 kHz).
Tone pips (25-ms duration with 3-ms on/off ramps) delivered at 2 pulses per second with a calibrated sound delivery system (Tucker-Davis Technologies, Gainesville, FL) were used to examine the organization of the A1. Extracellular neuronal spikes were recorded in the middle cortical layers of the right auditory cortices by using tungsten microelectrodes (1-μm tip diameter, 1-2 MΩ at 1 kHz, FHC, Bowdoinham, ME). At each sample site, single or multiple unit responses (spikes) evoked by randomly delivered stimuli ranging across a broad frequency and intensity continuum were recorded within cortical layers III-IV. Characteristic frequency (CF) was defined as the frequency that evoked reliable spike discharges at the lowest stimulus intensity. Tuning curves (TCs), were defined as continuous areas of responses to a finely graded (1/8th octave) series of test tones (tone pips) presented at graded intensity steps (10 dB). The receptive field (RF) irregularity index (19) was used to quantify eventual differences between control and experimental animals. The RF irregularity index was defined as [Corr(0, 0) - (Corr(1, 0) + Corr(0, 1))/2]/Corr(0, 0)1/2, where Corr(0, 0) represents the central term of the RF and Corr(0, 0) - (Corr(1, 0) + Corr(0, 1))/2 represents the periphery of the RF. A constant number of 3 was then subtracted from the value.
Thresholds were defined as the lowest intensity at which reliable responses were recorded. For the purposes of analysis, A1 neurons were subdivided into three main groups: (i) neurons with low-frequency “tuned” (LFT) CFs between 1 and 4 kHz; (ii) with middle-frequency tuned (MFT) CFs between 4 and 13 kHz; and (iii) with high-frequency tuned (HFT) CFs between 13 and 30 kHz. On average, each map was obtained by recordings from 113.6 ± 22.4 (mean ± SD, n = 5) cortical sites in control rats and 90.4 ± 14.0 in PA rats (n = 5). Recording locations in the cortex were marked on a digital image and assigned their specific CFs. CF maps were then reconstructed by using customized software.
Data are presented as the mean ± SD unless otherwise specified. The nonparametric sign test was used to compare the performances in the different time windows between the two groups. Repeated-measures two-way ANOVA was applied to test for the significance of change over time. The nonparametric Mann-Whitney U test was used in all other cases or as a supplemental statistical analysis. Values of P < 0.05 were considered significant.
Results
Auditory Behavior. Starting from P60, control and PA rats were trained on an auditory signal-detection task (Fig. 1A). The initial free-field intensity of the tone pip was set at ≈80 dB. After 4 days of operant training, the hit rate reached a value of 1.86 ± 0.47 RRs per min (mean ± SD, n = 4) for controls and 1.55 ± 0.83 RRs per min for perinatal anoxic rats (n = 4) (Fig. 1B Upper Left). Although the achievement of success was slower in PA rats, the corrected hit rates (total number of RRs divided by the total number of presentations given by the sum of RRs and misses; see Methods) reached 85-86% for both groups (Fig. 1B Upper Right). Two-way ANOVA did not yield a significant difference between groups in corrected hit rates (P = 0.122) (Fig. 1B Upper Right) but did show a highly significant effect of acquisition over time [F(3, 18) = 10.07; P < 0.001].
On the 5th day, the tone pip intensity was lowered to 50 dB (horizontal lines within the plots in Fig. 1B). The corrected hit rate immediately dropped for both groups to ≈71-73%. Within three sessions, the control rats' performances again reached 1.94 ± 0.26 RR per min, at a corrected hit rate of 84%. Over the following 15 behavioral sessions, the corrected hit rate rose further to a steady level near 2.44 ± 0.16 RR per min, at an equivalent corrected hit rate of 90-95%; this value was calculated for the five daily sessions between behavioral sessions 15 and 19 (Fig. 1B Upper Right).
By contrast, the PA rats' performances were significantly lower. After three behavioral sessions at 50 dB, PA rats performed at 1.59 ± 0.26 RR per min and, during the same time window (behavioral sessions 15 through 19), reached a mean level of 1.67 ± 0.14 RR per min (Fig. 1B Upper Left). The corrected hit rate showed a larger degree of daily variability, oscillating between 70% and 90% (Fig. 1B Upper Right). Two-way ANOVA yielded significant differences in corrected hit rates between the two groups [F(1, 5) = 35.95; P < 0.002] and over time [F(17, 85) = 6.00; P < 0.0001] but no two-way interaction (P > 0.4).
During the behavioral sessions at 50 dB, the average performance index (defined by PI = HR·S, where HR is the corrected hit rate and S is the safe rate correction factor and S = 1 - false-positive rate; see ref. 20) of control rats increased over time from 61% to >80%, whereas it fluctuated between 60% and 75% for PA rats. Two-way ANOVA showed a strong difference between the two conditions [F(1, 5) = 38.74; P < 0.002] and over time [F(17, 85) = 5.38; P < 0.0001] but no two-way interaction (P = 0.5).
After 4 weeks at 50 dB, the tone pip intensity was again returned to 80 dB. The control rats' performance promptly improved even further, with a corrected hit rate of more than three RRs per min (Fig. 1B Upper Left) and a performance index consistently higher than 80% (Fig. 1B Lower Right). Anoxic rats again exhibited a slower learning curve adjustment, with larger daily fluctuations but with a similar slope (Fig. 1B Lower Right). Although two-way ANOVA did not yield significant differences in corrected hit rates between groups (P > 0.13), it again showed significant differences over time (P < 0.025) but no two-way interaction [F(7, 42) = 1.41; P > 0.23]. A similar result was obtained for the statistical analysis of the performance index.
Although larger fluctuations were observed in PA rats, it might be noted that false-positive rates were always maintained <15% for both groups. False-positive rates did not significantly change over the course of this behavioral task (Fig. 1B Lower Left).
In summary, PA rats were slower in their behavioral responses and were always slower to improve performances at the three epochs when experimental conditions changed in this behavior. These rats showed a diminished capacity for processing these sound stimuli, which was especially notable when the stimuli were presented at lower intensity.
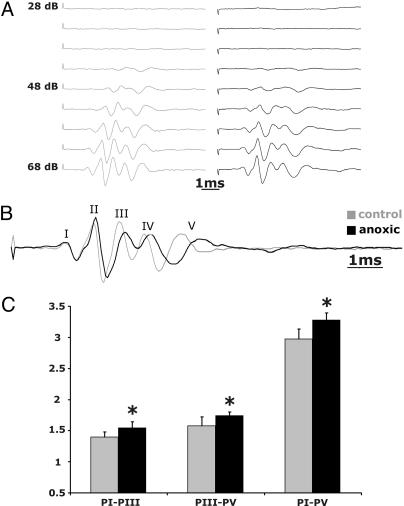
ABRs. ABRs were obtained by averaging the electroencephalographic “far field potentials” evoked by 1,000 clicks at a sampling rate of 50 kHz from six control and seven PA rats (Fig. 2 A and B) (see Methods). Thresholds were defined as the lowest click intensity levels eliciting reproducible responses. Although some differences in wave amplitudes and dynamics were observed in the ABRs of experimental vs. control animals (Fig. 2), the first detectable response was observed between 43 and 48 dB in both groups. The nonparametric Mann-Whitney U test showed no significant response threshold differences between control and PA rats (P > 0.05).
Fig. 2.
ABRs in normal control and anoxic rats. (A) ABRs were obtained by averaging the electroencephalographic signals to 1,000 presentations of an acoustic click. Threshold was defined as the lowest click intensity levels that elicited an evoked response. Representative recordings from a control rat (Left) and a PA rat (Right) are shown. The recordings resulted in the typical series of five identifiable waves (I-V shown at higher magnification in B). Although ABR peaks were of lower amplitude in PA rats, in both cases all wave peaks could be consistently observed in control and PA rats at 43 and 48 dB SPL. (C) Time intervals (ms) between peaks I and III (PI-PIII), III and V (PIII-PV), and I and V (PI-PV) were estimated and plotted. IPIs were significantly prolonged in anoxic animals (marked with asterisks). Values are means ± SD.
Response onsets and peak latencies varied as a function of click intensity and, as shown in earlier studies (e.g., see ref. 21), were shorter for louder stimuli. In control rats, peaks III and V were recorded at 3.0 ± 0.15 and 4.5 ± 0.15 ms and at 2.61 ± 0.2 ms and 4.22 ± 0.2 ms at 48 dB and 68 dB, respectively. In PA rats the same peaks were recorded at 3.29 ± 0.31 and 4.99 ± 0.17 ms and at 2.88 ± 0.13 and 4.63 ± 0.2 ms at 48 and 68 dB clicks, respectively. Differences were statistically significant for different intensities for both control and PA rats (P < 0.05; Mann-Whitney U test).
When latencies were compared at the same intensities, there were no significant differences in peak I latency between controls (e.g., at 68 dB, they were 1.40 ± 0.29 ms for controls and 1.39 ± 0.14 ms for PA rats). However, all subsequent peaks were significantly delayed, and interpeak intervals were all significantly longer in PA than in control rats (Fig. 2B). Interpeak intervals between peaks I and III, III and V, and I and V are summarized in Fig. 2C. Two-way ANOVA yielded a significant difference between groups [F(1, 110) = 25.88; P < 0.00001] and between waves [F(9, 110) = 566.98; P < 0.00001] and a significant two-way interaction [F(9, 110) = 2.89; P < 0.004].
ABRs provide a general index of peripheral and central auditory system integrity (22). Failure to record increased ABR thresholds suggests that brainstem auditory nuclei are at least substantially intact and functional and that the poorer performance recorded in these PA rats may have a substantially thalamic or cortical origin. Increased peak latencies may contribute to deficits in response rates recorded behaviorally, but it should be noted that these processing efficiency differences involve many tens or hundreds of millisecond differences in timing, whereas longer ABR latencies are on the scale of a millisecond or less.
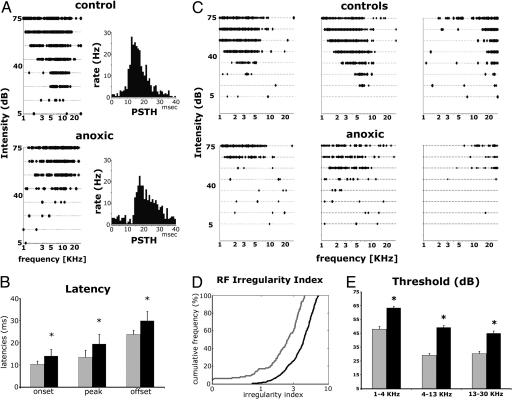
Cortical Recording. A1 neurons in control rats responded reliably to test tones, and their frequency intensity RFs or TCs were usually V-shaped, with an easily definable characteristic frequency (Fig. 3 A Upper Left and C a-c). A1 neurons could also be identified by their short stimulus-evoked response latencies, ranging between 7 and 20 ms (Fig. 3A Right) (18). Onsets, peaks, and offsets of responses in the poststimulus time histograms (PSTHs) in control rats occurred at mean poststimulus onset times of 10.33 ± 1.42, 13.47 ± 1.78, and 23.78 ± 3.1 ms, respectively (n = 6). The identification of A1 was more difficult in PA rats, and it was determined primarily on the basis of its caudorostral tonotopic organization, with LFT neurons (≤4 kHz) located at the caudal end of A1 (see Fig. 4A). TCs were degraded in multiple respects (Fig. 3 A Lower Left and C d-f). PSTH peaks were significantly delayed in PA rats and occurred at 13.94 ± 3.04, 19.43 ± 4.19, and 29.88 ± 4.32 ms (n = 9; Whitney-Mann U test; P < 0.005). Note that those delay differences not only paralleled but also amplified those recorded in the auditory brainstem.
Fig. 3.
Tone pip-evoked unit responses in the auditory cortex. (A) Left show representative examples of TCs obtained from one control (Upper) and one PA rat (Lower). Right show their PSTHs obtained in response to test tone pips. A1 neurons were identified by short-latency responses (6-7 to 20 ms) and tonotopy. (B) Bar graph showing average time (ms) to PSTH onset, peak, and offset obtained from four control (gray) and eight PA rats (black). (C) Representative example of TCs obtained from adult control (a-c) and PA (d-f) rats. A1 neurons in control rats responded continuously and reliably to test tones with a clearly delineable area or tuning curve. By contrast, discontinuous responses (lack of reliable responses to some of the test tones) and recurring blank domains within the TC increased (d) or decreased (e) sensitivity, and broad TCs (e and f) were recorded in PA rats. (D) A1 RFs were significantly degraded in PA rats as measured with the RF irregularity index. In control rats, the average RF irregularity index was 2.41 ± 0.13 and 4.20 ± 0.08 (mean ± SEM) for PA rats. An irregularity index >4, commonly observed in PA rats, was never seen in controls. (E) Response thresholds for three frequency ranges obtained from four control (gray) and eight PA rats (black). Asterisks mark significant difference bands.
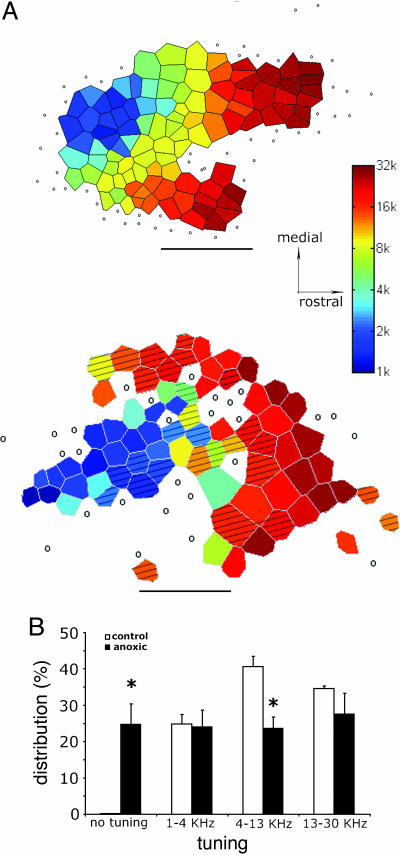
Fig. 4.
Disrupted representation in A1 of anoxic rats. (A) Cortical maps from a control rat (Upper) and an anoxic rat (Lower). Caudal is left and rostral is right. In control animals (n = 5), progressive changes in tonotopy were observed from caudal (low frequency) to rostral (high frequency). An increased number of nonresponsive and nonselectively responding sites (open circles) and a global reduction in sites responding to middle frequencies are evident in the A1 of PA rats (n = 5). (B) Distribution of CFs in the cortex of control and PA rats. Asterisks mark significant differences between control and PA rats (P < 0.05).
In striking contradiction to controls, recurring “blank” domains in the TCs and multiple-peaked RFs and broader tuning often accompanied delayed PSTH responses in PA rats (Fig. 3C d-f). These anomalies in RFs were quantified by using the RF irregularity index devised by Bao et al. (19) (Fig. 3D) (see Methods). PA rats' neurons resulted in a significantly higher RF irregularity indexes compared with control rats (Mann-Whitney U test; P < 0.01). The average RF irregularity index was 2.41 ± 0.13 (mean ± SEM) for control rats and 4.20 ± 0.08 for PA rats. Especially high RF irregularity index values (>5.50) were observed at several cortical sites in every PA rat (hatched areas in Fig. 4A). Moreover, neurons at most A1 recording sites responded only to relatively intense stimulation (>60 dB; Fig. 3 B and C). Average CF thresholds were 42.92 ± 2.09 for a low-frequency range (1-4 kHz), 24.00 ± 1.48 for a middle-frequency range (4-13 kHz), and 25.25 ± 1.8 dB for a high-frequency range (>13 kHz) in controls; and 58.47 ± 1.05, 44.24 ± 1.49, and 40.05 ± 1.62 dB SPL in anoxic rats. Differences between groups were significant for all frequency ranges [F(1, 156) = 33.41 and P < 0.00001 for LFT; F(1, 187) = 53.95 and P < 0.00001 for MFT; F(1, 175) = 25.94 and P < 0.00001 for HFT]. The greatest difference was observed in MFT neurons. Note that these large-scale, cortical-evoked unit response threshold differences between control and PA rats had no precedent in the more normal thresholds recorded for brainstem responses in PA rats.
Altered TCs and degraded spectral tuning in PA rats were also consistently associated with disrupted cortical tonotopy and a smaller putative A1 in PA rats (Fig. 4A). An increased number of nonresponsive and nonselectively responding sites (24.75% in PA rats vs. <1% in controls) at least partially accounts for this significant difference (P < 0.01; Mann-Whitney U test). The smaller responsive area may represent only a part of an anatomically intact A1. For example, only 23.67% of the cortical sites in PA rats vs. 40.6% of controls were tuned for frequencies between 4 and 13 kHz. A nonparametric Mann-Whitney U test yielded a significant difference between control and PA rats (P < 0.01) in this respect. It is worth stressing that some anomalies in cortical representations of LFT and HFT could have been “canceled out” by averaging representations recorded in different rats. In two cases, HFT sites were poorly represented, whereas in another case they were proportionally over-represented. In a third case, a poorer representation of MFT sites was associated with several nonresponsive cortical sites. In another case, LFT and HFT were represented adjacently, and the MFT occupied a more lateral part of the putative A1, an unusual tonotopy that has never been recorded in a control rat.
In conclusion, increased cortical thresholds and deficient temporal decoding associated with degraded TC properties and disrupted A1 representations were recorded in PA rats, suggesting a substantially forebrain origin for the increased behavioral thresholds and the reduced behavioral performance rates recorded in these rats.
Discussion
Data in the present study show that PA rats exhibited a decreased behavioral acoustic sensitivity, slower behavioral responses, and invariably slower adjustments to changing behavioral conditions. In parallel, there was a significant reduction of temporal precision at subcortical and especially cortical levels and a grossly degraded spectral resolution and representational order (tonotopy) recorded at the cortical level.
In the present study, a simple auditory signal-detection task was used to test consequences for the acoustic sensitivity of rats exposed to PA. PA rats' performances were reduced, with their behavioral differences exacerbated at a lower sound intensity, consistent with a diminished acoustic sensitivity. Reduced acoustic sensitivity, but not acquisition of auditory discrimination abilities, has been reported in primate and subprimate species exposed to PA (7, 16, 23, 24). Moreover, monkeys that experienced PA exhibited auditory, but not visual, modality-specific cortical-evoked potential differences (7, 16). Previous studies have also reported decreased, unchanged, or increased locomotor activity in rats exposed to PA (see ref. 18 for references), whereas Tomimatsu et al. (25) reported that reduced motor skills were correlated with reduced temporal specificity in their study animals' ABRs. Several other articles, including the present, have shown increased interpeak intervals but failed to detect changes in ABR thresholds after experimental PA (9, 25). Transient ABR threshold elevation has been recorded during controlled experimental hypoxia in rats (26). When normal air respiration returned, however, the ABR thresholds returned to normal. Similarly, human infants that suffered PA presented with a transient increase in ABR thresholds, which, at the age of 6 months, did not differ from the ABR thresholds of healthy controls (27, 28). In the present study, ABRs were recorded from 3-month and older rats, which correspond to the later human maturational stages (29). The failure to record a threshold difference in ABRs might also be due to the spectral nonspecificity of this measure, by which the threshold responses might be evoked from a less than completely intact auditory brainstem. Contrary to this interpretation, however, threshold differences were not observed in tone pip-evoked ABRs (data not shown).
ABRs represent useful measures of peripheral and central auditory system integrity (22). In the rat, the acoustic nerve generates peak I, the cochlear nucleus peak II, the superior olivary nucleus peak III, the lateral lemniscus peak IV, and the inferior colliculus peak V (30). Increased interpeak intervals may be an indication of anomalous myelination or nerve conduction as well as brainstem, thalamic, and/or cortical deficits (31, 32). Cochlear and brainstem auditory nuclei are highly sensitive to oxygen shortage (25, 33). Peak I, however, was not delayed in the present study, thus suggesting the integrity of the cochlea and auditory nerve. The modestly but highly consistently increased latency of peak III points to deficits in the cochlear nucleus and/or superior olivary complex or to a cumulative effect of slower conduction through these brainstem nuclear levels.
At postmortem examination, lesions of the especially highly metabolically active cochlear nuclei were a prominent and consistent finding after PA (6). Perinatal lesion of the cochlea leads to disrupted tonotopic representations in both the inferior colliculus and the A1 (34, 35), potentially explaining the increased latency recorded for peak V. The inferior colliculus is also one of the most metabolically active structures in the brain (36), and it therefore represents a vulnerable target to PA. Interestingly, neuronal proliferation in the inferior colliculus is prominent, and middle-frequency inputs are arriving in this nucleus in the immediate postnatal period in the rat (29, 37).
Finally, onset responses in the rat's inferior colliculus are significantly delayed when the A1 is pharmacologically inactivated (38), suggesting that the reduced temporal precision observed in the ABRs of PA rats may also have a cortical origin.
Increased thresholds together with delayed PSTH onsets, peaks, and offsets were all recorded at the cortical level. Thresholds were most strongly and more commonly increased for MFT neurons (4-13 kHz), suggesting a cortical role in the reduced hearing sensitivity at 9 kHz observed in the signal-detection task. Cortical-evoked potential alterations have also been documented in PA guinea pigs, in which they were more pronounced and prolonged than those alterations recorded in the ABRs (24).
Degraded auditory cortex spectral and temporal processing and disrupted cortical representations may account for part of the reduced hearing sensitivity exhibited by PA rats in the signal-detection task. Similar spectral and temporal degradation has been described in cortical neurons of noise-reared rats (4, 19). Interestingly, noise induces hypoxia at the cochlear level, and levels of hypoxia that alone would not affect the auditory system can enhance noise-induced hearing loss (39). These data suggest that chronic noise exposure during development may represent a “selective” form of chronic cochlear anoxia. This evidence is relevant because infants that are exposed often to unnatural noises like those of intensive care or preterm units are those that have also often suffered PA.
Finally, the differences in behavior between control and PA rats suggest that cognitive factors (i.e., executive dysfunction) extrinsic to the auditory system can also affect their performances. Indeed, executive dysfunctions, dopamine-signaling deficits, and memory deficits have been described in rats after PA (40). A delayed appearance of visual and locomotor behaviors has been reported in monkeys anoxic at birth. After these behaviors were established, however, there was little difference between anoxic and control animals, suggesting that the structures responsible for acquisition were more heavily damaged than those responsible for the behaviors per se (41).
In conclusion, PA, depending on the time window, may selectively induce domain-specific psychomotor slowing, have a more global effect on executive functions, and/or unmask or exaggerate inherited weaknesses (18). Second, reduced temporal processing in one system can also have a more global effect: cognitive functioning usually requires integration from multiple sensory modalities. The slower auditory processing concurrent with “normal” tactile information could eliminate simultaneity among sensory inputs, thus disrupting contingencies for basic associative learning. Finally, immature-like features, such as increased behavioral acoustic thresholds, reduced performance rate, and slower brainstem and cortical responses similar to those shown in PA rats, have been described in dyslexia (42) and autism (43, 44). Our findings suggest that PA in rats may represent a promising model that could allow us to understand the specific and global executive function deficits observed in children affected by neurodevelopmental disorders.
Acknowledgments
We thank Prof. Ehud Ahissar for his comments on the manuscript and Profs. Shaowen Bao, David Blake, and Dan Polley for their helpful suggestions during the preparation of the manuscript. This work was supported by funds from the Sandler Foundation (to M.M.M.), the MIND Foundation (to M.M.M.), Cure Autism Now (to M.M.M.), and National Institutes of Health Grant NS-10414 (to M.M.M.).
Author contributions: F.S. and A.R.d. designed research; F.S., E.F.C., and R.C.L. performed research; H.N. contributed new reagents/analytic tools; F.S. and B.H.B. analyzed data; and F.S. and M.M.M. wrote the paper.
Some parts of this study have been presented in abstract form [Strata, F., Chang, E. F., deIpolyi, A. R., Bonham, B. H., Nakahara, H., Liu, R. C. & Merzenich, M. M. (2004) in FENS Forum 2004 (Fed. Eur. Neurosci. Soc., Lisbon), Vol. 4, p. A148.24 (abstr.)].
Conflict of interest statement: No conflicts declared.
Abbreviations: PA, perinatal anoxia; SPL, sound pressure level; ABRs, auditory brainstem responses; A1, auditory cortex; CF, characteristic frequency; TCs, tuning curves; RF, receptive field; LFT, low-frequency tuned; MFT, middle-frequency tuned; HFT, high-frequency tuned; RRs, rewarded responses; PSTHs, poststimulus time histograms.
Footnotes
Kenet, T., Pessah, I. N. & Merzenich, M. M., Poster Presentation, 34th Annual Society for Neuroscience Meeting, Oct. 23-27, 2004, San Diego.
References
- 1.Smith, M., Woodroffe, A., Smith, R., Holguin, S., Martinez, J., Filipek, P. A., Modahl, C., Moore, B., Bocian, M. E., Mays, L., et al. (2002) Cytogenet. Genome Res. 98, 233-239. [DOI] [PubMed] [Google Scholar]
- 2.Rapin, I. & Dunn, M. (2003) Brain Dev. 25, 166-172. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, D. V. (2002) Am. J. Med. Genet. 114, 56-63. [DOI] [PubMed] [Google Scholar]
- 4.Glasson, E. J., Bower, C., Petterson, P., de Klerk, N., Chaney, G. & Hallmayer, J. F. (2004) Arch. Gen. Psychiatry 61, 618-627. [DOI] [PubMed] [Google Scholar]
- 5.Chang, E. F. & Merzenich, M. M. (2003) Science 300, 498-502. [DOI] [PubMed] [Google Scholar]
- 6.Hall, J. G. (1964) Acta Otolaryngol. 120, Suppl. 194, 1-93. [PubMed] [Google Scholar]
- 7.Berman, D., Karalitzky, A. R. & Berman, A. J. (1971) Exp. Neurol. 31, 140-149. [DOI] [PubMed] [Google Scholar]
- 8.Kaga, K., Ichimura, K., Kitazumi, E., Kodama, K. & Tamai, F. (1996) Int. J. Pediatr. Otorhinolaryngol. 36, 231-239. [DOI] [PubMed] [Google Scholar]
- 9.Rehn, A. E., Loeliger, M., Hardie, N. A., Rees, S. M., Dieni, S. & Shepherd, R. K. (2002) Hear. Res. 166, 159-165. [DOI] [PubMed] [Google Scholar]
- 10.Rance, G., Beer, D. E., Cone-Wesson, B., Shepherd, R. K., Dowell, R. C., King, A. M., Rickards, F. W. & Clark, G. M. (1999) Ear Hear. 20, 238-252. [DOI] [PubMed] [Google Scholar]
- 11.Bamiou, D. E., Musiek, F. E. & Luxon, L. M. (2001) Arch. Dis. Child. 85, 361-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noterdaeme, M., Amorosa, H., Mildenberger, K., Sitter, S. & Minow, F. (2001) Eur. Child Adolesc. Psychiatry 10, 58-66. [DOI] [PubMed] [Google Scholar]
- 13.Hultman, C. M., Sparen, P. & Cnattingius, S. (2002) Epidemiology 13, 417-423. [DOI] [PubMed] [Google Scholar]
- 14.Fuess, V. L., Bento, R. F. & da Silveira, J. A. (2002) Ear Nose Throat J. 81, 706-710, 712. [PubMed] [Google Scholar]
- 15.Hall, J. G. (1964) Acta Otolaryngol. 12, Suppl. 188, 331+.14146694 [Google Scholar]
- 16.Mirsky, A. F., Orren, M. M., Stanton, L., Fullerton, B. C., Harris, S. & Myers, R. E. (1979) Dev. Psychobiol. 12, 369-379. [DOI] [PubMed] [Google Scholar]
- 17.Brann, A. W., Jr., & Myers, R. E. (1975) Neurology 25, 327-338. [DOI] [PubMed] [Google Scholar]
- 18.Strata, F., Coq, J. O., Byl, N. & Merzenich, M. M. (2004) Neuroscience 129, 141-156. [DOI] [PubMed] [Google Scholar]
- 19.Bao, S., Chang, E. F., Davis, J. D., Gobeske, K. T. & Merzenich, M. M. (2003) J. Neurosci. 23, 10765-10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recanzone, G. H., Schreiner, C. E. & Merzenich, M. M. (1993) J. Neurosci. 13, 87-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levi, H. & Sohmer, H. (1995) J. Basic Clin. Physiol. Pharmacol. 6, 129-138. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson, J. T. (1985) An Overview of the Auditory Brainstem Response (College-Hill, San Diego).
- 23.Sohmer, H. & Freeman, S. (1991) Hear. Res. 55, 92-97. [DOI] [PubMed] [Google Scholar]
- 24.Makishima, K., Katz, R. B. & Snow, J. B., Jr. (1976) Ann. Otolaryngol. Rhinol. Laryngol. 85, 826-832. [DOI] [PubMed] [Google Scholar]
- 25.Tomimatsu, T., Fukuda, H., Endoh, M., Mu, J., Watanabe, N., Kohzuki, M., Fujii, E., Kanzaki, T., Oshima, K., Doi, K., et al. (2002) Brain Res. 926, 108-117. [DOI] [PubMed] [Google Scholar]
- 26.Cycowicz, Y., Schmuel, M., Freeman, S., Wanszelbaum, A. & Sohmer, H. (1988) Hear. Res. 33, 239-244. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, Z. D., Wang, J., Brosi, D. M., Shao, X. M. & Wilkinson, A. R. (2004) Acta Paediatr. 93, 82-87. [PubMed] [Google Scholar]
- 28.Kileny, P., Connelly, C. & Robertson, C. (1980) Int. J. Pediatr. Otorhinolaryngol. 2, 147-159. [DOI] [PubMed] [Google Scholar]
- 29.Bayer, S. A., Altman, J., Russo, R. J. & Zhang, X. (1993) Neurotoxicology 14, 83-144. [PubMed] [Google Scholar]
- 30.Funai, H. & Funasaka, S. (1983) Audiology 22, 9-19. [DOI] [PubMed] [Google Scholar]
- 31.Benstead, T. J., Dyck, P. J. & Low, P. (1988) J. Neuropathol. Exp. Neurol. 47, 599-608. [DOI] [PubMed] [Google Scholar]
- 32.Atis, S., Ozge, A. & Sevim, S. (2001) Respirology 6, 225-229. [DOI] [PubMed] [Google Scholar]
- 33.Mazurek, B., Winter, E., Fuchs, J., Haupt, H. & Gross, J. (2003) Hear. Res. 182, 2-8. [DOI] [PubMed] [Google Scholar]
- 34.Harrison, R. V., Ibrahim, D. & Mount, R. J. (1998) Exp. Brain Res. 123, 449-460. [DOI] [PubMed] [Google Scholar]
- 35.Harrison, R. V., Nagasawa, A., Smith, D. W., Stanton, S. & Mount, R. J. (1991) Hear. Res. 54, 11-19. [DOI] [PubMed] [Google Scholar]
- 36.Sokoloff, L. (1981) J. Cereb. Blood Flow Metab. 1, 7-36. [DOI] [PubMed] [Google Scholar]
- 37.Altman, J. & Bayer, S. A. (1981) Exp. Brain Res. 42, 411-423. [DOI] [PubMed] [Google Scholar]
- 38.Nwabueze-Ogbo, F. C., Popelar, J. & Syka, J. (2002) Physiol. Res. 51, Suppl. 1, S95-S104. [PubMed] [Google Scholar]
- 39.Chen, G. D. (2002) Hear. Res. 172, 186-195. [DOI] [PubMed] [Google Scholar]
- 40.Decker, M. J., Hue, G. E., Caudle, W. M., Miller, G. W., Keating, G. L. & Rye, D. B. (2003) Neuroscience 117, 417-425. [DOI] [PubMed] [Google Scholar]
- 41.Sechzer, J. A., Faro, M. D., Barker, J. N., Barsky, D., Gutierrez, S. & Windle, W. F. (1971) Science 171, 1173-1175. [DOI] [PubMed] [Google Scholar]
- 42.Nagarajan, S., Mahncke, H., Salz, T., Tallal, P., Roberts, T. & Merzenich, M. M. (1999) Proc. Natl. Acad. Sci. USA 96, 6483-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenhall, U., Nordin, V., Brantberg, K. & Gillberg, C. (2003) Ear Hear. 24, 206-214. [DOI] [PubMed] [Google Scholar]
- 44.McPartland, J., Dawson, G., Webb, S. J., Panagiotides, H. & Carver, L. J. (2004) J. Child. Psychol. Psychiatry 45, 1235-1245. [DOI] [PubMed] [Google Scholar]