Abstract
The larval brain of Drosophila is a useful model to study olfactory processing because of its cellular simplicity. The early stages of central olfactory processing involve the detection of odor features, but the coding mechanisms that transform them into a representation in higher brain centers is not clear. Here we examine the pattern of connectivity of the main neurons that process olfactory information in the calyx (dendritic region) of the mushroom bodies, a higher brain center essential for associative olfactory learning. The larval calyx has a glomerular organization. We generated a map of calyx glomeruli, using both anatomical criteria and the pattern of innervation by subsets of its input neurons (projection neurons), molecularly identified by GAL4 markers. Thus, we show that projection neurons innervate calyx glomeruli in a stereotypic manner. By contrast, subsets of mushroom body neurons (Kenyon cells) that are labeled by GAL4 markers show no clear preference for specific glomeruli. Clonal subsets of Kenyon cells show some preference for subregions of the calyx, implying that they receive distinct input. However, at the level of individual glomeruli, dendritic terminals of larval-born Kenyon cells innervate about six glomeruli, apparently randomly. These results are consistent with a model in which Kenyon cells process olfactory information by integrating different inputs from several calyx glomeruli in a combinatorial manner.
Keywords: Kenyon cells, MARCM, olfactory learning, projection neurons, antennal lobe
Much is known about the early steps in detection of olfactory signals and about the circuitry that processes them in the first olfactory relay center of the brain, the antennal lobe (AL) in Drosophila or the olfactory bulb in mammals (1). From these centers, information is carried by second-order olfactory neurons to the secondary olfactory centers: by projection neurons (PNs) to the mushroom body (MB) and lateral horn in Drosophila (2-4) and by mitral/tufted cells to a number of areas of olfactory cortex in mammals (5). Second-order neurons have stereotypic target areas within at least some of these secondary centers (2-4, 6, 7), and therefore carry a spatial representation of the olfactory map to there. However, the organization of the circuitry at secondary centers, which underlies information processing in both systems, is not well understood. Work in Drosophila and locusts suggests that, in contrast to the broad odor response tuning of PNs, the responses of MB neurons (Kenyon cells, KCs) to the same odors are usually rare and selective (8-10), and electrophysiological studies (9) suggest a model in which KCs act as coincidence detectors of odor input from PNs. In the mammalian piriform cortex, both anatomical and physiological data suggest that olfactory cortical neurons also integrate olfactory information in a combinatorial manner (reviewed in ref. 5).
To understand connectivity in the olfactory circuitry, Drosophila offers a simpler system with fewer cells than in mammals. In the larva, single olfactory sensory neurons (OSNs), each expressing a single olfactory receptor, transmit odor signals to single AL glomeruli, thus defining a spatial map of odor input as in other systems, but with a remarkable structural simplicity (11). Furthermore, larvae are able to discriminate odors and are capable of associative olfactory learning (12), thus providing a useful model for a comprehensive understanding of odor discrimination mechanisms from the anatomical to behavioral levels. A glomerular architecture of the larval calyx has been also found recently, in parallel to the work presented here, and analysis of random PN clones has suggested stereotypy in the connectivity between AL and MB (3).
The analysis of the connectivity of third-order neurons has been hampered partly by the lack of landmarks. Therefore, to understand the pattern of connnectivity between second- and third-order neurons of the olfactory system, we analyzed the simple calyx of the Drosophila third-instar larva, generated a map of calyx glomeruli based on PN GAL4 markers, and asked questions such as: Do identifiable classes of PN connect to identifiable classes of KC, analogously to OSN-PN connections in the AL? Do either PNs or KCs show stereotypic projection patterns? And what are the implications of the anatomical organization for current models of olfactory processing?
Our results show that calyx glomeruli have unique identities, defined by their PN input. However, in contrast to the highly stereotypic pattern of PN input into calyx glomeruli, the projection pattern of most KC dendrites in calyx glomeruli appears random. This pattern of connectivity suggests that one role of calyx glomeruli is to allow conversion of olfactory information from a limited number of channels transmitted by PNs, to a representation in the MB that potentially allows discrimination of a much larger palette of odor combinations. To our knowledge, this is the most complete characterization to date of connectivity in a secondary olfactory center.
Results
Supporting Information. For further details, see Supporting Text, Fig. 5, Tables 1 and 2, and Movies 1-11, which are published as supporting information on the PNAS web site.
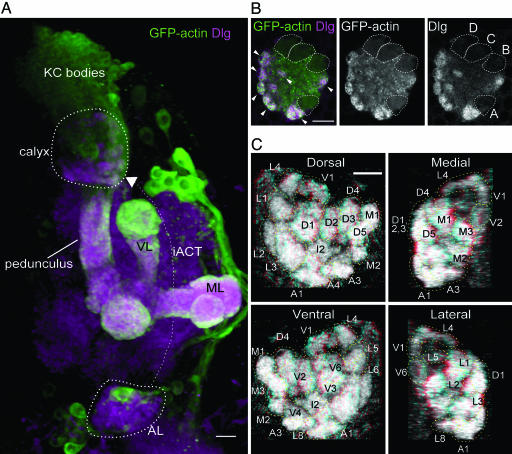
Glomerular Organization and Map of the Larval Mushroom Body Calyx. To study connectivity between PNs and KCs in the calyx, we first analyzed the general organization of the calyx. Therefore, we expressed GFP-actin (13) using the GAL4 line OK107, which drives expression in most larval KCs (14). GFP-actin was found enriched in calyx glomeruli (3) (Figs. 1B and 5A), where it colocalized with the synaptic scaffolding protein Discs-large (Dlg) and surrounded unlabeled presynaptic terminals. Glomeruli were distributed mainly over the surface of the calyx, except for areas occupied by the KC axon tracts, and only a few were localized internally (Figs. 1B and 5 and Movie 2). They occupied about two thirds of the volume of the calyx. The core region of the calyx was occupied mainly by nonglomerular regions of KC dendrites that joined the dendritic termini in the calyx glomeruli to the KC primary neurites. We analyzed five calyces from three individuals, using a dorsal view, and identified and named at least 34 glomeruli. The glomeruli identified by these anatomical criteria were highly stereotypic, although minor variations between individuals in size, position, and occurrence of glomeruli were found (Figs. 1C and 5A and Table 1). The number of identified glomeruli in this study is more than the 28 recently observed independently (3), probably because the dorsal view used here allows better resolution of calyx structures (which are posterodorsal) than the frontal view used in that study.
Fig. 1.
The larval mushroom body calyx. (A) Frontal view of a 3D reconstruction of part of the right brain hemisphere of a larva. GFP-actin is expressed in KCs under control of GAL4 OK107; brain neuropil is labeled by anti-Dlg antibody. Dorsal is to the top, medial to the right. The calyx of the mushroom body (inside a dashed line) lies just beneath the KC bodies in the dorsoposterior cortex of the brain. KCs have dendritic branches in the calyx, and their axons run posteriorly and medially to the calyx, to converge in the pedunculus ventral to the calyx. The axons in the pedunculus project anteroventrally, and branch into two main lobes, the vertical lobe (VL) and medial lobe (ML). The AL (in magenta) is shown inside a dashed line, partly obscured by two to three GAL4-expressing cell bodies. PNs have dendrites in the AL, and send axons dorsoposteriorly via the inner antennocerebral tract (iACT, fine dotted line with arrowhead) to the calyx of the mushroom body, and to the lateral horn (data not shown). In the calyx, they contact dendrites of Kenyon cells (KCs). (Scale bar, 10 μm.) (B) Expression of GFP-actin under control of GAL4 OK107 in KCs, and staining for Dlg reveal a glomerular organization of the calyx. Glomeruli (arrowheads) are seen mainly around the periphery of the calyx. The axonal tracts of each of the four clonal units of the mushroom body are outlined with dotted lines, and labeled A-D in Right. Panels show a horizontal section from around the middle of the calyx, anterior to the bottom and medial to the right. (Scale bar, 10 μm.) (C) Views of a calyx stained for Dlg, seen from dorsal, medial, ventral, and lateral perspectives, reconstructed from horizontal confocal sections. Glomeruli that are more distinct in each view, or closer to the observer, are labeled and outlined with dashed lines; because of overlap between glomeruli in the 2D projections and weaker staining of more ventral glomeruli, not all glomeruli can be seen distinctly. (Scale bar, 10 μm.) Panels can also be viewed as stereo images by using glasses with a red (left) and cyan, green, or blue (right) filter.
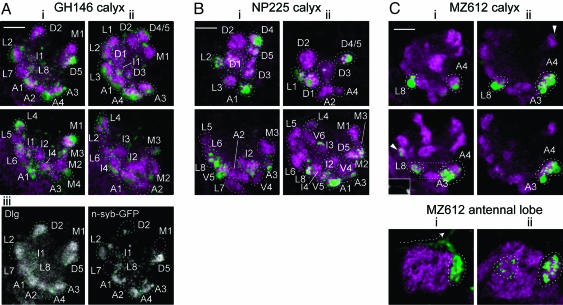
Conserved Identity of Calyx Glomeruli Defined by Projection Neuron Input. To test whether calyx glomeruli correspond to sites of input by specific PNs, we expressed n-syb-GFP (15) in PN terminals by using GAL4 lines that mark different subsets of PNs. We used the lines GH146 (2, 16, 17) and NP225 (6), which in the adult mark ≈83 and 70 PNs, respectively, from an estimated total number of 150-200. Approximately two thirds of larval calyx glomeruli were labeled (23 of 34 in GH146, n = 5; 23 of 36 in NP225, n = 9; Figs. 2 and 5 and Table 1).
Fig. 2.
Conserved identity of calyx glomeruli defined by projection neuron input. Expression of n-syb-GFP (green) by PN GAL4 lines GH146 (A), NP225 (B), and MZ612 (C) at presynaptic terminals in the larval calyx or AL (C Bottom). Two individuals (i and ii) are shown for each line. Confocal sections through the middle of calyces (two horizontal sections from slightly different levels) or larval antennal lobes (frontal sections) are shown. Glomeruli are visualized by using antibody to Dlg (magenta) and outlined by using dashed lines. In A and B, either green (for n-syb-GFP-positive) or magenta (for n-syb-GFP-negative) is used. In C, only n-syb-GFP-positive glomeruli are outlined, in white. In GH146, Aiii shows separate channels of Ai Upper; note the scattered spots of n-syb-GFP labeling, not in glomeruli, in the interior of the GH146 calyx. Further sections from GH146 and NP225 are shown in Fig. 5. (C) In many MZ612 calyces, a process can be seen linking L8 with A3/A4, (Ci Middle, dotted rectangular outline, green channel shown in Inset). Antennal lobes of MZ612 are each from the same brain hemisphere as the calyces shown above them. Both are innervated in a medial glomerulus (surrounded by a dashed line in the right half of each panel) by a PN that strongly expresses n-syb-GFP; its primary neurite can be seen coming from the direction of the cell body and projecting (dashed arrow in i) toward the inner antennocerebral tract. Weakly labeled AL glomeruli (dashed line in the left half of Cii) and calyx glomeruli (arrowheads in C) were sometimes also seen. Anterior (for calyces) or ventral (for antennal lobes) lies to the bottom, and medial is to the right in each panel. (Scale bars, 10 μm.)
In each of the GAL4 lines, NP225 and GH146, the positions of unlabeled glomeruli, and of glomeruli that were labeled by n-Syb-GFP more strongly, were consistent in most individuals (Fig. 5 and Table 1). In addition, the pattern of calyx glomeruli that were labeled and the levels of n-Syb-GFP expression in individual glomeruli were characteristic for each GAL4 line (Figs. 2 and 5 and Table 1). Some calyx glomeruli were not labeled in either GH146 or NP225 lines, and therefore could be innervated either by other PNs that are not labeled in any of these two GAL4 lines or by neurons originating from outside the olfactory system. The consistency in the level of n-syb-GFP labeling of individual glomeruli within each GAL4 line, and the differences in labeling between the two GAL4 lines, suggest a conserved identity for each calyx glomerulus that is specified by its PN innervation.
The conserved pattern of n-syb-GFP expression levels in these GAL4 lines also provided an additional mapping criterion of a molecular nature, and confirmed the map obtained by using only positional criteria with OK107 (Table 1).
Additional evidence for stereotypy of PN innervation of calyx glomeruli was obtained from the projection pattern of a third PN GAL4 line, MZ612 (Fig. 2). This line labels one larval PN (and AL glomerulus) per brain hemisphere strongly (18), and a variable number of additional PNs and AL glomeruli (up to four) weakly (Fig. 2C and data not shown). In 12 calyces examined, the same two or three strongly labeled glomeruli were always present. In nine calyces, glomeruli A3, A4, and L8 were labeled (Fig. 2C), whereas in the remaining three calyces, either A3 or A4 was unlabeled. An axonal process was often observed connecting L8 to A3 and A4, supporting the interpretation that they were innervated by the same PN (A3 and A4 were too close together to see any such process). In conclusion, the consistent labeling of these three calyx glomeruli, in a GAL4 line that reproducibly marks one specific PN molecularly, strongly supports the notion that specific calyx glomeruli are innervated by specific sets of PNs.
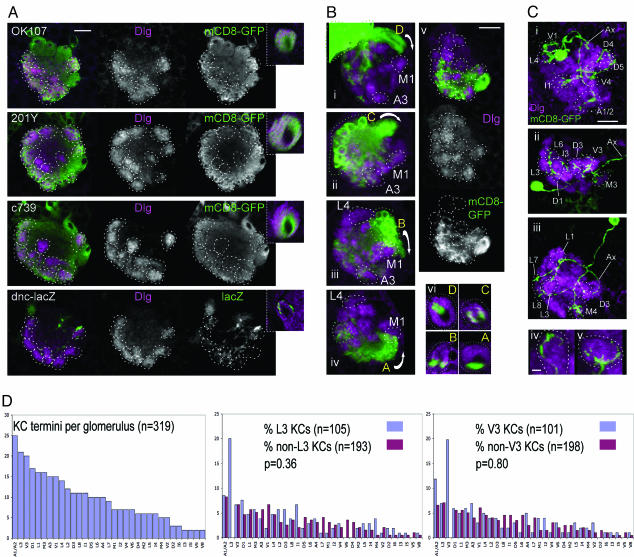
Pattern of KC Innervation of Calyx Glomeruli. Do KCs show a similar specificity for calyx glomeruli, as PNs do? To test for glomerular specificity of innervation by different classes of KC that are defined by gene expression patterns, we used four lines that label different but partially overlapping sets of KCs in third-instar larvae: GAL4 lines OK107, c739, and 201Y, and dnc-lacZ (14, 19-21). KCs labeled in each of these lines appear to project throughout the calyx to all glomeruli, although some of the internal glomeruli are labeled less intensely than the others (Fig. 3A). Even the KCs labeled by dnc-lacZ, which labels the fewest KCs among the four lines (14), appear to arborize in nearly all glomeruli to a small extent, rather than arborizing heavily in a small subset of glomeruli. Therefore, calyx glomeruli do not correspond to subsets of KCs that are defined by their gene expression patterns; rather, each glomerulus contains dendrites of a broad spectrum of different classes of KC.
Fig. 3.
Nonstereotypic innervation of calyx glomeruli by KCs. (A) KC subsets defined by gene expression project to glomeruli throughout the calyx. Each row shows a horizontal confocal section through a calyx stained for Dlg, and GAL4 insertion OK107, 201Y, or c739 driving expression of mCD8-GFP or dnc-lacZ; a cross-section through the pedunculus of each MB is shown in the right insets. Each subset of KCs appears to project to most or all glomeruli. Anterior is to the bottom, medial is to the left. Outlines of glomeruli are shown by dashed lines. (Scale bar, 10 μm.) (B) Progeny of individual mushroom body neuroblasts (labeled by mCD8-GFP using MARCM, green) arborize in most glomeruli (anti-Dlg, magenta), but preferentially in regions of the calyx (diffuse green labeling) that correspond to a similar position on the anterior-posterior axis to their respective axon tracts. (i-iv) Confocal reconstructions of calyces in which the neuroblasts giving rise to axon tracts D, C, B, and A, respectively, have been labeled. Rotations of each reconstruction around a longitudinal axis are shown in Movies 3-6, respectively. Arrows show the direction of axon projections from the cell bodies (enclosed in green dotted lines) toward the pedunculus; note that most cell bodies cannot be seen because dorsal sections have been removed before 3D reconstruction to avoid obscuring the calyx. Some glomeruli used as landmarks are labeled. (v) A horizontal section through the middle of a calyx, showing Dlg, and mCD8-GFP in the clonal progeny of a neuroblast that projects into axon tract A. (vi) Cross-sections through the pedunculi of larvae in which neuroblast progenies projecting into the pedunculus via axon tracts D, C, B, and A have been labeled. All preparations are viewed from dorsal; anterior is to the bottom, medial is to the right. (Scale bar, 10 μm.) (C) Projection patterns of individual KCs (expressing mCD8-GFP, green) to calyx glomeruli (anti-Dlg, magenta). (i-iii) Confocal reconstructions of three calyces, each showing the projections of a single KC. Calyx glomeruli where KC claws arborize are identified and outlined with dashed lines; three-dimensional positions of claws can be seen in rotations of these reconstructions in Movies 7-9, respectively. (iv and v) Confocal reconstructions showing the relationship of two different claws to two individual glomeruli. Rotations of reconstructions are shown in Movies 10 and 11, respectively. All panels are viewed from dorsal, with anterior to the bottom and medial to the right. Ax, KC axon, which projects to the pedunculus. (Scale bars, 10 μm in i-iii and 2 μm in iv and v.) (D) (Left) Numbers of claws from 55 labeled single KCs that innervate each calyx glomerulus. A1 and A2 were combined because they could only be distinguished reliably by using NP225 or GH146 GAL4 lines as markers (Fig. 5). (Center) For glomerulus L3, the frequencies (y axis) with which other glomeruli (x axis) are innervated either by KCs that also innervate L3 (left blue bars), or by KCs that do not innervate L3 (right red bars), are compared. Frequencies are expressed as a percentage of all claws in the respective category, excluding claws in L3. The total number of claws in each category is shown, together with the P value from a χ2 comparison of the two distributions. (Right) A similar analysis for glomerulus V3. Original data on claw distributions are supplied in Table 2.
The KCs consist of four clonal subsets, each of which is derived from a separate neural stem cell (neuroblast) (22). Because there has been no precise map of the calyx, it has been impossible to test whether these subsets are functionally equivalent (22). Therefore, we visualized each of the four larval KC clones by using the GAL4 OK107 strain with MARCM (mosaic analysis with a repressible cell marker) induced in early first-instar larvae (23). Progeny of each of the four neuroblasts were identified by the position of their axonal projections, in one of four bundles, designated A-D in an anterior to posterior direction around the medial side of the calyx (Fig. 3B i-iv), and in the pedunculus (Fig. 3Bvi). Each clonally related set of dendrites projected preferentially to a different calyx region, in an order that reflected the anteroposterior positions of the corresponding axonal bundles, with dendrites from neuroblast A progeny innervating mainly the most anterior region of the calyx, and those from neuroblast D progeny innervating mainly the most posterior region (Fig. 3B i-iv and Movies 3-6). However, the regional preference of dendritic calyx arborization by each neuroblast clone was not absolute, because some arborization was found outside the heavily innervated region of each neuroblast clone (Fig. 3B i-v and Movies 3-6). Therefore, each clonally related set of KCs receives input preferentially from a region of the calyx, but there is not an absolute correlation between each clone and a group of glomeruli. The regional preferences of the four clonal subsets of KCs in the larval calyx are in contrast to an earlier report that no such preferences exist in the larva, but consistent with restricted arborization of these subsets in the adult calyx (21). Our data imply that these subsets of KCs are not strictly functionally equivalent, as has been suggested from their similar axonal projection patterns and the labeling of all four subsets by all enhancer-trap lines tested (22); rather, because different glomeruli are innervated by different PNs, each clonal subset of KCs receives a different spectrum of olfactory inputs.
To examine the distribution of single KC dendrites among identified calyx glomeruli, we used the MARCM method (mosaic analysis with a repressible cell marker) (23) to induce labeling of KCs in first-instar larvae. This procedure resulted in labeling of multiglomerular KCs, typical of larval-born KCs (3) that comprise the majority of those found in third-instar larvae (24), and similar to most adult KCs, which also have multiple dendritic termini (6, 21, 25). Each labeled KC had about six claw-like dendritic termini (5.80 ± 0.14, n = 55; minimum of two, maximum of seven; all but four had between five and seven). Each terminus (or “claw”) contacted a single glomerulus (Fig. 3C iv and v). In most cases, the dendritic projections of a single KC were not confined to an identifiable region, but apparently distributed throughout much of the calyx (Fig. 3C i-iii and Table 2).
To detect any preferences of KCs to innervate specific combinations of glomeruli, we used morphological criteria to identify the glomeruli innervated by the 55 individual KCs, a total of 319 glomeruli. Strikingly, for 10 of the most commonly innervated glomeruli, there was no significant preference for either coinnervation or avoidance of other glomeruli (χ2 tests, P values all between 0.31 and 0.95; Table 2 and Fig. 3D). A higher frequency of KC claws was found in the larger glomeruli (compare Fig. 3D with the panels showing specific calyx glomeruli in Figs. 1, 2, and 5). This result is consistent with the large glomerular size being due to the volume of dendritic termini of a large number of KCs. In conclusion, in contrast to the high specificity of PN subsets innervating particular calyx glomeruli, there is no strict glomerular specificity in dendritic projections of KCs.
Discussion
Here we have described a stereotypic input of PNs, and apparently random innervation by KC dendrites, in the MB calyx, organized in a conserved glomerular pattern. Individual calyx glomeruli are sites of synaptic divergence, from single PNs (3) to hundreds of KCs. KCs are units of synaptic convergence, receiving input from several glomeruli. Electron microscopic analysis of adult Drosophila calyx has also shown synaptic contacts between a single presynaptic PN and many KC dendrites in individual glomeruli (26).
Our data show that larval calyx glomeruli are sites of input for specific PNs, and that connectivity is correlated with the molecular properties of PNs. These data are consistent with the conclusion of Ramaekers et al. (3) that larval calyx glomeruli appear to be sites of specific PN input. Their conclusion is based on seven calyx glomeruli, and for most of these, it depends on a shared anatomy of two or three randomly generated PN clones. PN GAL4 lines provide an additional molecular criterion that helps to identify a larger number of calyx glomeruli, and our analysis of these glomeruli suggests that this conclusion is generally true. These GAL4 lines also provide tools for targeted expression in identified calyx glomeruli, which should aid investigation of the mechanisms of connectivity and olfactory processing there.
KCs have been proposed as detectors of coincident olfactory input (9). Our observations clearly link the model of coincidence detection to calyx glomeruli. In the context of this model, the coincident inputs required to fire a KC would come from different glomeruli. Stimulation in one glomerulus alone is unlikely to fire a multiglomerular KC, because our data suggest that each glomerulus is innervated on average by ≈300 of the 1,800 (24) larval-born KCs in the third-instar larva (1,800 × 5.8/34), whereas KC responses to odors are actually rare and selective (8, 9). If odors stimulated small numbers of calyx glomeruli, the best odor discrimination would be achieved if about four of the six claws of a KC must be stimulated to fire it; in this case, each combination of four stimulated claws would fire an average of just under one KC per calyx, i.e., 1,800/[34!/(6!28!)/30/(2!28!)]. However, real odors may stimulate the calyx in as many as 60% of PNs, and hence calyx glomeruli (9, 10), and even a requirement for stimulation in four claws would still lead to firing of a large fraction of KCs; it is therefore likely that KCs would require stimulation in more than four claws to fire, for agreement with observations that the numbers of KCs that respond to a given odor are low (8, 9).
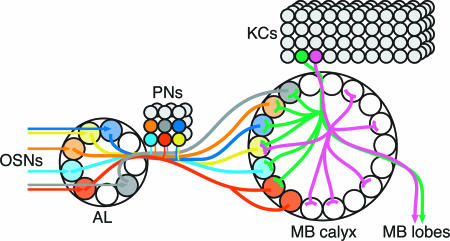
Although both the calyx and the AL have a glomerular organization, the logic of the connectivity is very different (Fig. 4). AL glomeruli have stereotypic and preprogrammed connectivity between defined olfactory neurons and defined PNs (3, 11, 27-29) (Fig. 2C). By contrast, calyx glomeruli have stereotypic PN input, but nonstereotypic KC connectivity that appears not to be “hard-wired.” The random but combinatorial innervation of glomeruli by KCs should allow individual KCs to respond to a specific combination of odor qualities, and the MB as a whole could discriminate among a much larger combination of odors than might be expected from the lower specificity of OSNs and PNs (10, 11).
Fig. 4.
Connectivity in the larval mushroom body calyx. PNs receive olfactory input from OSNs in specific glomeruli of the AL, and send axonal projections to specific glomeruli in the MB calyx and to the lateral horn (not shown). In the MB calyx, larval KCs have dendrites ending in claw-like processes in ≈6 of 34 glomeruli, implying that information from ≈6 calyx glomeruli can converge to a single KC. KCs have a nonstereotypic arborization pattern. Even KCs that arborize in the same glomerulus have other arborizations in different sets of glomeruli (magenta and green KCs), implying that information from single PNs diverges to multiple KCs, where it is integrated with information from other PNs.
The multiglomerular KC dendritic arborization patterns described here are broadly typical of most larval and adult KCs (6, 21, 25). However, a minority of KCs has fewer dendrites than those described here, embryonic-born KCs in the larval MB (3) and KCs born in a narrow window of pupal development in the adult MB (21). To date, it is not known whether the connectivity of these KCs is stereotypic. They could potentially allow the MB to form associations with odors that activated only a small number of calyx glomeruli, or with other forms of sensory input that are carried through fewer channels than olfactory stimuli.
Because the adult calyx has KCs that have multiple dendritic termini (6, 21, 25, 30) and glomeruli, albeit not until now recognized as having a stereotypic organization (26), it may have a fundamentally similar organization to the larval calyx. There are ≈150-200 adult PNs (31), and if there is a glomerulus at each of the 2-11 PN termini (2), there would be ≈1,000 glomeruli in the adult calyx. Multiple termini of the same PN could allow the MB to detect a broader spectrum of odor combinations than would otherwise be possible, given the preferences of clonally related dendrites for one region of the calyx (21). They could also allow the same olfactory inputs to be transmitted to different functional subsets of adult KCs whose dendrites innervate different regions of the calyx; for example, adult KCs whose axons innervate the γ lobes of the MB have a preferential distribution of dendrites in the center of the calyx (6). Detailed design differences mapped on a fundamentally similar architecture will allow both larvae (12) and adults (1, 32) to exhibit odor discrimination learning in ways that are adapted to their specific needs.
In conclusion, we have described the pattern of connectivity between second- and third-order neurons at the single-cell level in a secondary olfactory center essential for olfactory learning in the Drosophila larval brain. The pattern of stereotypic and divergent input and convergent dendritic projections loosely resembles that of the anterior piriform cortex of mammals (5), although the latter has not been described at single-cell resolution and does not have recognizable glomeruli. In both systems, it can provide the basis for combinatorial recognition of odor qualities. Most odors, even monomolecular ones, activate a number of glomeruli in the primary olfactory centers of both insects (33, 34) and mammals (35, 36). A combinatorial recognition system in secondary olfactory centers, such as we have described, is therefore essential to achieve specific behavioral responses to odors.
Materials and Methods
KC clones were generated at 25-27 h after egg-laying by MARCM (23), and were examined by dissecting brains from wandering third-instar larvae of genotype hsFLP P{UAS-mCD8::GFP}/y w; P{FRT(whs)}G13 P{UAS-mCD8::GFP}LL5/P{FRT(whs)}G13 P{tubP-GAL80}LL2; OK107/+, followed by immunolabeling and confocal microscopy. Confocal sections were processed by using lsm (Carl Zeiss, Tokyo), imagej (http://rsb.info.nih.gov/ij), and photoshop (Adobe Systems, San Jose, CA) software to generate image stacks, 3D projections, and montages, which were all used to visualize neuronal projections and identify glomeruli. To compare the glomerular distribution of claws between different sets of calyces, innervated glomeruli were identified by using morphological criteria (Table 1), and distributions were tested for independence by using χ2 tests in excel (Microsoft), as summarized in Table 2. Detailed experimental procedures, and details of fly and antibody stocks, are provided in Supporting Text.
Supplementary Material
Acknowledgments
We thank Y. Uchijima for continual informatic support; the National Institute of Child Health and Human Development Developmental Studies Hybridoma Bank (University of Iowa) for supplying Dlg 4F3 antibody; Drs. K. Furukubo-Tokunaga (University of Tsukuba, Tsukuba, Japan), K. Ito (University of Tokyo), M. Ramaswami (University of Arizona, Tucson), R. Stocker (University of Fribourg, Fribourg, Switzerland), V. Verkhusha (Tsukita Cell Axis Project, ERATO, Osaka), L. Luo (Stanford University, Stanford, CA), and the Drosophila Stock Center (Bloomington, IN) for fly stocks; Drs. K. Ito, N. Uchida, H. Kashiwadani, and S. Laughlin for critical reading of the manuscript and helpful discussions; Dr. T. Tabata (University of Tokyo) for fly and microscopy facilities; Dr. S. Takahashi for financial and institutional support of L.M.M.-N.; and Dr. K. Ito for support of N.K.T. C.J.O'K. was supported by a Biotechnology and Biological Sciences Research Council Research Development Fellowship.
Author contributions: L.M.M.-N. and C.J.O'K. designed research; L.M.M.-N. and N.K.T. performed research; L.M.M.-N. and C.J.O'K. analyzed data; and L.M.M.-N. and C.J.O'K. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: AL, antennal lobe; PN, projection neuron; MB, mushroom body; KC, Kenyon cell; OSN, olfactory sensory neuron.
References
- 1.Davis, R. L. (2004) Neuron 44, 31-48. [DOI] [PubMed] [Google Scholar]
- 2.Wong, A. M., Wang, J. W. & Axel, R. (2002) Cell 109, 229-241. [DOI] [PubMed] [Google Scholar]
- 3.Ramaekers, A., Magnenat, E., Marin, E. C., Gendre, N., Jefferis, G. S. X. E., Luo, L. & Stocker, R. F. (2005) Curr. Biol. 15, 982-992. [DOI] [PubMed] [Google Scholar]
- 4.Marin, E. C., Jefferis, G. S. X. E., Komiyama, T., Zhu, H. & Luo, L. (2002) Cell 109, 243-255. [DOI] [PubMed] [Google Scholar]
- 5.Neville, K. R. & Haberley, L. B. (2004) in The Synaptic Organization of the Brain, ed. Shepherd, G. M. (Oxford Univ. Press, Oxford), pp. 415-444.
- 6.Tanaka, N. K., Awasaki, T., Shimada, T. & Ito, K. (2004) Curr. Biol. 14, 449-457. [DOI] [PubMed] [Google Scholar]
- 7.Zou, Z., Horowitz, L. F., Montmayeur, J.-P., Snapper, S. & Buck, L. B. (2001) Nature 414, 173-179. [DOI] [PubMed] [Google Scholar]
- 8.Wang, Y., Guo, H.-F., Pologruto, T. A., Hannan, F., Hakker, I., Svoboda, K. & Zhong, Y. (2004) J. Neurosci. 24, 6507-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Orive, J., Mazor, O., Turner, G. C., Cassenaer, S., Wilson, R. I. & Laurent, G. (2002) Science 297, 359-365. [DOI] [PubMed] [Google Scholar]
- 10.Wilson, R. I., Turner, G. C. & Laurent, G. (2004) Science 303, 366-370. [DOI] [PubMed] [Google Scholar]
- 11.Kreher, S. A., Kwon, J. Y. & Carlson, J. R. (2005) Neuron 46, 445-456. [DOI] [PubMed] [Google Scholar]
- 12.Scherer, S., Stocker, R. F. & Gerber, B. (2003) Learn. Mem. 10, 217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkhusha, V. V., Tsukita, S. & Oda, H. (1999) FEBS Lett. 445, 395-401. [DOI] [PubMed] [Google Scholar]
- 14.Kurusu, M., Awasaki, T., Masuda-Nakagawa, L. M., Kawauchi, H., Ito, K. & Furukubo-Tokunaga, K. (2002) Development (Cambridge, U.K.) 129, 409-419. [DOI] [PubMed] [Google Scholar]
- 15.Ito, K., Suzuki, K., Estes, P., Ramaswami, M., Yamamoto, D. & Strausfeld, N. J. (1998) Learn. Mem. 5, 52-77. [PMC free article] [PubMed] [Google Scholar]
- 16.Python, F. & Stocker, R. F. (2002) J. Comp. Neurol. 445, 374-387. [DOI] [PubMed] [Google Scholar]
- 17.Stocker, R. F., Heimbeck, G., Gendre, N. & de Belle, J. S. (1997) J. Neurobiol. 32, 443-456. [DOI] [PubMed] [Google Scholar]
- 18.Marin, E. C., Watts, R. J., Tanaka, N. K., Ito, K. & Luo, L. (2005) Development (Cambridge, U.K.) 132, 725-737. [DOI] [PubMed] [Google Scholar]
- 19.Connolly, J. B., Roberts, I. J. H., Armstrong, J. D., Kaiser, K., Forte, M., Tully, T. & O'Kane, C. J. (1996) Science 274, 2104-2107. [DOI] [PubMed] [Google Scholar]
- 20.Yang, M. Y., Armstrong, J. D., Vilinsky, I., Strausfeld, N. J. & Kaiser, K. (1995) Neuron 15, 45-54. [DOI] [PubMed] [Google Scholar]
- 21.Zhu, S., Chiang, A.-S. & Lee, T. (2003) Development (Cambridge, U.K.) 130, 2603-2610. [DOI] [PubMed] [Google Scholar]
- 22.Ito, K., Awano, W., Suzuki, K., Hiromi, Y. & Yamamoto, D. (1997) Development (Cambridge, U.K.) 124, 761-771. [DOI] [PubMed] [Google Scholar]
- 23.Lee, T. & Luo, L. (1999) Neuron 22, 451-461. [DOI] [PubMed] [Google Scholar]
- 24.Technau, G. & Heisenberg, M. (1982) Nature 295, 405-407. [DOI] [PubMed] [Google Scholar]
- 25.Lee, T., Lee, A. & Luo, L. (1999) Development (Cambridge, U.K.) 126, 4065-4076. [DOI] [PubMed] [Google Scholar]
- 26.Yusuyama, K., Meinertzhagen, I. A. & Schürmann, F.-W. (2002) J. Comp. Neurol. 445, 211-226. [DOI] [PubMed] [Google Scholar]
- 27.Vosshall, L. B., Wong, A. M. & Axel, R. (2000) Cell 102, 147-159. [DOI] [PubMed] [Google Scholar]
- 28.Jefferis, G. S. X. E., Marin, E. C., Stocker, R. F. & Luo, L. (2001) Nature 414, 204-208. [DOI] [PubMed] [Google Scholar]
- 29.Gao, Q., Yuan, B. & Chess, A. (2000) Nat. Neurosci. 3, 780-785. [DOI] [PubMed] [Google Scholar]
- 30.Strausfeld, N. J., Sinakevitch, I. & Vilinsky, I. (2003) Microsc. Res. Tech. 62, 151-169. [DOI] [PubMed] [Google Scholar]
- 31.Stocker, R. F., Lienhard, M. C., Borst, A. & Fishbach, K. F. (1990) Cell Tissue Res. 262, 9-34. [DOI] [PubMed] [Google Scholar]
- 32.Heisenberg, M. (2003) Nat. Rev. Neurosci., 266-275. [DOI] [PubMed]
- 33.Ng, M., Roorda, R. D., Lima, S. Q., Zemelman, B. V., Morcillo, P. & Miesenböck, G. (2002) Neuron 36, 463-474. [DOI] [PubMed] [Google Scholar]
- 34.Wang, J. W., Wong, A. M., Flores, J., Vosshall, L. B. & Axel, R. (2003) Cell 112, 271-282. [DOI] [PubMed] [Google Scholar]
- 35.Rubin, B. D. & Katz, L. C. (1999) Neuron 23, 499-511. [DOI] [PubMed] [Google Scholar]
- 36.Uchida, N., Takahashi, Y. K., Tanifuji, M. & Mori, K. (2000) Nat. Neurosci. 3, 1035-1043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.