Abstract
Repeated induction of pre- and postsynaptic action potentials (APs) at a fixed time difference leads to long-term potentiation (LTP) or long-term depression (LTD) of the synapse, depending on the temporal order of pre- and postsynaptic activity. This phenomenon of spike-timing-dependent plasticity (STDP) is believed to arise by nonlinear processes that lead to larger calcium transients (and thus LTP) when presynaptic APs precede postsynaptic APs and smaller calcium transients (and thus LTD) when postsynaptic APs precede presynaptic APs. In contrast to predictions from such calcium-peak-detector models, we show that constitutively or artificially broadened APs in layer II/III pyramidal cells of entorhinal cortex (EC) lead to an increase in the dendritic calcium transient and shift the balance of STDP toward LTD. STDP in entorhinal pyramidal cells is NMDA-receptor-dependent and modulated by the CaV1Ca2+ channel-blocker nifedipine. Results are consistent with an elaboration of the calcium-peak-detector model in which downstream signals from voltage-dependent Ca2+ channels suppress LTP relative to LTD. Our results suggest that modulation of AP width is a potent way to adjust the rules of synaptic plasticity in the EC.
Keywords: spike-timing-dependent plasticity, NMDA receptors, nifedipine, calcium imaging, entorhinal cortex
The temporal order of repeated pairing of pre- and postsynaptic action potentials (APs) plays a key role in determining the direction of synaptic plasticity (1–11). This phenomenon of spike-timing-dependent plasticity (STDP) has been shown to be Ca2+-dependent (1, 3, 9). Although the details of the underlying Ca2+ mechanisms of STDP are not fully understood, it is believed to arise via a calcium-peak-detector model (3, 9, 12–14). In this model, long-term depression (LTD) is induced when presynaptic APs lag postsynaptic APs, generating small dendritic Ca2+ transients; and long-term potentiation (LTP) is induced when presynaptic APs precede postsynaptic APs, generating larger Ca2+ transients (15).
A key prediction of the peak-detector model is that broader dendritic APs should enhance timing-dependent LTP by increasing the Ca2+ flux through NMDA receptors and postsynaptic voltage-gated Ca2+ channels. We tested this prediction in layer II/III pyramidal cells of the rat entorhinal cortex (EC), where classical LTP and LTD have been observed in response to periodic stimulation of input fibers (16). We confirmed that EC cells exhibit STDP and found that constitutively or artificially broadened postsynaptic APs lead to an increase in the dendritic calcium transient but shift the balance of STDP toward LTD, in contrast to what one would predict from the peak-detector model. STDP is NMDA receptor-dependent and modulated by the CaV1 Ca2+ channel-blocker nifedipine. Our results are consistent with an elaboration of the calcium peak-detector model in which downstream signals from voltage-dependent Ca2+ channels suppress LTP, making LTD more prominent.
Materials and Methods
Slice Preparation. All protocols were approved by the Boston University Animal Care and Use Committee. Brain slices of EC were prepared from Long–Evans rats (14–19 days old) as described in ref. 17. Rats were anesthetized with isoflurane, and the brain was removed after decapitation. The EC and hippocampus were dissected rapidly and transferred to a chamber filled with ice-cold oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (126 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgCl2, 26 mM NaHCO3, 25 mM dextrose, and 2 mM CaCl2). Transverse slices of EC (300 μm) were cut with a tissue slicer (Vibratome 1000 Plus, Ted Pella, Inc., Redding, CA) and incubated at room temperature for at least 1 h before being transferred to the recording chamber at 34–36°C.
Electrophysiological Recording. Whole-cell recordings of layer II/III pyramidal neurons were performed on brain slices of EC. Layer II/III pyramidal neurons were identified under infrared differential interference contrast optics based on their morphology and patched with 4- to 6-MΩ glass pipettes filled with artificial intracellular fluid (120 mM potassium gluconate, 10 mM KCl, 10 mM Hepes, 4 mM Mg-ATP, 0.3 mM Tris·GTP, 10 mM sodium phosphocreatine, and 20 units/ml creatine kinase). Pipettes were connected to the headstage of an AxoClamp 2B amplifier (Axon Instruments, Foster City, CA), and the bridge mode was used to obtain current-clamp recordings. An extracellular glass stimulating electrode filled with artificial cerebrospinal fluid was placed at the same layer of the pyramidal cell 100–200 μm away from the recording site. Presynaptic axons were stimulated by using current-pulse stimuli [duration = 150 μs, amplitude = 5–20 μA, frequency = 0.1 Hz for measurement of excitatory postsynaptic potentials (EPSPs) before and after conditioning] delivered by a constant-current stimulator. Because STDP typically requires cooperative inputs (10), we adjusted the stimulation intensity to elicit EPSPs with amplitudes >1.5 mV. A glass pipette (tip diameter 20 μm) filled with 5 mM bicuculline was placed near the soma of the pyramidal neuron being recorded to reduce local inhibition without inducing epileptiform activity (5). In a handful of cases, we bath-applied bicuculline (5 μM) instead. To induce STDP, we paired EPSPs with postsynaptic spikes for 50 repetitions (5, 10) at 0.1–1 Hz. Postsynaptic APs were induced by using periodic current-pulses (duration = 5 ms) injected via the recording electrode into the soma. After 50 pairs, test stimuli were delivered to the presynaptic axons at 0.1 Hz in the absence of postsynaptic spikes for at least 30 min. To measure STDP, we compared the maximal rising slope of the evoked EPSP, recorded between the end of the stimulus artifact and the peak of the EPSP, before and after the conditioning pairs. Results were the same when we examined changes in EPSP amplitude (data not shown). Spike half-width was defined as a spike's duration at half-spike amplitude, measured from threshold voltage (defined as the voltage at which the spike induced an inflection in the trace) to peak. Reported half-widths are from isolated spikes (i.e., spikes evoked without accompanying EPSPs).
Input resistance, resting membrane potential, and spike half-width were monitored throughout the recording session to ensure stability of recordings. Except in cases in which we purposefully modulated spike width, data were discarded when any of these measures changed by more than 10% during the recording session. Data are reported statistically as mean ± SEM.
Optical Imaging. Neurons were loaded intracellularly for 1 h with intracellular fluid containing 20 μM calcium green-1, hexapotassium salt (KD = 190 nM; Molecular Probes). Neurons were imaged by using an Axioskop 2FS+ microscope (Zeiss) equipped with a 150-W arc-lamp from Opti-Quip (Highland Mills, NY) and an epifluorescence illumination cube (exciter = 480/40 nm, dichroic = 505 nm, and emitter = 535/50 nm; Chroma Technology, Rockingham, VT) and imaged with a NeuroCCD-256-SM charge-coupled device camera (Red Shirt Imaging, Fairfield, CT). The region of interest (ROI) was selected around the apical dendrite of the cell. Before the experiment, we verified that the baseline fluorescence within the ROI had stabilized, and the change in fluorescence from each AP fluctuated by <5% from trial to trial. The protocol for the experiment consisted of taking sets of five trials containing 70 frames recorded at 25–100 Hz while simultaneously recording the electrical activity of the cell at 6.4 kHz. The neuron was stimulated at the onset of the 20th frame with a pulse of current (duration = 10 ms, amplitude = 2–5 nA). The calcium signal was measured by subtracting the average baseline ΔF/F from the peak ΔF/F observed in a 50-ms window after the AP. Trials were taken every 3 seconds, and a set of five trials was taken every minute. A baseline of three to five sets was established before 10 mM tetraethylammonium (TEA) was washed in for 3 min and then washed off. In TEA, cells occasionally fired bursts or did not fire; only trials in which a single AP was recorded were included in the results. Results were corrected for photobleaching by detrending using an exponential fit to the baseline and wash-out data. Results were normalized by the baseline response.
Modeling. In the proposed model, described in detail in Supporting Text, which is published as supporting information on the PNAS web site, and shown in cartoon form in Fig. 3c, simulated NMDA receptors included glutamate-mediated activation and instantaneous magnesium block (18). Postsynaptic APs were modeled as biexponential waveforms that matched dendritic recordings (19); the decay-time constant was adjusted to match APs of normal and broad half-width. The LTD signal was linearly related to the peak Ca2+ waveform from NMDA receptors; the LTP signal was generated by passing the peak Ca2+ concentration through a quadratic nonlinearity. The Ca2+ signal from voltage-dependent Ca2+ channels (VDCCs) suppressed the input signal from NMDA receptors to the LTP pathway. This suppression signal was a biexponential signal with a delay of 0.5 ms, a rising-time constant of 2 ms, and a decay-time constant of 20 ms. Although our implementation was generated under the assumption that changes in dendritic-spike width mirror those measured in the soma, the only critical feature is that dendritic Ca2+ transients covary with somatic AP width (Fig. 3a).
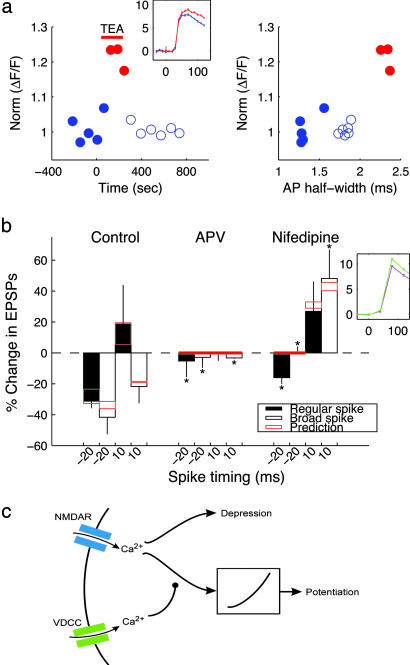
Fig. 3.
AP-induced Ca2+ transients modulate STDP. (a)Ca2+ transients covary with AP half-width. (Left) Average values of normalized ΔF/F(n = 5 trials for each point). Wash-in of 10 mM TEA (red bar) increased ΔF/F by 20% (red data points). (Inset) Two averaged traces (n =5 trials, mean ± SEM) recorded under control conditions (blue) and in 10 mM TEA (red). Units: y axis, ΔF/F (%); x axis, ms. (Right) ΔF/F vs. AP half-width from the same data set. The relationship is approximately linear (R = 0.78). (b) STDP is NMDA-dependent and modulated by CaV1 Ca2+ channels. Bar graphs (mean ± SD, n = 3–13) show changes in EPSPs vs. spike timing ≈-20 and +10 ms. Two-amino-5-phosphonovalerate (APV) (30 μM) blocked STDP under both regular and broad AP conditions. Nifedipine (10 μM) tilted the balance of STDP toward LTP. Red boxes indicate predictions based on the model from c for the range of spike timing recorded in each case. (Inset) Nifedipine suppresses Ca2+ transients. Two averaged traces (n =5 trials, mean ± SEM) recorded under control conditions (green) and in 10 mM nifedipine (magenta). Units: y axis, ΔF/F (%); x axis, ms. Statistical significance vs. control: *, P < 0.05, Wilcoxon rank-sum test. (c) A proposed biophysical model accounts for the effects of spike width, APV, and nifedipine on STDP. Predictions from the model are shown in Fig. 2b (solid and dashed lines) and b (this figure) (red bars).
Results
STDP Depends on Spike Width in Layer II/III Pyramidal Neurons of the Rat EC. In brain slices of the EC, we recorded from layer II/III pyramidal cells. To test whether the timing of pre- and postsynaptic activation contributes to the direction of synaptic modification in the superficial layer of EC, we paired pre- and postsynaptic APs at a given time difference. When the onset of EPSPs preceded the peak of postsynaptic APs (positive spike timing), we typically observed a persistent enhancement of the slope of EPSPs after pairing (Fig. 1a; EPSP slope in this example before pairing, 0.77 ± 0.024 V/s; after pairing, 1.09 ± 0.023 V/s; P < 0.001, Wilcoxon rank-sum test). In contrast, pairing with negative spike timing typically led to long-lasting depression of the slope of EPSPs (Fig. 1b; EPSP slope in this example before pairing, 1.77 ± 0.034 V/s; after pairing, 1.30 ± 0.020 V/s; P < 0.001, Wilcoxon rank-sum test). The relationship between synaptic modifications and the relative timing of pre- and postsynaptic activation under these conditions is shown in Fig. 2b (blue symbols). The STDP profile is generally very similar to those seen in other cortical areas (5, 10).
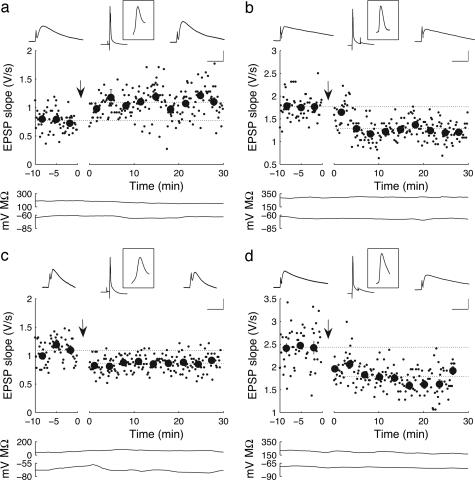
Fig. 1.
STDP depends on spike width in layer II/III pyramidal neurons of the rat EC. (a) LTP is induced when EPSPs and postsynaptic APs are paired with positive timing (+10 ms) in a cell with AP half-width of 1.3 ms. Small data points show the maximum rising slope of EPSPs elicited by test stimuli (0.1 Hz); large data points are the mean ± SD of groups of 20 measurements. Traces above represent average (n = 20) EPSPs recorded 5 min before pairing, average AP-EPSP pairs recorded during pairing, and average EPSPs recorded 20 min after pairing. (Inset Top) The average spike paired with the EPSPs. (Bottom) Traces show the input resistance and resting potential. [Scale bar =1.2 mV, 30 ms (EPSPs); 22 mV, 75 ms (pairing); 22 mV, 3.7 ms (Inset).] (b) LTD is induced with negative timing (-30 ms) in a cell with spike half-width of 1.2 ms. (Scale bar as in a, except that the scale bar for EPSPs is 3 mV.) (c) LTD is induced in a cell with broad spikes (1.7 ms), even though APs and synaptic inputs were paired with positive timing (+20 ms). (Scale bar as in a.) (d) LTD is induced by pairing with negative timing (-20 ms) in a cell with spike half-width of 1.6 ms. (Scale bar as in a, except that the scale bar for EPSPs is 4 mV.)
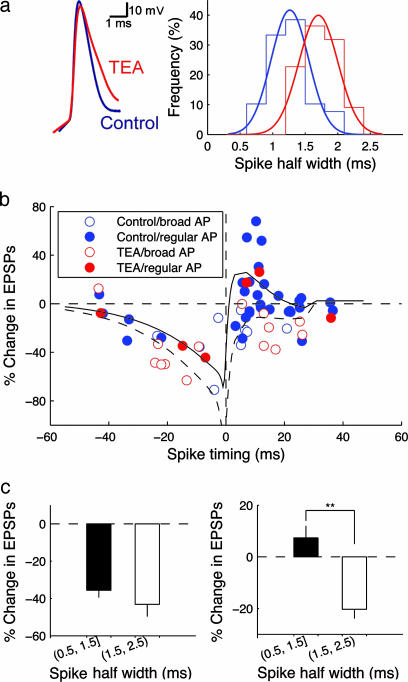
Fig. 2.
Broad postsynaptic APs favor LTD induction. (a) Intracellular TEA (10 mM) broadens APs in layer II/III pyramidal cells. (Left) Representative traces of control and TEA-broadened spikes. (Right) distributions of spike half-widths under control (blue) and TEA-broadened (red) conditions. (b) The form of STDP depends on AP width; changes in EPSP slope are plotted vs. spike timing. Symbol color and type indicate the pharmacological condition and AP half-width (regular, <1.5 ms; broad, >1.5 ms). Solid and dashed lines show results from the model in Fig. 3c for regular and broad APs, respectively. (c) STDP is significantly different between broad and regular spikes paired with preceding EPSPs. (Left) Negative time intervals (-30 to 0 ms); broad spikes induce an insignificant increase in LTD (regular spike, -35.6 ± 3.9%, n = 4; broad spike, -43.2 ± 6.5%, n = 9; P > 0.05, Wilcoxon rank-sum test). (Right) Positive timing (0–30 ms) broad spikes cause a significant change in STDP (regular spike, 7.36 ± 4.6%, n = 27; broad spike, -20.3 ± 3.6%, n = 12; **, P < 0.01, Wilcoxon rank-sum test).
Surprisingly, there is a correlation between the direction of synaptic modification and the half-width of the postsynaptic AP measured at the soma (Fig. 1). For cases in which the half-width of the AP exceeded 1.5 ms (1.75 ± 0.12 ms, n = 4), pairing with positive timing led to LTD instead of LTP in 4 of 4 cases (e.g., Fig. 1c; EPSP slope in this example before pairing, 1.09 ± 0.026 V/s; after pairing, 0.86 ± 0.011 V/s; P < 0.001, Wilcoxon rank-sum test). For cases with AP half-width <1.5 ms (1.18 ± 0.044 ms, n = 19), positive timing induced clear LTD in only 3 of 19 cases. Pairing in the negative timing window gave rise to LTD for cells with both broad APs (3 of 3 cases, e.g., Fig. 1d; EPSP slope in this example before pairing, 2.13 ± 0.12 V/s; after pairing,1.79 ± 0.028 V/s; P < 0.001, Wilcoxon rank-sum test) or regular APs (5 of 6 cases). Broad and regular-width spikes did not differ in amplitude (for regular-width spikes, 68.7 ± 2.3 mV, n = 19; for broad spikes, 69.5 ± 2.6 mV, n = 4).
To examine the relationship between spike width and STDP in a larger number of cases, we broadened APs by using intracellular TEA (10 mM), which blocks K+ channels intracellularly or extracellularly with similar affinity (20). On average, intracellular TEA broadened AP half-width by 50% (Fig. 2a). Over the population of recordings in control conditions and in TEA, LTP can be induced only when the half-width of APs is <1.5 ms (filled red and blue symbols, Fig. 2b). For cases with AP half-width >1.5 ms (open red and blue symbols, Fig. 2b), the STDP curve is more dominated by LTD for both negative and positive timing. The difference in STDP outcomes based on spike width is statistically highly significant (P < 0.01, Wilcoxon rank-sum test; Fig. 2c).
AP-Induced Ca2+ Transients Modulate STDP. The fact that broader somatic spikes lead to enhanced LTD is counter to what one would predict from the Ca2+ peak-detector model. Manipulations that broaden somatic spikes are likely to broaden dendritic spikes as well (21). Broadened dendritic spikes should induce larger dendritic Ca2+ transients (21), because the broader spike exposes both NMDA receptors and VDCCs to depolarized potentials for a longer period. These enhanced Ca2+ transients should, in turn, enhance LTP, in accordance with the calcium-peak-detector model commonly used to model both conventional and timing-dependent synaptic plasticity (3, 12–14, 22).
We directly examined the effects of somatic spike width on the amplitude of the dendritic Ca2+ transient in imaging experiments. In these experiments, we washed in TEA (10 mM) and examined the relationship between somatic spike half-width and the peak of the dendritic Ca2+ transient in single cells. In many cases, we blocked ionotropic synaptic transmission to ensure that the observed effects of TEA on spike width and dendritic Ca2+ transients were not induced by network effects. In 6 of 6 cases (e.g., Fig. 3a), the Ca2+ transient was significantly larger (P < 0.01, Wilcoxon rank-sum test) in the presence of TEA than in control conditions. The relationship between AP half-width and the peak of the Ca2+ transient is approximately linear (average R = 0.77; n = 6). These results demonstrate that manipulations of AP width lead to expected increases in the amplitudes of Ca2+ transients, implying that timing-dependent LTP and LTD do not operate by simply thresholding the dendritic Ca2+ transient.
We explored the role of Ca2+ in STDP in more detail. Intracellular administration of the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (10 mM) reduces the amount of STDP-induced LTP for Δt = 15 ms from 17.5 ± 17.5 (n = 4) to -5.69 ± 4.5 (n = 5). This reduction in LTP is significant (P < 0.05) when analyzed by using the one-tailed Wilcoxon rank-sum test. The two most likely sources of dendritic Ca2+ influx are NMDA receptors and VDCCs. Two-amino-5-phosphonovalerate (30 μM), which blocks Ca2+ influx through NMDA receptors, prevented the induction of STDP in all conditions (Fig. 3b), indicating that STDP in EC is NMDA-receptor-dependent. Nifedipine (10 μM), an L-type (CaV 1) Ca2+ channel blocker, generally tilts the balance of STDP toward potentiation (compare results in nifedipine with those in control in Fig. 3b), implying that Ca2+ flux through postsynaptic VDCCs provides feedback that inhibits LTP and/or enhances LTD. In 3 of 3 cases, the spike-evoked Ca2+ transient was significantly inhibited (P < 0.01, Wilcoxon rank-sum test) by nifedipine (Fig. 3b Inset). Blocking CaV1 Ca2+ channels did not affect synaptic transmission and AP generation: both AP duration (99.5 ± 3.2% of the control, n = 4; P ≈ 1, paired Wilcoxon rank-sum test) and EPSP slope (98.04 ± 10.6% of the control, n = 4; P ≈ 1, paired Wilcoxon rank-sum test) remained constant after wash-in of nifedipine. The effects of nifedipine on STDP are statistically significant in most cases (P < 0.05, Wilcoxon rank-sum test). In particular, entorhinal pyramidal cells show exclusive LTP for positive timing in nifedipine. For negative timing, blocking CaV1 channels reduces or eliminates LTD in our preparation (Fig. 3b) as well as visual cortex (23) and hippocampal culture (2).
Based on our results from manipulations of spike width and dendritic Ca2+ channels, we modified the Ca2+peak-detector model to include feedback from VDCCs that favors LTD over LTP. The proposed model is shown in Fig. 3c. We build peak detection into the model by making LTP and LTD depend quadratically and linearly, respectively, on the Ca2+ waveform through NMDA receptors. Thus, in the absence of feedback from VDCCs, small transients generate a larger LTD signal, whereas large transients generate a larger LTP signal. This nonlinear gain for LTP is qualitatively compatible with the autocatalytic nature of calcium-calmodulin kinase II, known to be crucial for mediating LTP (24). The final outcome, expressed as a percent change in synaptic strength, is the simple arithmetic difference of the LTP and LTD signals. To account for the effects of spike width and nifedipine, we propose that Ca2+ entering via CaV1 channels selectively inhibits the LTP pathway. In the model, this inhibition takes the form of a delayed, time-dependent suppression of the Ca2+-driven signal that is passed through the quadratic nonlinearity in Fig. 3c. This model fits our data well (see the solid and dashed lines in Fig. 2b; root mean square (RMS) error of 23.6% over the data set, compared with 18.1% for the best-fit 4th-order polynomial, which indicates the lower bound on RMS error). In the model, broadened spikes favor LTD by increasing the amount of feedback from VDCCs. When VDCCs are blocked, the suppression signal is not present, and the only effect of broader spikes is to increase the NMDA-mediated Ca2+ flux (by 20% for a 50% increase in spike width in the model). In this case, the model predicts elimination of LTD when the spike timing is negative and a conversion of LTD to LTP when EPSPs precedes the broadened APs (Fig. 3b, red boxes). For three of four conditions, the quantitative match between the model and the data in Fig. 3b is excellent. In the fourth case (regular spikes, negative timing), the model predicts the trend correctly but overestimates the size of the effect of blocking VDCCs. Interestingly, blocking VDCCs eliminates left-hand-side LTD in other experimental studies (2, 23).
Discussion
By investigating STDP on pyramidal neurons with constitutively or pharmacologically broadened APs, we showed that the postsynaptic AP width is critical for the direction of synaptic modification in the EC. Broad postsynaptic APs shift the balance of STDP toward depression. When the half-width of an AP exceeds 1.5 ms, no timing-dependent LTP is observed. Ca2+ imaging experiments on the proximal dendrites showed that dendritic Ca2+ transients are proportional to the half-width of APs recorded from the soma. Our results imply that enhanced VDCC-mediated Ca2+ transients are correlated with diminished LTP and enhanced LTD. This result contradicts the Ca2+ threshold-detector model, the most prevalent model linking Ca2+ signals to LTP. Other results call the peak-detector model into question as well. First, the peak-detector model predicts LTD when presynaptic APs precede postsynaptic APs by a sufficiently long time interval; such results are not seen in most experimental data, including ours. (See ref. 8 for a potential resolution of this discrepancy.) Second, triplets of pre-postpresynaptic (or post-pre-postsynaptic) APs, which are likely to lead to larger Ca2+ transients in the dendrite than do doublets of APs, often induce LTD (10, 25, 26).
The essential feature of our algorithm for STDP is that VDCC-mediated Ca2+ fluxes, evoked by postsynaptic APs, shift the balance in metabolic signals leading to LTP and LTD toward LTD. The duration of this effect determines the temporal extent of the LTD window for negative timing. Such an algorithm could arise from a number of mechanisms, including (as pictured in Fig. 3c) divergent signaling pathways, driven by a single NMDA-mediated Ca2+ flux but differentially modulated by VDCC-driven transients. Another possibility involves multiple pools or classes of NMDA receptors, tied to different outcomes in terms of plasticity (27, 28) and differentially modulated by feedback from VDCCs. A model of this form would require modification of the diagram in Fig. 3c to include these two pools, perhaps in different cellular locations (28) but would not necessarily require any changes in the computational model.
Although we are not in a position to construct a detailed, mechanistic model of STDP induction, we can rule out some simple alternatives to the model of Fig. 3c. Models that did not include VDCC-mediated suppression of the LTP pathway were unable to account for the enhancement of LTD in response to spike broadening. Models in which VDCC-mediated currents generally suppressed NMDA receptors (29–31) and reduced both LTP and LTD were also ineffective in fitting our data. Although these models could partially account for enhanced LTD, with spike broadening for positive timing, they could not simultaneously account for the depression-enhancing effects of spike broadening for negative timing (data in Fig. 3b; simulation results not shown). Our data are also incompatible with the specific multidetector recently proposed by Bi and colleagues (25, 26), in which a low-Ca2+-threshold mechanism leads to a “veto” of LTD. Like the standard Ca2+ peak-detector model, we believe that the Bi model is incapable of accounting for increasing LTD with increased spike width, because spike broadening and subsequent enhancement of the Ca2+ transient would only increase the degree of veto of LTD. However, our data are supportive of the general hypothesis from Bi and Buonomano (25, 26, 32) that multiple, spatially segregated Ca2+ signals are necessary to account for the properties of STDP. In particular, we require that the modulatory VDCC-mediated Ca2+ transient be segregated from NMDA-mediated signal. Imaging data support such segregation: In neocortical pyramidal cells, Ca2+ transients evoked by postsynaptic APs give rise to Ca2+ transients in the dendritic trunk and spines, whereas EPSP-evoked transients are confined to the spine head (15).
Our findings suggest that modulation of spike width (e.g., through phosphorylation- or history-dependent mechanisms) could provide a way to alter the properties of long-term synaptic plasticity. Feedback from VDCC-mediated Ca2+ transients is likely to be an important factor in other circumstances as well. For example, induction of LTP in CA1 pyramidal cells leads to local enhancement of the dendritic Ca2+ transient evoked by back-propagating APs near the potentiated synapse (33). Under these circumstances, feedback from VDCCs may tilt the balance of potentiated synapses back toward synaptic depression.
Supplementary Material
Acknowledgments
We thank B. G. Burton, M. E. Hasselmo, D. Johnston, and K. Lillis for helpful discussions and comments. This work was supported by National Institutes of Health Grant NS34425.
Author contributions: Y.-D.Z., K.S., and J.A.W. designed research; Y.-D.Z., C.D.A., and T.I.N. performed research; Y.-D.Z., C.D.A., T.I.N., and J.A.W. analyzed data; and Y.-D.Z. and J.A.W. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: AP, action potential; EC, entorhinal cortex; EPSP, excitatory postsynaptic potential; LTD, long-term depression; LTP, long-term potentiation; STDP, spike-timing-dependent plasticity; TEA, tetraethylammonium; VDCC, voltage-dependent calcium channel.
References
- 1.Bi, G. & Poo, M. (2001) Annu. Rev. Neurosci. 24, 139-166. [DOI] [PubMed] [Google Scholar]
- 2.Bi, G. Q. & Poo, M. M. (1998) J. Neurosci. 18, 10464-10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan, Y. & Poo, M. M. (2004) Neuron 44, 23-30. [DOI] [PubMed] [Google Scholar]
- 4.Debanne, D., Gahwiler, B. H. & Thompson, S. M. (1998) J. Physiol. (London) 507, 237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman, D. E. (2000) Neuron 27, 45-56. [DOI] [PubMed] [Google Scholar]
- 6.Magee, J. C. & Johnston, D. (1997) Science 275, 209-213. [DOI] [PubMed] [Google Scholar]
- 7.Markram, H., Lubke, J., Frotscher, M. & Sakmann, B. (1997) Science 275, 213-215. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama, M., Hong, K., Mikoshiba, K., Poo, M. M. & Kato, K. (2000) Nature 408, 584-588. [DOI] [PubMed] [Google Scholar]
- 9.Sjöström, P. J. & Nelson, S. B. (2002) Curr. Opin. Neurobiol. 12, 305-314. [DOI] [PubMed] [Google Scholar]
- 10.Sjöström, P. J., Turrigiano, G. G. & Nelson, S. B. (2001) Neuron 32, 1149-1164. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, L. I., Tao, H. W., Holt, C. E., Harris, W. A. & Poo, M. (1998) Nature 395, 37-44. [DOI] [PubMed] [Google Scholar]
- 12.Abarbanel, H. D., Huerta, R. & Rabinovich, M. I. (2002) Proc. Natl. Acad. Sci. USA 99, 10132-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artola, A. & Singer, W. (1993) Trends Neurosci. 16, 480-487. [DOI] [PubMed] [Google Scholar]
- 14.Shouval, H. Z., Bear, M. F. & Cooper, L. N. (2002) Proc. Natl. Acad. Sci. USA 99, 10831-10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koester, H. J. & Sakmann, B. (1998) Proc. Natl. Acad. Sci. USA 95, 9596-9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun, S. H., Mook-Jung, I. & Jung, M. W. (2002) J. Neurosci. 22, RC214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netoff, T. I., Banks, M. I., Dorval, A. D., Acker, C. D., Haas, J. S., Kopell, N. & White, J. A. (2005) J. Neurophysiol. 93, 1197-1208. [DOI] [PubMed] [Google Scholar]
- 18.Destexhe, A., Mainen, Z. F. & Sejnowski, T. J. (1998) in Methods in Neuronal Modeling: from Ions to Networks, eds. Koch, C. & Segev, I. (MIT Press, Cambridge, MA), pp. 1-26.
- 19.Hoffman, D. A., Magee, J. C., Colbert, C. M. & Johnston, D. (1997) Nature 387, 869-875. [DOI] [PubMed] [Google Scholar]
- 20.Hille, B. (2001) Ion Channels of Excitable Membranes (Sinauer, Sunderland, MA), 3rd Ed., pp. 555-556.
- 21.Korngreen, A., Kaiser, K. M. & Zilberter, Y. (2005) J. Physiol. (London) 562, 421-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisman, J. (1989) Proc. Natl. Acad. Sci. USA 86, 9574-9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Froemke, R. C., Poo, M. M. & Dan, Y. (2005) Nature 434, 221-225. [DOI] [PubMed] [Google Scholar]
- 24.Malenka, R. C. & Nicoll, R. A. (1999) Science 285, 1870-1874. [DOI] [PubMed] [Google Scholar]
- 25.Rubin, J. E., Gerkin, R. C., Bi, G. Q. & Chow, C. C. (2005) J. Neurophysiol. 93, 2600-2613. [DOI] [PubMed] [Google Scholar]
- 26.Bi, G. Q. & Rubin, J. (2005) Trends Neurosci. 28, 222-228. [DOI] [PubMed] [Google Scholar]
- 27.Liu, L., Wong, T. P., Pozza, M. F., Lingenhoehl, K., Wang, Y., Sheng, M., Auberson, Y. P. & Wang, Y. T. (2004) Science 304, 1021-1024. [DOI] [PubMed] [Google Scholar]
- 28.Sjöström, P. J., Turrigiano, G. G. & Nelson, S. B. (2003) Neuron 39, 641-654. [DOI] [PubMed] [Google Scholar]
- 29.Legendre, P., Rosenmund, C. & Westbrook, G. L. (1993) J. Neurosci. 13, 674-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong, G., Shepherd, D. & Jahr, C. E. (1995) Science 267, 1510-1512. [DOI] [PubMed] [Google Scholar]
- 31.Zucker, R. S. (1999) Curr. Opin. Neurobiol. 9, 305-313. [DOI] [PubMed] [Google Scholar]
- 32.Karmarkar, U. R. & Buonomano, D. V. (2002) J. Neurophysiol. 88, 507-513. [DOI] [PubMed] [Google Scholar]
- 33.Frick, A., Magee, J. & Johnston, D. (2004) Nat. Neurosci. 7, 126-135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.