Abstract
Intracranial self-stimulation (ICS) is a motivated behavior that results from contingent activation of the brain reward system. ICS with stimulating electrodes placed in the medial forebrain bundle (MFB) is particularly robust. However, the neurons that course through this pathway use a variety of neurotransmitters including dopamine and GABA. For this reason, the neurotransmitters that are central to this behavior, and the specific roles that they subserve, remain unclear. Here, we used extracellular electrophysiology and cyclic voltammetry at the same electrode in awake rats to simultaneously examine cell firing and dopamine release in the nucleus accumbens (NAc) during ICS and noncontingent stimulation of the MFB. ICS elicited dopamine release in the NAc and produced coincident time-locked changes (predominantly inhibitions) in the activity of a subset of NAc neurons. Similar responses were elicited with noncontingent stimulations. The changes in firing rate induced by noncontingent stimulations were reversed by the GABAA receptor antagonist bicuculline. Most time-locked unit activity was unaffected by D1 or D2-like dopamine-receptor antagonists, or by inhibition of evoked dopamine release, although, for a minority of units, the D1 dopamine-receptor antagonist SCH23390 attenuated neural activity. Thus, neurons in the NAc are preferentially inhibited by GABAA receptors after MFB stimulation, a mechanism that may also be important in ICS.
Keywords: cyclic voltammetry, electrophysiology, nucleus accumbens, motivated behavior, behaving rat
The nucleus accumbens (NAc) is an important neural component of the brain reward system (1-4). It integrates information related to goal-directed behaviors and contributes to behavioral selection in response to predictive cues, sequencing of behaviors, and learning (5-7). Single-unit electrophysiological recordings have shown that information is rapidly processed by NAc neurons regardless of reinforcer type (8, 9). A particularly interesting reinforcer is intracranial self-stimulation (ICS) in which the neural substrates mediating reward are directly activated (10). In this paradigm, animals lever press to deliver electrical stimulation to select brain regions. Like natural rewards, ICS causes time-locked changes in unit activity in the NAc (11, 12).
The medial forebrain bundle (MFB), a major axonal tract for both ascending and descending projections, is a site that supports robust ICS. One set of axons that occupy the MFB are the ascending neurons of the mesolimbic dopamine system that project to the NAc from their origin in the ventral tegmental area (VTA) (13, 14). Because electrical stimulation with parameters that are used in ICS causes antidromic firing of dopaminergic neurons (15) and dopamine-receptor antagonists applied to the NAc inhibit ICS reinforcement (16), dopamine neurotransmission in the NAc is considered important in reward processing (17). Modern views of reward-related behavior ascribe an alerting or learning role to dopamine, rather than direct mediation of hedonia (2, 14, 18, 19). However, several other neuronal systems project through the MFB and are activated by its stimulation. For example, a descending, myelinated neuronal pathway has been identified as central to maintenance of ICS (20). A GABAergic pathway, comprising 36% of VTA projection neurons (21), ascends to the NAc (22) and can also be activated in anticipation of a reinforced response for ICS (23). Because of these complexities, the signaling roles of GABA, dopamine, and other neurotransmitters during ICS remain unclear.
To address this issue, we have combined electrophysiology and fast-scan cyclic voltammetry at the same carbon-fiber electrode in awake, unrestrained rats to allow simultaneous monitoring of neuronal activity and dopamine release at the same location within the NAc. The combined approach, pioneered by Millar and coworkers (24), has only been used in anesthetized animals. During ICS, we found time-locked responses of NAc single units, and these were accompanied by dopamine release. Similar responses were found with noncontingent delivery of the same stimulation. Because systemic delivery of dopamine antagonists interferes with ICS (25), pharmacological investigations of the neurotransmitters responsible for the electrophysiological changes were done with experimenter-delivered stimulations. The results reveal that the unit responses are preferentially responsive to GABA, not dopamine release.
Materials and Methods
Animals and Surgery. Male Sprague-Dawley rats (n = 28), implanted with jugular vein catheters (Charles River Breeding Laboratories) and weighing between 300 and 350 g, were used. Rats, housed individually, had ad libitum access to food and water with lighting provided on a 0700-1900 h cycle. Surgeries were performed as described (26). A guide cannula (Bioanalytical Systems, West Lafayette, IN) was positioned above the NAc core (+1.3 mm anteriorposterior, +1.3 mm mediolateral, relative to bregma). An Ag/AgCl reference electrode was placed in the contralateral hemisphere. A detachable microdrive containing a cylindrical carbon-fiber microelectrode (50- to 100-μm length of exposed T-650 fiber, Amoco, Greenville, SC) and the electrode was lowered into the NAc core. A bipolar stimulating electrode placed above the MFB (-4.6 mm anteriorposterior, +1.4 mm mediolateral, and -7.7 mm dorsoventral) was lowered in 0.2-mm increments until electrically evoked dopamine release was detected (between -8.0 and -8.8 mm dorsoventral). The components were permanently affixed with dental cement. The carbon-fiber electrode was removed and replaced with a stylet.
ICS. Experimental sessions began 3 days after surgery in a behavioral chamber (Med Associates, St Albans, VT) that contained a retractable lever with a cue light above it. For ICS, the animal depressed the lever on a FR1 schedule, and each response delivered a stimulation train to the MFB (24 biphasic pulses, 60 Hz, 125 μA, 2 ms per phase). The stimulus train was delivered 300 ms after a lever press. Training began with a 1-s time out (TO1″) in which the lever was retracted after each press. During the time-out, the cue light was off, the house light was on, and a 67-dB (1-kHz) tone was presented. During training, the time out was increased to 10 s (FR1, TO10″). Criterion responding was achieved when animals had 30 stimulations per 5-min session, with between one and five sessions per animal. For noncontingent stimulation sessions, rats were given one stimulation every 60 s for 10 min either with the ICS stimulation train or with a shorter, lower-frequency train (six biphasic pulses, 30 Hz, 125 μA, 2 ms per phase).
Combined Electrophysiology/Electrochemistry. A carbon-fiber electrode was lowered into the NAc core, and the carbon-fiber and Ag/AgCl electrodes were connected to head-mounted operational amplifiers. Signals were routed through an electrical swivel (Med-Associates), which allowed free movement of the animal, to custom-built amplifiers (Chemistry Department Electronics Facility, University of North Carolina, Chapel Hill). Cyclic voltammograms were generated at 5 Hz by using a triangular waveform (-0.6 to + 1.4 V, 400 V/s) with custom-written labview software (National Instruments, Austin, TX). The electrode was conditioned with the waveform applied at 60 Hz for 15 min. A solid-state relay in the headstage alternated between a current amplifier for voltammetric scans and a voltage follower for unit recording. The unit recordings had a ≈20 ms gap every 180 ms when the voltammograms were collected (Fig. 5, which is published as supporting information on the PNAS web site). Both signals were referenced to the Ag/AgCl electrode that was connected to ground. Recordings were made at sites where single units were isolated and dopamine release could be detected. After collection of data at one site, the electrode was lowered ≈300 μm until another unit and release site were found.
Units recorded from carbon-fiber electrodes (impedance at the tip: ≈500 kΩ at 1 KHz) were amplified (×1,000) and bandpass-filtered (300-3,000 Hz). They were digitized with commercially available software (digitizer, Plexon, Dallas). Discrimination of waveforms was accomplished by using principal component analysis in offline sorter (Plexon). Typically, one to two neurons could be discriminated at each location. Custom-written software (labview, National Instruments) recorded behavioral time stamps in the electrochemical record, whereas electrophysiological and behavioral time stamps were combined for offline analyses by using neuroexplorer (Plexon).
Data Analysis. Dopamine was identified from the cyclic voltammograms that were background subtracted from 10 cyclic voltammograms recorded before the stimulation. They were displayed with time as the abscissa, voltage as the ordinate, and the background subtracted current encoded in false color. Dopamine changes were extracted from the current at the peak for oxidation of dopamine (approximately +0.6 V vs. Ag/AgCl) once pH-corrected (27).
Neural activity was characterized with raster displays and perievent histograms (PEHs) across distinct time domains that bracketed the electrical stimulation. Each PEH was partitioned into three epochs [baseline (-8 to 0 s before the stimulation), response (0 to 4 s after stimulation), and recovery (+4 to +8 s after the stimulation)] to allow measurement of changes relative to the stimulation. Statistical differences in unit activity and signal-to-baseline ratios across epochs were evaluated as described in Supporting Text, which is published as supporting information on the PNAS web site.
Results
Neuronal Firing Patterns and Dopamine Release Dynamics in the NAc During ICS. Rats (n = 11) exhibited stable ICS on the FR1, TO10″ schedule (Fig. 1A Top). Across all animals, the mean latency to respond after lever extension was 1.1 ± 0.2 s. Simultaneous electrochemical/electrophysiological measurements were made during ICS when the animals had reached this level of responding.
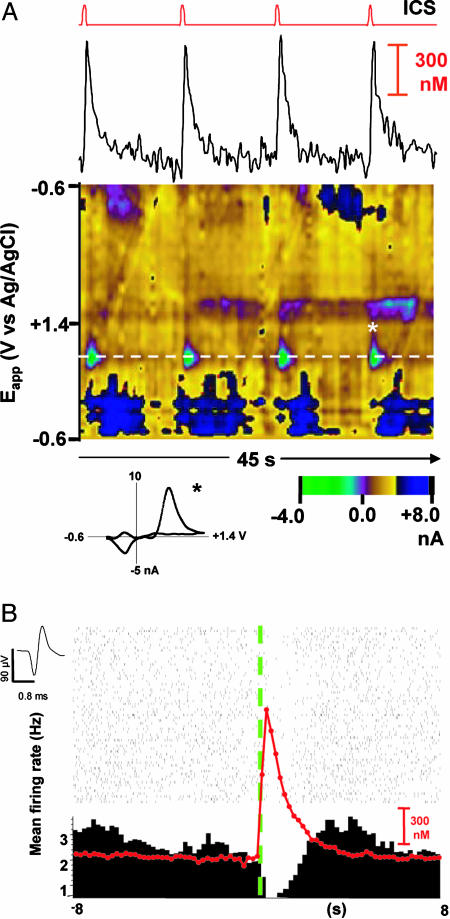
Fig. 1.
Responses measured in the NAc during ICS. (A) Dopamine release in the NAc evoked by ICS (24 pulses, 60 Hz, 125 μA) of the MFB using an FR1, TO10″ schedule. The top line indicates the time of each reinforced response. Each lever press caused a stimulation followed by a retraction of the lever for 10 s. The dopamine temporal response, corrected for pH changes, is shown below. The color representation shows all of the voltammetric currents; the white dashed line is at the potential for oxidation of dopamine. The green feature centered on the dashed line is due to the release of dopamine. The blue features are due to a basic pH shift after dopamine release. A cyclic voltammogram obtained at the peak of the last response (asterisk) is shown below the color plot. (B) Representative perievent raster and PEH centered on the ICS lever press (green dashed line). Each tick on the raster plot represents an extracellularly recorded action potential (average waveform shown to the top left). The red line is the evoked dopamine signal averaged from the 63 trials shown. Bin width was 200 ms for both measurements.
The color representation of a set of background-subtracted cyclic voltammograms recorded at one site is shown in Fig. 1A Bottom. The oxidation of dopamine is apparent by the green features at the potential where it is oxidized (dashed line). These are seen after each time out and subsequent lever press. The shape of the individual cyclic voltammograms further confirms dopamine detection; the one after the fourth lever press (asterisk) is shown below the color plot. In addition, longer lasting changes (blue features) accompany the dopamine release that are due to changes in extracellular pH (27). Removal of the pH component provides the dopamine concentration evoked by the stimulation (≈700 nM in this example, Fig. 1A). Thus, unlike ICS with a continuous reinforcement schedule that resulted in suppression of dopamine release during the session (28), on the FR1 TO10″ schedule, each lever press and its accompanying stimulus train evoked robust dopamine release that was stable across the behavioral session.
The PEH and associated raster plot centered on the beginning of the electrical stimulation (green dashed line) shown in Fig. 1B displays the activity of the unit (waveform to the left) that was simultaneously recorded at the site where the voltammograms shown in Fig. 1A were collected. This unit was consistently inhibited for the 63 trials shown, and the overlaid trace (red) shows the average of all of the dopamine signals.
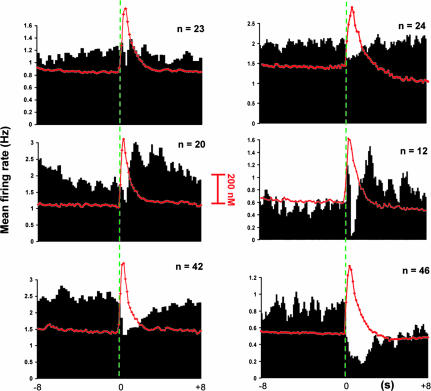
Of the 85 NAc neurons recorded during ICS, three categories of neuronal firing patterns were identified that were time-locked to the stimulation resulting from the lever press (Fig. 2 Left). U type(n = 23) were unaffected (Fig. 2, Top Left) with similar firing during the baseline, response, and recovery epochs (Table 1; F2,68 = 0.16; P > 0.05). I/E cells (n = 20, Fig. 2 Middle Left) showed a mixed response with an inhibition followed by excitatory components. I/E-type cells had a mean baseline firing rate that differed significantly from that measured at the peak but not from that measured in the recovery epoch (Table 1; F2,59 = 2.50; P ≤ 0.05). I-type (n = 42), the most prevalent, were inhibited in the response epoch (Fig. 2 Bottom Left). Onset of recovery from inhibition was 4.7 ± 1.2 s (mean ± SEM). The amplitude of dopamine release did not differ significantly (F2,297 = 1.88; P ≤ 0.15) at the different cell categories.
Fig. 2.
Responses measured in the NAc during ICS and noncontingent stimulations. (Left) Neural activity ± 8s around the ICS lever press (green dashed line at time 0; 24 pulses, 60 Hz, 125 μA). (Right) Neural activity displayed in the same way obtained during noncontingent stimulations. Data are shown as composite PEHs. Histograms were made by summing data from each unit over individual trials, and sorting according to responses obtained [Top, unaffected (U-type); Middle, inhibited/excited (I/E-type); Bottom, inhibited (I-type)]. The overlaid trace (red) shows the time course of average extracellular dopamine concentration changes measured at the same loci where neurons were recorded after the stimulation. Bin width was 200 ms for all histograms.
Table 1. Neuronal firing patterns time-locked to ICS and noncontingent MFB stimulation.
| Neuron population
|
||||||
|---|---|---|---|---|---|---|
| Stimulation | U-type | I-type | E-type | I/E-type | ||
| ICS (n) | 24p 60 Hz (23) | 24p 60 Hz (42) | 24p 60 Hz (0) | 24p 60 Hz (20) | ||
| Baseline | 1.12 ± 0.3 | 2.48 ± 0.3 | – | 1.91 ± 0.5 | ||
| Response | 1.28 ± 0.4 | 1.77 ± 0.2 | – | 0.62 ± 0.3, 2.77 ± 0.7* | ||
| Recovery | 1.05 ± 0.5 | 2.32 ± 0.3 | – | 2.12 ± 0.6 | ||
| Noncontingent (n) | 6p 30 Hz (26) | 24p 60 Hz (24) | 6p 30 Hz (40) | 24p 60 Hz (46) | 6p 30 Hz (14) | 24p 60 Hz (12) |
| Baseline | 1.26 ± 0.2 | 1.99 ± 0.8 | 0.99 ± 0.1 | 0.84 ± 0.1 | 2.29 ± 0.5 | 0.54 ± 0.2 |
| Response | 1.25 ± 0.5 | 1.88 ± 0.9 | 0.28 ± 0.1 | 0.36 ± 0.1 | 3.52 ± 0.3 | 0.29 ± 0.1, 0.86 ± 0.2* |
| Recovery | 1.38 ± 0.3 | 2.04 ± 0.8 | 0.57 ± 0.2 | 0.52 ± 0.3 | 2.97 ± 0.5 | 0.81 ± 0.2 |
Response components are separated into the short inhibition (upper) and the delayed excitation (lower)
Neuronal Firing Patterns and Dopamine Release in the NAc During Noncontingent MFB Stimulation. Dopamine release and single-unit activity were also simultaneously monitored in the NAc during noncontingent (response independent) stimulations (n = 13 animals) using the ICS stimulation train. The composite PEHs are remarkably similar to those obtained during ICS (Fig. 2 Right). The majority of NAc cells (n = 46) were I-type (Fig. 2 Bottom Right, Table 1; F2,125 = 2.19; P ≤ 0.05), whereas 24 NAc neurons were U type (Fig. 3, Table 1; F2,71 = 0.14; P > 0.05). The average recovery time for cell firing of I-type neurons was 5.1 ± 0.6 s. I/E-type (n = 12) were also observed (Fig. 2 Middle Right; Table 1; F2,35 = 4.6; P ≤ 0. 05). The early excitatory component seen in the composite PEH is due to antidromic activation of the axons (latency 8.33 ± 0.01 ms). Similar activation has been observed during ICS (11, 29). The maximal dopamine concentration (530 ± 47 nM) did not correlate with the different categories of cells (U, I, E, or I/E) simultaneously recorded (F2,243 = 1.6, P > 0.05).
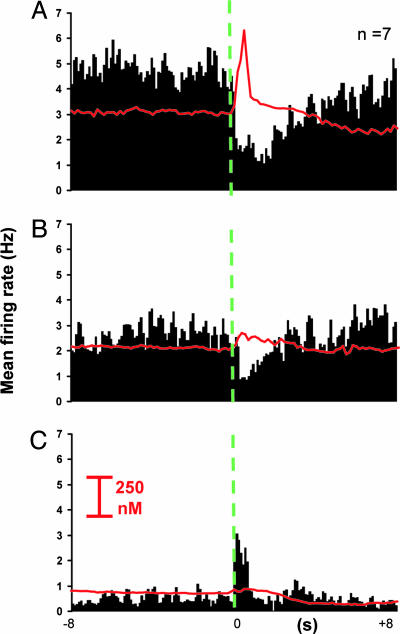
Fig. 3.
Inhibitory responses are independent of vesicular dopamine release but modulated by GABAA receptors. (A) PEH obtained during noncontingent stimulation (green dashed line at time 0; 24 pulses, 60 Hz, 125 μA) with average dopamine response (red line) superimposed. (B) Inhibition of action potential-driven dopamine release with RO (1 mg·kg-1) decreases background firing rates, but does not eliminate the inhibition of firing caused by the stimulation. (C) Blockade of GABAA receptors with BIC (200 μg·kg-1) after RO uncovers a time-locked excitation, whereas background firing is further decreased and dopamine release remains undetectable (Bottom). Bin width was 200 ms, all drugs were delivered intravenously.
Pharmacological Effects on NAc Neuronal Inhibitions Time-Locked to Noncontingent MFB Electrical Stimulation. Dopaminergic effects on noncontingent, I-type responses were probed by administration of RO4-1284 (RO; 1 mg·kg-1, i.v.; n = 7), an inhibitor of vesicular monoamine transporter 2, the transporter for catecholamine (30), indoleamine, and histaminergic vesicles. I-type units were selected because they are the predominant phasic cells and are devoid of antidromicity confounds. RO reduced stimulated dopamine release from 243 ± 91 nM to undetectable levels in ≈120 s (Fig. 3). However, the stimulation-induced inhibition of firing persisted (Fig. 3A, pre-RO firing rate at trough: 2.11 ± 0.5 Hz vs. post-RO, Fig. 3B: 1.87 ± 0.7; t = -3.1; P > 0.05), although baseline firing rate significantly decreased (pre-RO baseline firing rate: 4.8 ± 0.7 Hz vs. post-RO: 2.67 ± 0.5 Hz, t = 10.1; P ≤ 0.01). This inhibition in baseline activity was accompanied by a significant decrease in the signal-to-baseline ratio of the inhibitory response after RO (0.74 ± 0.1 to 0.41 ± 0.2; t = -2.6; P ≤ 0.05).
To investigate GABA responses, after RO administration, bicuculline (BIC, a GABAA receptor antagonist) was administered at a nonepileptogenic dose (200 μg·kg-1, i.v.). Surprisingly, this combination further decreased baseline firing rate to 0.54 ± 0.1 Hz (t = 13.4; P ≤ 0.0001, Fig. 3C). Presumably, this paradoxical response is due to the combined presence of BIC and RO that suppresses all monoamine release. More importantly, I-type responses modified their patterned discharge to a poststimulation excitation (E-type response; change from 0.54 ± 0.1 Hz at baseline to 1.17 ± 0.1 Hz at the peak; t = -1.4; P ≤ 0. 001), whereas evoked dopamine changes remained undetectable. There was no evidence for antidromic activation because latencies varied from one pulse to the next (98 ± 16 ms; range 38-125 ms).
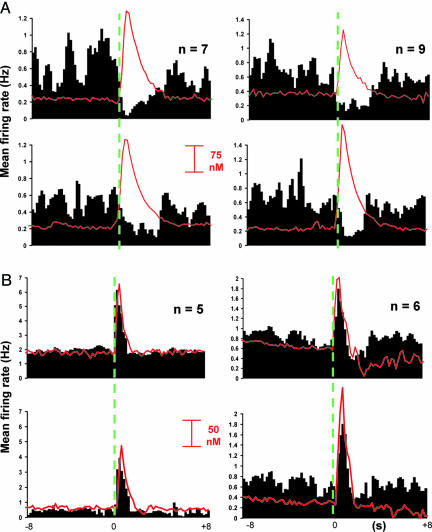
Effect of Dopamine Receptor Antagonists on NAc Neuronal Responses Time-Locked to Short Noncontingent MFB Electrical Stimulation. To further probe dopamine effects, “short” stimulations (six biphasic pulses, 30 Hz) were used. Although these can support ICS (31), they evoke less dopamine release (124 ± 21 nM; significantly lower than with the long stimulation, t = 7.5; P ≤ 0.01), and, therefore, unit responses mediated by dopamine should be more sensitive to antagonists. With this stimulation, both U-type (n = 26) and I-type (n = 40) cells were found (see Table 1 and Fig. 6, which is published as supporting information on the PNAS web site). Again, the firing rate of U-type cells was not modified by the stimulation (F2,77 = 0.07; P > 0.05). I-type neurons displayed a significant inhibition relative to stimulation onset (Fig. 4A, Table 1; F2,125 = 5.13; P ≤ 0.05). I/E responses were not observed with the short stimulation train; however, some neurons (n = 14) showed E-type responses (Table 1 and Fig. 6; F2,41 = 5.5; P ≤ 0.05) lasting 3.4 ± 0.4 s. Peak firing rate for E-type cells was significantly higher than the rebound excitation elicited by I/E cells (Table 1; t = -7.1; P ≤ 0.05). However, many E-type units (64.2%) were antidromically driven (11, 29) by the short electrical stimulation as evidenced by their short and invariant latency to fire (16.7 ± 0.04 ms) after each stimulus pulse (Fig. 6). Again, the dopamine concentration was uncorrelated with the different categories of simultaneously recorded cells (F2,237 = 3.6, P > 0.05).
Fig. 4.
Composite PEHs showing the effects of dopamine receptor antagonists on inhibitory and excitatory responses time-locked to electrical stimulation of the MFB. Stimulation initiated at time indicated by the green dashed line. (A) Time-locked inhibitions of I-type neurons are unchanged between before (Upper) and after (Lower) administration of SCH23390 (SCH, 40 μg·kg-1; Left) and raclopride (Raclo, 80 μg·kg-1, Right). Dopamine release is also unchanged after SCH, but Raclo increases electrically evoked release. (B) The overall response of E-type cells was diminished after SCH treatment, whereas dopamine release was unaltered (Left). Although the time-locked excitation was unchanged by Raclo treatment (Right), dopamine release was again increased (Right). Bin width was 200 ms for all PEHs, all drugs were given intravenously.
Antagonists were administered systemically to further examine dopamine effects on cell firing, and recordings were begun 90 s after they were given. D1-like dopamine receptor blockade with SCH23390 (SCH, 40 μg·kg-1, i.v.; ref. 32) significantly enhanced the signal-to-baseline ratio for E-type responses (1.24 ± 0.2 to 1.97 ± 0.3; t = 2.1; P ≤ 0.05) but not for I-type responses. In contrast, the D2-like dopamine-receptor antagonist raclopride (RACLO; 80 μg·kg-1, i.v.; ref. 32) did not significantly alter signal-to-baseline ratios (all P values > 0.05). SCH administration did not alter I-type (P > 0.05) but attenuated E-type (P ≤ 0.05) firing patterns (Fig. 4A Left). RACLO did not modify the time-locked response of I-type or E-type (both P > 0.05) cells (Fig. 4B). It was evident that RACLO was reaching its molecular target by 90 s after injection because it significantly elevated evoked maximal dopamine recorded near I-type and E-type cells (from 137 ± 18 nM to 219 ± 37 nM; t =-1.6; P ≤ 0.05 and from 108 ± 11 nM to 205 ± 32 nM; t = -3.6; P ≤ 0.01, respectively, Fig. 4 A and B Right), consistent with its known actions on dopamine release (33).
Discussion
Here, we adapted technology developed by Millar and coworkers (24) to obtain the first simultaneous, subsecond measurements of both dopamine release and neuronal firing in awake, behaving animals. This approach affords unparalleled insight into both chemical and electrical signaling during behavior. Using this technique, we examined the regulation of NAc neuronal activity and concurrent dopamine release during ICS, a potent reinforcer. Although dopamine release is time-locked to the reinforcing electrical stimulation, its predominant effect appears to be regulation of baseline firing. However, the principal finding is that time-locked inhibitions, the predominant cellular response, are mediated by GABA.
Most investigators concur that dopamine neurotransmission in the NAc plays an essential, but as yet, undefined role in ICS. Evidence for dopamine's importance in ICS includes the reduced responding in rats with lesions of dopaminergic neurons (34), the blockade of ICS by NAc microinjections of a dopaminergic antagonist (16), and the impaired ICS in D1 knockout mice (12). However, measurements of dopamine release during ICS indicate that it is not a necessary condition for ICS (35). Indeed, with a continuous ICS schedule, dopamine release is suppressed (28, 36, 37). Although direct activation of dopaminergic neurons is unnecessary for ICS (20), we purposely used stimulation parameters that orthodromically activate dopaminergic neurons (15). These parameters, used in conjunction with the FR1, TO10″ schedule, support stable dopamine release during ICS and complement those of Yavich and Tiihonen (37), who showed that stable dopamine release can occur during ICS on an FR8 schedule.
Robust changes in cell firing of many NAc units were observed with ICS, noncontingent stimulations with the same waveform, and short stimulations. In all three cases, the changes in firing rate were time-locked to the stimulation train and concurrent with dopamine release. The firing patterns of NAc neurons during ICS reported here are similar to those reported in mice during ICS (12, 29). Similarly, dopamine release evoked by ICS was indistinguishable from the responses observed during non-contingent stimulations. However, after elimination of dopamine release by using an inhibitor of the vesicular monoamine transporter (Fig. 3), the inhibitory response was still present. Similarly, I-type cells were unaltered by D1- or D2-like dopamine-receptor antagonists. The D1-like dopamine-receptor antagonist SCH23390 modified baseline firing of E-type cells, a relatively small subset of all time-locked cells, in a manner consistent with previous reports (38, 39), and also altered their time-locked activity. Thus, the results during noncontingent stimulations suggest a limited role for evoked dopamine on the observed unit activity.
With noncontingent stimulations similar to the ICS train that we used, Williams and Millar (40) found that striatal units were excited upon dopamine release, and the elevated firing continued for tens of seconds after the stimulation. These responses were suppressed after dopamine depletion by synthesis inhibition. Excitation, mediated by D1 receptors, was also observed by Gonon within 200 ms of the stimulation in a subpopulation of striatal neurons (41). In contrast to these prior reports in anesthetized animals, the predominant response seen here in the NAc of awake rats was an inhibition. Thus, these contradictory findings may be due to anesthesia where different striatal populations may be sampled (42, 43).
Unlike classical neurotransmitters whose receptors control ion channels, dopamine receptors are G protein coupled and operate through second messengers. Dopamine modulates the actions of other neurotransmitters (44) through such interactions. The limited dopamine-mediated responses observed here may arise from the slow onset of G protein receptor responses. Indeed, in the prefrontal cortex, the actions of dopamine after VTA stimulation occur over minutes (45). Similarly, D1 receptor-mediated inactivation of sodium currents is slow both in onset (46) and time to reach a steady-state (≈15 min, ref. 47). However, presynaptic D2 receptor-mediated autoinhibition of pulsatile dopamine release is much faster, requiring only a few hundred ms for full expression with a duration of seconds or less (48-50). Indeed, the existence of rapid dopamine transients intuitively suggests a role for rapid actions of dopamine on the efficacy of fast synaptic transmission during behavior (51).
After inhibition of dopamine release, bicuculline reversed the time-locked inhibitory responses evoked by noncontingent stimulations. GABAergic neurons from the VTA project to the NAc along with the ascending dopamine systems (21). Thus, their activation by the noncontingent stimulation or during ICS could lead to inhibitions similar to those found in the prefrontal cortex and NAc during electrical stimulation of the VTA (12, 29, 52). Other GABAergic afferents such as local neurons may also contribute to the observed responses. Thus, our results support the view that GABA may play an underappreciated role in ICS (23). Recently, it has been proposed that glutamate is coreleased with dopamine (45, 53). This mechanism could lead to the time-locked excitation seen after inhibition of dopamine release with RO and GABAA receptor blockade with BIC (Fig. 3C). The observed E-type responses (Fig. 6) may be the critical first-stage neurons for ICS (20). However, we did not pharmacologically investigate their GABAergic properties.
The use of a single electrode to monitor simultaneously both a neurotransmitter and single-unit activity provides unique information about neurotransmission on a behaviorally relevant time scale. The measured events are both obtained from a volume of tissue that has dimensions of a few micrometers. Dopamine, an extrasynaptic messenger, diffuses from its release site to target receptors (or the electrode), but the DAT constrains its spread to the local area (54). Similarly, unit responses are restricted to <40 μm from the cell body by the limited spread of their electric field (40). With this technique, we have revealed that dopamine is unequivocally released during ICS with the 10-s time out, but we suggest that the predominant time-locked responses to stimulations that support ICS in the NAc are GABAA receptor-mediated.
Nevertheless, subtle changes in cell firing result from dopamine's postsynaptic actions on NAc neurons. For example, reductions in baseline activity were observed after both RO (Fig. 3B) and D1-like dopamine-receptor antagonist pretreatment (Fig. 4). Thus, rapid dopamine release events may contribute to steady-state (tonic) dopamine that influences baseline activity of NAc neurons. Furthermore, although pretreatment with RO did not abolish the inhibitory response of NAc cells to MFB stimulation, the duration of the inhibition was attenuated (Fig. 3B). This observation indicates that dopamine may be involved in modulating the phasic activation of NAc neurons to MFB stimulation, although GABA release clearly predominates in this process. Although not directly tested here, rapid dopamine release may modulate changes in unit activity in the NAc only when other circuit components are engaged (i.e., as is the case during ICS). Indeed, fast intracellular responses mediated by dopamine in the prefrontal cortex or NAc may not directly increase cell firing but may make neurons more susceptible to afferent activation (55). Exactly this role has been ascribed to dopamine during the learning of ICS (25). Overall, these findings bolster the view that GABA release occurs during ICS-like stimulations, and suggests that ICS is a behavior involving extensive neuronal circuitry, not solely involving dopamine.
Supplementary Material
Acknowledgments
We thank John Peterson and Colin McKinney from the University of North Carolina Department of Chemistry Electronics Facility for instrumentation, Kate Wassum and Minar Kim for technical assistance, and Drs. Paul Phillips and Garret Stuber for discussions. This work was supported by U.S. Army Medical Research and Materiel Command Grant 03281055 (to P.A.G.) and National Institute of Drug Abuse Grants 10900 (to R.M.W. and R.M.C.) and 017318 (to R.M.C. and R.M.W.).
Author contributions: J.F.C., M.L.A.V.H., R.M.C., and R.M.W. designed research; J.F.C. and M.L.A.V.H. performed research; J.F.C., M.L.A.V.H., and R.M.C. analyzed data; and J.F.C., P.A.G., R.M.C., and R.M.W. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: NAc, nucleus accumbens; ICS, intracranial self-stimulation; VTA, ventral tegemental area; MFB, medial forebrain bundle; PEH, perivent histogram; BIC, bicuculline.
References
- 1.Berridge, K. C. & Robinson, T. E. (1998) Brain Res. Rev. 28, 309-369. [DOI] [PubMed] [Google Scholar]
- 2.Schultz, W., Dayan, P. & Montague, P. R. (1997) Science 275, 1593-1599. [DOI] [PubMed] [Google Scholar]
- 3.Self, D. W. & Nestler, E. J. (1998) Drug Alcohol Depend. 51, 49-60. [DOI] [PubMed] [Google Scholar]
- 4.Bassareo, V. & Di Chiara, G. (1999) Neuroscience 89, 637-641. [DOI] [PubMed] [Google Scholar]
- 5.Mogenson, G. J., Jones, D. L. & Yim, C. Y. (1980) Prog. Neurobiol. 14, 69-97. [DOI] [PubMed] [Google Scholar]
- 6.Koob, G. F. & Bloom, F. E. (1988) Science 242, 715-723. [DOI] [PubMed] [Google Scholar]
- 7.Robbins, T. W. & Everitt, B. J. (1996) Curr. Opin. Neurobiol. 6, 228-236. [DOI] [PubMed] [Google Scholar]
- 8.Carelli, R. M. & Wightman, R. M. (2004) Curr. Opin. Neurobiol. 14, 763-768. [DOI] [PubMed] [Google Scholar]
- 9.Deadwyler, S. A., Hayashizaki, S., Cheer, J. F. & Hampson, R. E. (2004) Neurosci. Biobehav. Rev. 27, 703-711. [DOI] [PubMed] [Google Scholar]
- 10.Bielajew, C. & Shizgal, P. (1986) J. Neurosci. 6, 919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolske, M., Rompre, P. P., Wise, R. A. & West, M. O. (1993) J. Neurosci. 13, 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran, A. H., Tamura, R., Uwano, T., Kobayashi, T., Katsuki, M. & Ono, T. (2005) Proc. Natl. Acad. Sci. USA 102, 2117-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redgrave, P. & Mitchell, I. (1982) Neuroscience 7, 885-894. [DOI] [PubMed] [Google Scholar]
- 14.Ikemoto, S. & Panksepp, J. (1999) Brain Res. Rev. 31, 6-41. [DOI] [PubMed] [Google Scholar]
- 15.Kuhr, W. G., Wightman, R. M. & Rebec, G. V. (1987) Brain Res. 419, 122-128. [DOI] [PubMed] [Google Scholar]
- 16.Stellar, J. R. & Corbett, D. (1989) Brain Res. 477, 126-143. [DOI] [PubMed] [Google Scholar]
- 17.Wise, R. A. (1996) Annu. Rev. Neurosci. 19, 319-340. [DOI] [PubMed] [Google Scholar]
- 18.Hyland, B. I., Reynolds, J. N., Hay, J., Perk, C. G. & Miller, R. (2002) Neuroscience 114, 475-492. [DOI] [PubMed] [Google Scholar]
- 19.Comoli, E., Coizet, V., Boyes, J., Bolam, J. P., Canteras, N. S., Quirk, R. H., Overton, P. G. & Redgrave, P. (2003) Nat. Neurosci. 6, 974-980. [DOI] [PubMed] [Google Scholar]
- 20.Gallistel, C. R., Shizgal, P. & Yeomans, J. S. (1981) Psychol. Rev. 88, 228-273. [PubMed] [Google Scholar]
- 21.Van Bockstaele, E. J. & Pickel, V. M. (1995) Brain Res. 682, 215-221. [DOI] [PubMed] [Google Scholar]
- 22.Steffensen, S. C., Svingos, A. L., Pickel, V. M. & Henriksen, S. J. (1998) J. Neurosci. 18, 8003-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steffensen, S. C., Lee, R., Stobbs, S. H. & Henriksen, S. J. (2001) Brain Res. 906, 190-197. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong-James, M., Millar, J. & Kruk, Z. L. (1980) Nature 288, 181-183. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima, S. & O'Regan, N. B. (1991) Pharmacol. Biochem. Behav. 39, 465-468. [DOI] [PubMed] [Google Scholar]
- 26.Cheer, J. F., Wassum, K. M., Heien, M. L., Phillips, P. E. & Wightman, R. M. (2004) J. Neurosci. 24, 4393-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venton, B. J., Michael, D. J. & Wightman, R. M. (2003) J. Neurochem. 84, 373-381. [DOI] [PubMed] [Google Scholar]
- 28.Garris, P. A., Kilpatrick, M., Bunin, M. A., Michael, D., Walker, Q. D. & Wightman, R. M. (1999) Nature 398, 67-69. [DOI] [PubMed] [Google Scholar]
- 29.Tran, A. H., Tamura, R., Uwano, T., Kobayashi, T., Katsuki, M., Matsumoto, G. & Ono, T. (2002) Proc. Natl. Acad. Sci. USA 99, 8986-8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, S. R., Gainetdinov, R. R., Wightman, R. M. & Caron, M. G. (1998) J. Neurosci. 18, 1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yavich, L. & Tiihonen, J. (2000) J. Neurosci. Methods 104, 55-63. [DOI] [PubMed] [Google Scholar]
- 32.Shi, W. X., Smith, P. L., Pun, C. L., Millet, B. & Bunney, B. S. (1997) J. Neurosci. 17, 7988-7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonon, F. G. & Buda, M. J. (1985) Neuroscience 14, 765-774. [DOI] [PubMed] [Google Scholar]
- 34.Fibiger, H. C., LePiane, F. G., Jakubovic, A. & Phillips, A. G. (1987) J. Neurosci. 7, 3888-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miliaressis, E., Emond, C. & Merali, Z. (1991) Behav. Brain. Res. 46, 43-48. [DOI] [PubMed] [Google Scholar]
- 36.Kilpatrick, M. R., Rooney, M. B., Michael, D. J. & Wightman, R. M. (2000) Neuroscience 96, 697-706. [DOI] [PubMed] [Google Scholar]
- 37.Yavich, L. & Tiihonen, J. (2000) Neurosci. Lett. 293, 41-44. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez-Lopez, S., Bargas, J., Surmeier, D. J., Reyes, A. & Galarraga, E. (1997) J. Neurosci. 17, 3334-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West, A. R. & Grace, A. A. (2002) J. Neurosci. 22, 294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams, G. V. & Millar, J. (1990) Neuroscience 39, 1-16. [DOI] [PubMed] [Google Scholar]
- 41.Gonon, F. (1997) J. Neurosci. 17, 5972-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West, M. O. (1998) J. Neurosci. 18, 9055-9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Douarin, C., Penit, J., Glowinski, J. & Thierry, A. M. (1986) Brain Res. 363, 290-298. [DOI] [PubMed] [Google Scholar]
- 44.Nicola, S. M., Surmeier, J. & Malenka, R. C. (2000) Annu. Rev. Neurosci. 23, 185-215. [DOI] [PubMed] [Google Scholar]
- 45.Lavin, A., Nogueira, L., Lapish, C. C., Wightman, R. M., Phillips, P. E. & Seamans, J. K. (2005) J. Neurosci. 25, 5013-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan, Z., Hsieh-Wilson, L., Feng, J., Tomizawa, K., Allen, P. B., Fienberg, A. A., Nairn, A. C. & Greengard, P. (1999) Nat. Neurosci. 2, 13-17. [DOI] [PubMed] [Google Scholar]
- 47.Hayashida, Y. & Ishida, A. T. (2004) J. Neurophysiol. 92, 3134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips, P. E., Hancock, P. J. & Stamford, J. A. (2002) Synapse 44, 15-22. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy, R. T., Jones, S. R. & Wightman, R. M. (1992) J. Neurochem. 59, 449-455. [DOI] [PubMed] [Google Scholar]
- 50.Benoit-Marand, M., Borrelli, E. & Gonon, F. (2001) J. Neurosci. 21, 9134-9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greengard, P. (2001) Science 294, 1024-1030. [DOI] [PubMed] [Google Scholar]
- 52.Pirot, S., Godbout, R., Mantz, J., Tassin, J.-P., Glowinski, J. & Thierry, A.-M. (1992) Neuroscience 49, 857-865. [DOI] [PubMed] [Google Scholar]
- 53.Chuhma, N., Zhang, H., Masson, J., Zhuang, X., Sulzer, D., Hen, R. & Rayport, S. (2004) J. Neurosci. 24, 972-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cragg, S. J. & Rice, M. E. (2004) Trends Neurosci. 27, 270-277. [DOI] [PubMed] [Google Scholar]
- 55.Goto, Y. & O'Donnell, P. (2001) J. Neurosci. 21, 4498-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.