Abstract
Remodeling chromatin is essential for cAMP-regulated gene expression, necessary not only for development but also for memory storage and other enduring mental states. Histone acetylation and deacetylation mediate long-lasting forms of synaptic plasticity in Aplysia as well as cognition in mice. Here, we show that histone acetylation by the cAMP-response element binding protein (CREB)-binding protein (CBP) mediates sensitivity to cocaine by regulating expression of the fosB gene and its splice variant, ΔfosB, a transcription factor previously implicated in addiction. Using the chromatin immunoprecipitation assay with antibodies against histone H4 or CBP, we find that CBP is recruited to the fosB promoter to acetylate histone H4 in response to acute exposure to cocaine. We show that mutant mice that lack one allele of the CBP gene and have normal levels of fosB expression are less sensitive to chronic (10-day) administration of cocaine than are wild-type mice. This decreased sensitivity is correlated with decreased histone acetylation and results in decreased fosB expression and diminished accumulation of ΔfosB. Thus, CBP, which forms part of the promoter complex with CREB, mediates sensitivity to cocaine by acetylating histones.
Keywords: addiction, chromatin modulation, memory storage, Rubenstein-Tabi syndrome
Similar molecular mechanisms underlie the formation of long-term memory and cocaine addiction. In long-term facilitation of sensory-to-motor neuron synapses underlying defensive reflexes in Aplysia, an established cellular model for long-term memory, the facilitatory neurotransmitter serotonin (5-HT) activates the cAMP-dependent protein kinase (PKA) (1) to phosphorylate cAMP-response element-binding protein (CREB) (2). After recruiting CREB-binding protein (CBP) (3), phospho-CREB leads to the induction of two immediate early genes, C/EBP (4) and ubiquitin C-terminal hydrolase (5), as well as several late effector genes including eEF1A and the RII subunit of PKA (6, 7). In addition to serving as a scaffold protein in CREB promoter complexes, the recruited CBP also has intrinsic histone acetyltransferase activity and can modify histones at promoters, resulting in the decondensation of DNA and thus enabling gene expression (3, 8). Recently, Kumar et al. (9) showed that histone acetylation is involved in the cellular and molecular changes induced in neurons by cocaine. They found that histones at the promoter of cFos, a gene induced after acute cocaine administration, are acetylated acutely, and that chronic cocaine treatment involves acetylation at the promoters of BDNF and Cdk5, genes that are expressed in rodent models of cocaine addiction.
Activation of CREB also underlies cocaine addiction (for review, see refs. 10-12); its overexpression in the striatum of mice decreases cocaine's immediate rewarding effects; conversely, its inhibition increases the effect of reward (13, 14). FosB, an immediate early gene, also influences the response to cocaine. Thus, manipulation of fosB and ΔfosB expression in the striatum of transgenic mice increases the behavioral response to cocaine and to other drugs of abuse (15, 16). We now provide evidence that fosB is induced by activating CREB and recruiting CBP to the fosB promoter. CBP was first identified as a nuclear protein that binds specifically to phosphorylated CREB (8); later, it was found to be mutated in patients with Rubinstein-Taybi syndrome, a disorder characterized by mental retardation, facial abnormalities, broad thumbs, and big toes (17). In addition to phosphorylated CREB, CBP can form complexes with several other transcription factors (c-Jun, c-Fos, and ATF2) and is involved in cell growth, development, and oncogenesis (18). CBP is also essential for activity-dependent gene expression in the hippocampus and cortex (19-21). Several CBP knockout or dominant-negative mouse mutants have been generated, all of which display normal short-term memory but are deficient in certain forms of long-term memory (22-24).
In this paper, we find that cocaine administration causes the recruitment of CBP to the fosB promoter. CBP acetylates histone H4 and increases fosB expression. Compared with the wild type, in CBP haploinsufficient mice, less CBP is recruited to the fosB promoter, resulting in decreased histone acetylation and fosB expression. Less expression of fosB results in decreased accumulating ΔfosB, and decreased sensitization after cocaine challenge. Thus, we provide direct evidence that the recruitment of CBP to the fosB promoter and the resulting histone acetylation are essential for inducing the gene expression that underlies cocaine addiction.
Materials and Methods
Animals. Tanaka et al. (25) described the generation of CBP haploinsufficient mice. We observed a progressively reduced transmission of the mutation with mice repeatedly backcrossed with C57BL/6J strain (after four backcrossings, many heterozygous males did not transmit the silenced CBP allele). Those that did transmit this allele exhibited a rate lower than 50%). Therefore, we carried out our experiments in a genetic background similar to the one used by Tanaka et al. (25) and crossed C57BL/6J mutant males with BALB/c females to generate the F1 hybrids. The wild-type mice used as controls were littermates of the mutants. Mice were maintained and bred under standard conditions consistent with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committees.
Chromatin Immunoprecipitation (ChIP). The ChIP assay was performed as described in ref. 3 with some modifications. The striatum was dissected from mice anesthetized with 100 mg/kg ketamine and perfused with 4% paraformaldehyde for 30 min, washed in 0.125 M glycine and PBS, each for 12 min at 4°C, and homogenized. After sonication, the average size of DNA fragments was ≈600 bp. Chromatin was used for immunoprecipitation with specific antibodies, anti-acetylated histone H4 (Upstate Biotechnology, Lake Placid, NY) and anti CBP (Santa Cruz Biotechnology). The presence of the fosB promoter was analyzed by real-time PCR with the promoter-specific primer pair GGTCCCGGAGGCATAAATTC (forward) and TCACGCCTCCAAGAAGAAGAA (reverse) using actin sequences to control for specificity of histone acetylation and transcription. Real-time PCR was performed with the GeneAmp 5700 Sequence Detection System (PE Corporation, Norwalk CT). Amounts of specific histone modifications at the fosB promoter were determined by measuring the amount of acetylated histone-associated DNA using quantitative real-time PCR with actin as control. No differences were seen between animals injected with saline and animals killed that had not been injected. Input and immunoprecipitated DNA amplification reactions were run in triplicate in the presence of SYBR-Green (Applied Biosystems). Normalized reporter (Rn) values [fluorescence detected during PCR (GeneAmp 5700 Sequence Detection System manual, PE Corporation) from each sample] were obtained by using sequence detector 1.1 software (see Fig. 1). Relative quantification of template was done as described by the manufacturer (Applied Biosystems). Values (mean ± SEM) were analyzed by two-tailed paired t tests (adjusted for multiple comparisons) for statistical significance (P < 0.05). PCRs were run in triplicate for each brain sample, and at least three independent sample pairs were used for each statistical analysis.
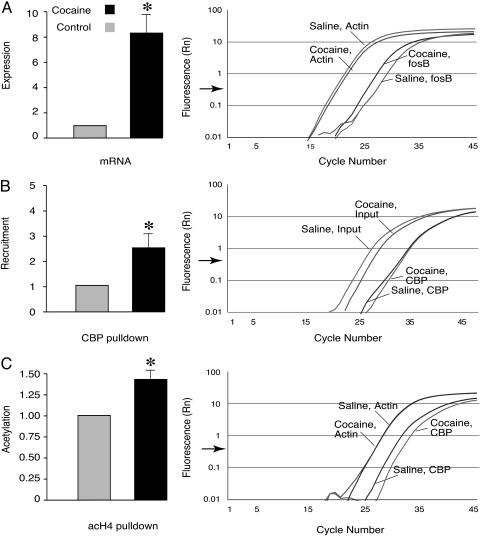
Fig. 1.
Cocaine induces fosB expression through CBP recruitment and histone acetylation at the fosB promoter. (A) Real-time RT-PCR shows that fosB is induced in C57BL/6J mouse striata 2 h after injecting 30 mg/kg cocaine i.p. (n = 3 in each group; actin set at 1; cocaine, 7.88 ± 0.15; P < 0.001). (B) ChIP assays show increased CBP recruitment to the fosB promoter 20 min after injecting 30 mg/kg cocaine i.p. [n = 7 in each group; control set at 1; cocaine, 2.45 ± 0.65; P < 0.05, input (nonimmunoprecipitated DNA) was used as control]. (C) The acetylation of histone H4 at the fosB promoter was increased 20 min after injecting 30 mg/kg cocaine i.p. (n = 6 mice pairs; control set at 1; cocaine, 1.41 ± 0.14; P < 0.05). Normalized reporter (Rn) represents the fluorescence detected. Data are expressed as mean ± SEM, P values were measured by Student's t test. Representative data from real-time PCR experiments are shown to the right of each bar chart. The quantifying of fosB cDNA (A), and CBP and acetylated histone H4 antibody pulldown (B and C) was done by normalizing Ct values (Ct value is the number of cycles at which the fluorescence crosses a threshold indicated by the arrow at the abscissa) of cDNA and immunoprecipitated DNA (saline control vs. cocaine) to Ct values of actin and input DNA. Acetylated histone H4 at the actin promoter and levels of actin cDNA were unaffected by cocaine.
Measuring mRNA by Real-Time PCR. RNA from the striata of mice injected with 30 mg/kg cocaine was extracted by using Trizol reagent (Invitrogen) and precipitated with isopropanol. mRNA was reverse transcribed by using a SuperScript III First-Strand Synthesis kit (Invitrogen). The amount of cDNA was quantified by using real-time PCR. The primers used to amplify specific cDNA regions of the transcripts of interest were: fosB 5′-ACAGATCGACTTCAGGCGGA-3′ and 5′-GTTTGTGGGCCACCAGGAC-3′; as internal control for normalization actin, 5′-ATGGTGGGAATGGGTCAGAAG-3′ and 5′-TCTCCATGTCGTCCCAGTTG-3′. Fold differences of mRNA over control values were calculated by using the Rn method as described by the manufacturer (Applied Biosystems).
Antibodies and Immunoblotting. Mice were injected with 30 mg/kg cocaine i.p. for 10 days and killed 24 h later. Striata were dissected in PBS on ice and homogenized in 0.25% Triton X-100, 0.5% Nonidet P-40, 10 mM EDTA, 0.5 mM EGTA, 10 mM Tris·HCl (pH 8.0), and 1 mM PMSF. Protein was measured by a Lowry-based assay (Bio-Rad); 30 μg of protein was used for detection of ΔfosB and separated by SDS/10% PAGE. After electrophoresis, gels were transferred to nitrocellulose membranes (Protran, Schleicher & Schuell) and probed with fosB antibody (H-75) (Santa Cruz Biotechnology), overnight at 4°C. Blots were incubated with an anti-rabbit IgG-HRP as secondary antibody (1:1,000) (Sigma) for 1 h at room temperature and quantified by using ECL (Amersham Pharmacia). Some blots were later stripped with 0.2 M glycine (pH 2.8) and 0.5 M NaCl for 25 min at room temperature and reprobed as indicated. To verify the accuracy of sample loading, selected blots were reprobed with a monoclonal antibody to β-tubulin (1:20,000; Sigma). A single band was observed at 55 kDa, and the intensity of the signal was similar in all lanes. Relative optical density readings for the ΔfosB and β-tubulin bands were determined by using a computer-assisted densitometry program (NIH image 1.63 software) from three independent sample pairs. The optical density of components corresponding to the 35- to 37-kDa ΔfosB protein was normalized with the optical density of β-tubulin-specific bands for each sample by dividing the optical density obtained from ΔfosB-specific bands by the optical density obtained from β-tubulin-specific bands for each animal.
Behavior. For all tasks, we used adult male mutant mice and control littermates. Statistical analyses by ANOVAs and means ± SEM are presented in Fig. 5. Experimenters were blind to the genotype in all studies. Motor activity in an open field was quantified in four Plexiglas open field boxes 43 × 43 cm2 (MED Associates, Georgia, VT). Two sets of 16 pulse-modulated infrared photobeams were placed on opposite walls 2.5 cm apart to record ambulatory movements. Activity chambers were computer interfaced for data sampling at 100-ms resolution. The computer-defined grid lines that divided each open field into center and surround regions, with each of four lines being 11 cm from each wall. Overall motor activity was quantified as the total distance traveled (m) over the test session. In a behavioral sensitization paradigm, mice were divided into four groups (two groups of wild-type and two of mutants), and their activities were measured for 1 h after cocaine or saline injection. For the first 3 days, mice (+/+, n = 14; +/-, n = 13) were placed in the chambers immediately after saline injections. From the fourth to ninth day, mice were given either saline or cocaine and were placed in the chambers [cocaine: 30 mg/kg: +/+, n = 7; +/-, n = 7; vehicle (saline): +/+, n = 7; +/-, n = 6]. On day 16, all of the mice received saline injections and were placed in the test chambers, and their locomotor activity was measured for 1 h.
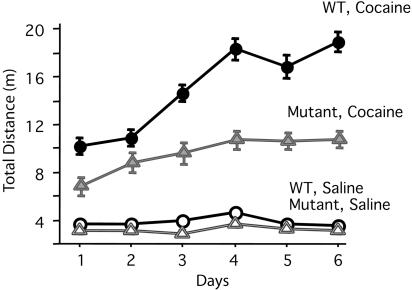
Fig. 5.
CBP haploinsufficient mice show decreased sensitivity to cocaine. Sensitivity assessed by locomotor activity: total (±SEM) distance traveled (in meters) of wild-type and CBP haploinsufficient mice during six consecutive days of cocaine injection [cocaine: 30 mg/kg: +/+, n = 7; +/-, n = 7; vehicle (saline): +/+, n = 7; +/-, n = 6]. There was no difference in activity between mutant and wild-type groups that were injected with saline. Activity was greater in wild-type than in cocaine-injected CBP haploinsufficient mice (P < 0.05 for all days), whereas there was no difference between the wild-type and mutant controls.
Results
Cocaine Induces fosB, Recruits CBP, and Causes Histone Acetylation. FosB is induced in the rodent striatum by cocaine administration (26, 27). We confirmed this observation by using real-time RT-PCR and found that, 2 h after injecting 30 mg/kg cocaine, the fosB gene was induced ≈8-fold in the striatum of wild-type C57BL/6J mice (Fig. 1A).
Does cocaine cause chromatin remodeling at the promoters of genes that have been implicated in addiction? To address this question, we adapted the ChIP assay to mouse brain (see ref. 3). Our working hypothesis was that chromatin remodeling, which underlies memory storage in Aplysia, is likely to be important also for the formation of stable addictive behaviors. The fosB gene was examined because the expression of its truncated splice variant ΔfosB has a robust effect on sensitivity to cocaine (15). Activation of the fosB promoter depends on the cAMP-dependent PKA phosphorylation of CREB (28). CREB in turn recruits CBP to induce immediate early genes. CBP is a histone acetyltransferase (HAT) that activates transcription by acetylating specific lysine residues in histones of nucleosomes, thereby opening up repressive chromatin structures (8, 23). Using the ChIP assay, we found an increase in the recruitment of CBP to the fosB promoter and increased acetylation of histone H4 20 min after injecting cocaine (Fig. 1 B and C). The increase in acetylation was selective: both the acetylation at actin's promoter and transcription of the actin gene were unaffected by cocaine (Fig. 1 A and C).
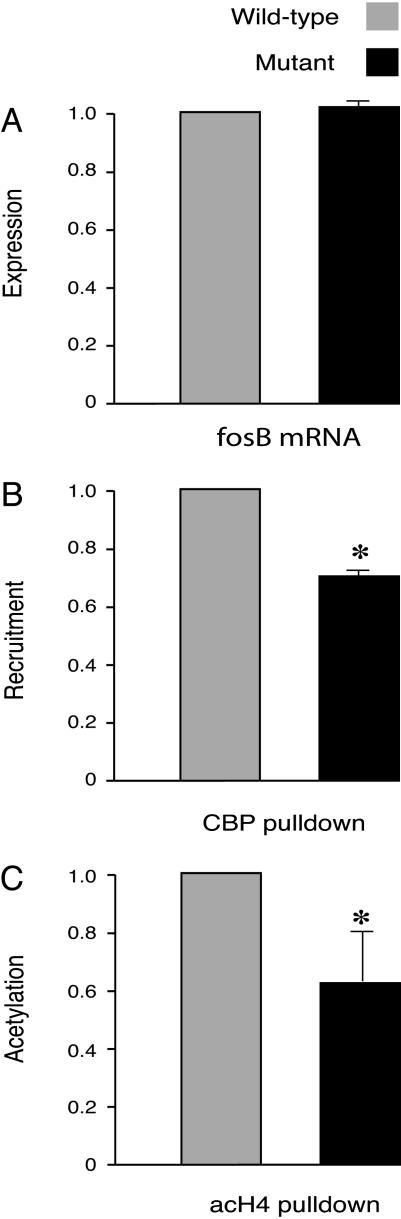
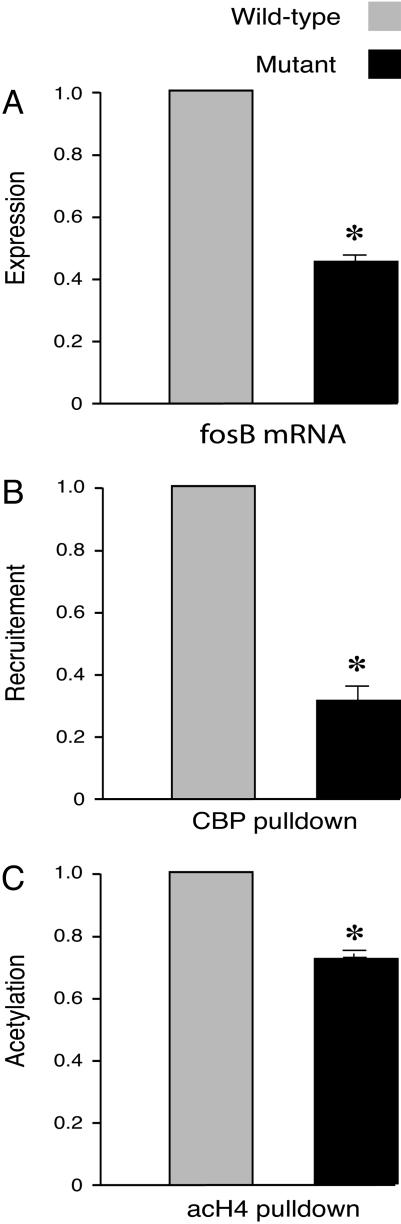
We next examined CBP haploinsufficient mice. This mutant carries only one functional cbp allele. The expression of fosB is similar in mutant and wild type at baseline, even though the recruitment of CBP to the fosB promoter and histone acetylation are decreased in the mutant (Fig. 2). We next asked how cocaine affects the recruitment of CBP, histone acetylation, and fosB expression. After a cocaine challenge, CBP and histone H4 acetylation were reduced at the fosB promoter of the mutant mouse compared to that in the wild type. This reduction of CBP recruitment and histone acetylation correlates with the reduction in fosB expression in the mutant (Fig. 3).
Fig. 2.
Basal recruitment of CBP and histone H4 acetylation at the fosB promoter in CBP haploinsufficient and in wild-type mice. (A) The basal expression of fosB in the mutant and wild-type were equivalent (n = 3 pairs; wild type set at 1; mutant, 1.061 ± 0.041; P > 0.05). Note that the values are set relative to those of the wild-type. The fosB expression was 4- to 8-fold less than that observed after treatment with cocaine (also see Fig. 1A). (B) CBP recruitment was less in the mutant (n = 5 in each group, wild type set at 1; mutant, 0.68 ± 0.06; P < 0.01; input DNA was used as control). (C) Basal histone H4 acetylation at the fosB promoter in the mutant also was less than that in the wild type (n = 5 in each group; wild type set at1; mutant, 0.63 ± 0.17; P < 0.05; input DNA was used as control).
Fig. 3.
Cocaine-induced CBP recruitment and histone H4 acetylation are decreased at the fosB promoter, resulting in decreased fosB expression in CBP haploinsufficient mice. (A) Cocaine was less effective in inducing fosB in the mutant mice. Values were from the mice 2 h after injection of 30 mg/kg cocaine i.p. (n = 3 mice pairs, control, 1; cocaine, 0.448 ± 0.033; P < 0.01). (B and C) ChIP assays show that, 20 min after 30 mg/kg cocaine injection, there is a decrease in CBP recruitment to the fosB promoter in mutant mouse striatum compared to wild type (n = 3 in each group, wild type set at 1, mutant 0.31; P < 0.05, input DNA as control) (B) and a decrease in acetylated histone H4 at the fosB promoter (n = 3 in each group; wild type set at 1; mutant 0.67; P = 0.014, input DNA as control) (C). Data are expressed as mean ± SEM.
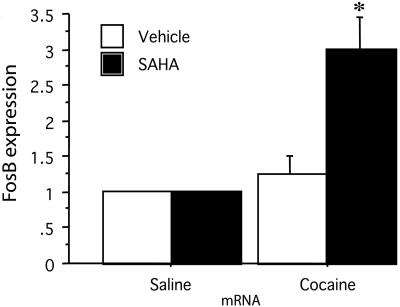
Suberoylanilide Hydroxamic Acid (SAHA), a Histone Deacetylase Inhibitor, Enhances fosB Expression in C57BL/6J Mice Striatum After Cocaine Treatment. To examine whether histone acetylation plays a role in the induction of gene expression by cocaine, we used a potent and specific histone deacetylase inhibitor, SAHA, that we and others have found to pass the blood brain barrier and to exert its effect on the brain (29). We find that SAHA caused a 2-fold increase in the expression of fosB after cocaine treatment. SAHA administration did not affect the expression of fosB in the absence of cocaine, suggesting that histone acetylation plays an important role in the gene expression induced by cocaine. Thus, by inhibiting the deacetylating process, which counteracts the CBP's histone acetyltransferase activity, SAHA causes an increase in fosB expression (Fig. 4).
Fig. 4.
Expression of fosB in the striatum of mice treated with a histone deacetylase inhibitor is enhanced after cocaine treatment. Real-time PCR shows a 3-fold increase in fosB expression after cocaine injection in mice that were pretreated with SAHA. Treatment with SAHA without cocaine administration did not change fosB expression. C57BL/6J were injected with SAHA (25 mg/kg) or vehicle (DMSO) 2 h before receiving cocaine (20 mg/kg). Mice were killed 2 h after the injection of cocaine. n = 18; control set at 1; DMSO and cocaine, 1.25 ± 0.001; SAHA plus cocaine, 3 ± 0.002; *, P < 0.05.
Mutant Mice Accumulate Less ΔfosB After Chronic Cocaine Administration. Drug addiction is thought to depend on the buildup of ΔfosB protein that takes place during chronic administration of cocaine (15). Before we injected the drug, the amount of ΔfosB protein was undetectable in both CBP haploinsufficient and wild-type mice. After six daily injections, we also failed to detect any ΔfosB. By 10 days, however, ΔfosB immunoblotting revealed that the mutants have relative optical density (ROD) of 0.72 ± 0.059, and wild-type ROD was 1.476 ± 0.069 (n = 3 pairs; P < 0.05 Student t test).
The Response to Cocaine Is Reduced in CBP Haploinsufficient Mice. We next examined the response to cocaine in a behavioral sensitization paradigm, which is a widely used index of addictive behavior in rodents (30-32). For the first 3 days, all mice received saline i.p., and no differences between wild-type and mutants were observed (P < 0.05). Next, mice were divided into four groups and injected with either saline or cocaine each day for 6 days. We found that the response to cocaine was reduced in mutant mice. In contrast, wild-type littermates showed a typical pattern of enhanced sensitization to cocaine with the expected increase in locomotor activity (Fig. 5).
Discussion
Many drugs of abuse act by increasing dopamine in the striatum (33, 34). Dopamine then stimulates the synthesis of cAMP through D1 receptors, and the cAMP activates PKA. The kinase phosphorylates CREB, a constitutive transcription factor. CREB is resident at CRE-containing promoter sites. Upon phosphorylation, CREB recruits CBP to form the active promoter complex, and the fosB gene is induced. Using ChIP assays, we show directly that cocaine administration leads to the induction of the fosB gene by recruiting CBP. The binding protein then acetylates histones, modifying chromatin and resulting in the decondensation of DNA. The accumulation of a splice variant, ΔfosB, has been shown to be important for the formation of addictive behavior in rodents (14). We found that the CBP haploinsufficient mutants, in response to cocaine, accumulate less ΔfosB protein in the striatum than do wild-type mice. This difference correlates with the decrease in CBP recruitment and histone acetylation at the fosB promoter.
The accumulation of ΔfosB has been shown to be important in the formation of addictive behaviors in rodents (35). Because levels of fosB and ΔfosB expression are the same in both wild-type and CBP haploinsufficient mice before a cocaine challenge, the mutant is better suited for studying the effect of the fosB gene on the response to cocaine than the two other mice mutant strains that were used in the past [mice that overexpress ΔfosB (15), and fosB knockout mice (16)]. In these two strains, expression of fosB and ΔfosB was altered before cocaine administration. One strain had increased ΔfosB protein before cocaine administration and the other had diminished fosB expression at baseline. Both strains showed increased sensitivity to cocaine. These results appear to be paradoxical because, on the one hand, the mutant that overexpressed ΔfosB was sensitive to cocaine, and on the other, the knockout mutant with no fosB expression and no ΔfosB protein was also more sensitive. With CBP mutant mice, we confirmed that, when starting with normal fosB expression levels before cocaine was administered, a decrease in fosB expression in response to cocaine (influenced by the lack of CBP) correlates with decreased sensitivity to cocaine. However, it remains unclear why fosB mutant mice exhibit increased sensitization in the absence of fosB expression.
Alarcon et al. (23) studied deficits in long-term memory in CBP haploinsufficient mice. These mice phenocopy a well described syndrome in humans: the Rubinstein-Taybi syndrome. Long-term memory and long-term potentiation, both of which are impaired in these mutants, can be significantly improved by pharmacological compensation for the lack of CBP with histone deacetylase (HDAC) inhibitors. This finding suggests that these deficits are due to the requirement of CBP throughout life and not to the consequences of altered brain development leading to permanent anatomical abnormalities. It is possible that the altered sensitization that we observed in mutant mice may also be pharmacologically reversed as with the memory deficits (23).
The increase in ΔfosB expression induced by the cAMP-dependent phosphorylation of CREB and the recruitment of CBP is a common pathway in the formation of several different addictive behaviors caused by many drugs and situations (36). Although CREB-mediated gene expression has been examined in higher animals (37, 38), in Aplysia the connection between molecular events and behavior are clearer. In Aplysia, treatment with serotonin (5-HT) results in the recruitment of CREB1 and CBP to the C/EBP promoter and acetylation of histones inducing downstream effector genes needed for producing long-term facilitation (3). The facilitation is blocked by the inhibitory neuropeptide FMRFamide, which acts through p38 mitogen-activated protein kinase phosphorylation of the inhibitory transcription factor CREB2 (39). CREB2 displaces CREB1 and recruits HDAC5 to the C/EBP promoter, blocking the expression of effector genes and leading to long-term depression. Two avenues of pharmacological intervention seem promising: agents that result in blocking the recruitment of CBP (as, for example, FMRFamide in Aplysia) and drugs that cause deacetylation of the fosB gene. These agents may not only prevent the formation of new behaviors, but may also reverse downstream synaptic changes that are induced by previously learned behavior. It is not clear why agents that are expected to act at many sites of gene induction might target the fosB gene specifically. Nevertheless, recent clinical trials with chromatin modifying drugs have produced specific therapeutic affects despite their ability to influence many areas of the genome (40, 41).
Acknowledgments
We thank Rene Hen, Stephanie Dulawa, Lee Zuckerman, Jinmin Liu, Alejandro I. Hernandez, and Jason Wolk for help with this paper. This work was supported by National Institute of Mental Health Grants MH15174 (to J.H.S.) and MH60387 (to J.H.S.), and National Institute of Neurological Disorders and Stroke Grants MH048850 and NS29255 (to J.H.S.).
Author contributions: A.A.L., Z.G., E.R.K., and J.H.S. designed research; A.A.L., Z.G., and S.X. performed research; A.B. contributed new reagents/analytic tools; A.A.L. and Z.G. analyzed data; and A.A.L. and Z.G. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: CREB, cAMP-response element-binding protein; CBP, CREB-binding protein; ChIP, chromatin immunoprecipitation; SAHA, suberoylanilide hydroxamic acid.
References
- 1.Kandel, E. R. & Schwartz, J. H. (1982) Science 218, 433-443. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch, D., Casadio, A., Karl, K. A., Serodio, P. & Kandel, E. R. (1998) Cell 95, 211-223. [DOI] [PubMed] [Google Scholar]
- 3.Guan, Z., Giustetto, M., Lomvardas, S., Kim, J. H., Miniaci, M. C., Schwartz, J. H., Thanos, D. & Kandel, E. R. (2002) Cell 111, 483-493. [DOI] [PubMed] [Google Scholar]
- 4.Alberini, C. M., Ghirardi, M., Metz, R. & Kandel, E. R. (1994) Cell 76, 1099-1114. [DOI] [PubMed] [Google Scholar]
- 5.Hegde, A. N., Inokuchi, K., Pei, W., Casadio, A., Ghirardi, M., Chain, D. G., Martin, K. C., Kandel, E. R. & Schwartz, J. H. (1997) Cell 89, 115-126. [DOI] [PubMed] [Google Scholar]
- 6.Giustetto, M., Hegde, A. N., Si, K., Casadio, A., Inokuchi, K., Pei, W., Kandel, E. R. & Schwartz, J. H. (2003) Proc. Natl. Acad. Sci. USA 100, 13680-13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu, J. & Schwartz, J. H. (2003) Brain Res 959, 68-76. [DOI] [PubMed] [Google Scholar]
- 8.Chrivia, J. C., Kwok, R. P., Lamb, N., Hagiwara, M., Montminy, M. R. & Goodman, R. H. (1993) Nature 365, 855-859. [DOI] [PubMed] [Google Scholar]
- 9.Kumar, A., Choi, K. H., Renthal, W., Tsankova, N. M., Theobald, D. E., Truong, H. T., Russo, S. J., Laplant, Q., Sasaki, T. S., Whistler, K., et al. (2005) Neuron 48, 303-314. [DOI] [PubMed] [Google Scholar]
- 10.Nestler, E. J. (2004) Trends Pharmacol. Sci. 25, 210-218. [DOI] [PubMed] [Google Scholar]
- 11.Terwilliger, R. Z., Beitner-Johnson, D., Sevarino, K. A., Crain, S. M. & Nestler, E. J. (1991) Brain Res. 548, 100-110. [DOI] [PubMed] [Google Scholar]
- 12.Kano, T., Suzuki, Y., Shibuya, M., Kiuchi, K. & Hagiwara, M. (1995) NeuroReport 6, 2197-2200. [DOI] [PubMed] [Google Scholar]
- 13.Carlezon, W. A., Jr., Thome, J., Olson, V. G., Lane-Ladd, S. B., Brodkin, E. S., Hiroi, N., Duman, R. S., Neve, R. L. & Nestler, E. J. (1998) Science 282, 2272-2275. [DOI] [PubMed] [Google Scholar]
- 14.Walters, C. L. & Blendy, J. A. (2001) J. Neurosci. 21, 9438-9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelz, M. B., Chen, J., Carlezon, W. A., Jr., Whisler, K., Gilden, L., Beckmann, A. M., Steffen, C., Zhang, Y. J., Marotti, L., Self, D. W., et al. (1999) Nature 401, 272-276. [DOI] [PubMed] [Google Scholar]
- 16.Hiroi, N., Brown, J. R., Haile, C. N., Ye, H., Greenberg, M. E. & Nestler, E. J. (1997) Proc. Natl. Acad. Sci. USA 94, 10397-10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrij, F., Giles, R. H., Dauwerse, H. G., Saris, J. J., Hennekam, R. C., Masuno, M., Tommerup, N., van Ommen, G. J., Goodman, R. H., Peters, D. J., et al. (1995) Nature 376, 348-351. [DOI] [PubMed] [Google Scholar]
- 18.Goodman, R. H. & Smolik, S. (2000) Genes Dev. 14, 1553-1577. [PubMed] [Google Scholar]
- 19.Hardingham, G. E., Chawla, S., Cruzalegui, F. H. & Bading, H. (1999) Neuron 22, 789-798. [DOI] [PubMed] [Google Scholar]
- 20.Hu, S. C., Chrivia, J. & Ghosh, A. (1999) Neuron 22, 799-808. [DOI] [PubMed] [Google Scholar]
- 21.Impey, S., Fong, A. L., Wang, Y., Cardinaux. J. R., Fass, D. M., Obrietan, K., Wayman, G. A., Storm, D. R., Soderling, T. R. & Goodman, R. H. (2002) Neuron 34, 235-244. [DOI] [PubMed] [Google Scholar]
- 22.Oike, Y., Hata, A., Mamiya, T., Kaname, T., Noda, Y., Suzuki, M., Yasue, H., Nabeshima, T., Araki, K. & Yamamura, K. (1999) Hum. Mol. Genet. 8, 387-396. [DOI] [PubMed] [Google Scholar]
- 23.Alarcon, J. M., Malleret, G., Touzani, K., Vronskaya, S., Ishii, S., Kandel, E. R. & Barco, A. (2004) Neuron 42, 947-959. [DOI] [PubMed] [Google Scholar]
- 24.Korzus, E., Rosenfeld, M. G. & Mayford, M. (2004) Neuron 42, 961-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka, Y., Naruse, I., Maekawa, T., Masuya, H., Shiroishi, T. & Ishii, S. (1997) Proc. Natl. Acad. Sci. USA 94, 10215-10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hope, B., Kosofsky, B., Hyman, S. E. & Nestler, E. J. (1992) Proc. Natl. Acad. Sci. USA 89, 5764-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, D., Zhang, L., Lou, D. W., Nakabeppu, Y., Zhang, J. & Xu, M. (2002) J. Neurochem. 82, 1453-1464. [DOI] [PubMed] [Google Scholar]
- 28.Inoue, D., Kido, S. & Matsumoto, T. (2004) J. Biol. Chem. 279, 49795-49803. [DOI] [PubMed] [Google Scholar]
- 29.Hockly, E., Richon, V. M., Woodman, B., Smith, D. L., Zhou, X., Rosa, E., Sathasivam, K., Ghazi-Noori, S., Mahal, A., Lowden, P. A., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 2041-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson, T. E. & Berridge, K. C. (1993) Brain Res. Brain Res. Rev. 18, 247-291. [DOI] [PubMed] [Google Scholar]
- 31.Izenwasse, S., French, D., Carroll, F. I. & Kunko, P. M. (1999) Behav. Brain Res. 99, 201-208. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas, P. W. & Duffy, P. (1993) J. Neurosci. 13, 266-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyman, S. E. & Malenka, R. C. (2001) Nat. Rev. Neurosci. 2, 695-703. [DOI] [PubMed] [Google Scholar]
- 34.Kalivas, P. W. & Volkow, N. D. (2005) Am. J. Psychiatry 162, 1403-1413. [DOI] [PubMed] [Google Scholar]
- 35.Chao, J. & Nestler, E. J. (2004) Annu. Rev. Med. 55, 113-132. [DOI] [PubMed] [Google Scholar]
- 36.Impey, S., Smith, D. M., Obrietan, K. Donahue, R., Wade, C. & Storm, D. R. (1998) Nat. Neurosci. 1, 595-601. [DOI] [PubMed] [Google Scholar]
- 37.Barrot, M., Olivier, J. D., Perrotti, L. I., DiLeone, R. J., Berton, O., Eisch, A. J., Impey, S., Storm, D. R., Neve, R. L., Yin, J. C., et. al. (2002) Proc. Natl. Acad. Sci. USA 99, 11435-11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nestler, E. J., Barrot, M., DiLeone, R. J., Eisch, A. J., Gold, S. J. & Monteggia, L. M. (2002) Neuron 34, 13-25. [DOI] [PubMed] [Google Scholar]
- 39.Guan, Z., Kim, J. H., Lomvardas, S., Holick, K., Xu, S., Kandel, E. R. & Schwartz, J. H. (2003) J. Neurosci. 23, 7317-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly, W. K. & Marks, P. A. (2005) Nat. Clin. Pract. Oncol. 2, 150-157. [DOI] [PubMed] [Google Scholar]
- 41.Lehrman, G., Hogue, I. B., Palmer, S., Jennings, C., Spina, C. A., Wiegand, A., Landay, A. L., Coombs, R. W., Richman, D. D., Mellors, J. W., et. al. (2005) Lancet 366, 549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]