Abstract
Context: Cryotherapy is commonly used for a variety of purposes; however, the body's response to cryotherapy immediately postexercise is unknown.
Objective: To investigate the effect of prior exercise on crushed-ice–bag treatment of a large muscle group.
Design: 2 × 3 repeated-measures design on depth (1 cm and 2 cm below adipose tissue) and treatment (exercise followed by ice, exercise followed by no ice, and no exercise followed by ice).
Setting: Sports Injury Research Laboratory.
Patients or Other Participants: Six physically active, uninjured male volunteers.
Intervention(s): For the 2 exercise conditions, subjects rode a stationary cycle ergometer at 70% to 80% of their age-predicted maximum heart rate, as calculated by the Karvonen method. For the no-exercise condition, subjects lay supine on a treatment table. The cryotherapy treatment consisted of a 1-kg ice bag applied to the anterior mid thigh. For the no-ice condition, subjects lay supine on a treatment table.
Main Outcome Measure(s): Time required for the intramuscular temperatures at the 1-cm and 2-cm depths below adipose tissue to return to pre-exercise baseline and time required to cool the 1-cm and 2-cm depths to 10°C below the pre-exercise temperature.
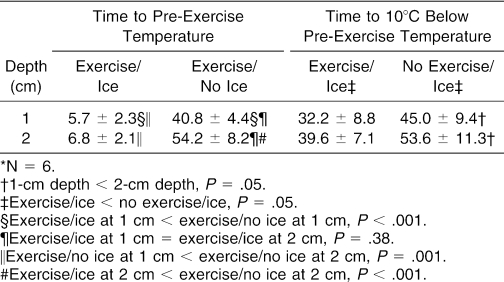
Results: The time to cool the rectus femoris to the pre-exercise temperature using a crushed-ice–bag treatment was reduced by approximately 40 minutes (P < .001). The ice bag cooled the 1-cm and 2-cm depths to the pre-exercise temperature within 7 minutes (P = .38), but the 2-cm tissue depth took nearly 13.5 minutes longer to cool than the 1-cm depth when no ice was applied (P = .001). The 1-cm depth cooled to 10°C below the pre-exercise temperature about 8 minutes sooner than the 2-cm depth, regardless of whether the tissue was exercised or not (P < .001). Exercise shortened the cooling time to 10°C below the pre-exercise temperature by approximately 13 minutes (P = .05).
Conclusions: Exercise before cooling with a crushed-ice bag enhanced the removal of intramuscular heat.
Keywords: cryotherapy, cold modalities, intramuscular temperature, stationary cycle ergometer
Cryotherapy is commonly used to decrease surface interface temperature,1,2 intramuscular temperature,1–11 cellular metabolism,12,13 pain,14,15 muscle spasm,16,17 and blood flow.18,19 Previous authors have investigated the effects of various types of cooling modalities,20–24 site of application,1,3,21–23 and thickness of adipose tissue2,3,25 on decreasing subcutaneous and skin interface temperature at rest. Although this research has been critical, it is important to understand how exercise may affect the body's response to cryotherapy.
Currently, ice application is routinely used by some athletes after training and competitive bouts and is commonly prescribed during the rehabilitation of muscle strains or contusions. Although these practices are frequent, information in the literature regarding cooling exercised muscle is lacking, and so we questioned the efficacy of a cold modality to cool exercised muscle tissue. Because physical activity increases body heat, thereby elevating tissue temperatures, even after the exercise bout has ended,25 we believed that cooling times would be lengthened. Moreover, we believed that the deeper tissues would need more time to cool. Thus, it is important to understand how long it takes a cryotherapy treatment to cool tissues to a target temperature after exercise.
Additionally, most of what we know about tissue cooling has been learned through ice application to rested subjects. Even though this model has provided us with information about muscle tissue temperature responses to cooling, the effect of exercise before cooling on muscle tissue temperature has not been adequately reported. Therefore, one purpose of our study was to evaluate how long it takes to return the rectus femoris (RF) musculature at 1 cm and 2 cm deep to pre-exercise temperatures after exercise, with and without the use of a cryotherapy treatment. Secondly, we wanted to determine the effect of pre-cryotherapy exercise on the time to cool the RF muscle to 10°C below the pre-exercise temperature at the same depths.
METHODS
Design
A 2 × 3 repeated-measures design was used to guide this study. The independent variables were depth (1 cm and 2 cm below adipose tissue) and treatment (exercise followed by ice [Ex/Ice], exercise followed by no ice [Ex/No Ice], and no exercise followed by ice [No Ex/Ice]). The dependent variables were the length of time it took the RF tissue depths to return to the pre-exercise temperatures after exercise with and without ice-bag application and the length of time it took the exercised and nonexercised RF muscle to cool to 10°C below baseline temperature using an ice-bag application.
Subjects
Six physically active, uninjured volunteers (age = 26.0 ± 3.2 years, height = 181.6 ± 2.4 cm, mass = 92.9 ± 9.1 kg, anterior mid thigh skinfold = 25.4 ± 2.7 mm) who were currently involved in an exercise program participated in this study. Before inclusion, each subject completed a health history questionnaire and was familiarized with the study and was screened. Subjects were excluded if they reported any circulatory problems, bloodborne or clotting diseases, vascular disorders, hepatitis, hypersensitivity to cold, or cold-related conditions that might bother them during the study. Moreover, to make sure temperature readings were not affected by different superficial tissue thicknesses, only subjects with a right anterior mid thigh skinfold measurement between 20 and 30 mm were included. Each subject gave informed consent, and the School of Health and Human Performance Human Subjects Review Committee approved this study.
Instrumentation
We used a skinfold caliper (Beta Technology, Inc, Cambridge, MD) to measure skinfold thickness. Two type-T implantable thermocouples (Columbus Instruments Intl, Columbus, OH) were inserted through two 18-gauge × 4.78-cm intravenous catheters (Alliance Medical, Russellville, MO). The thermocouples were connected to a portable temperature data-acquisition device (Datalogger MMS 3000-T6V4, Commtest Instrument Ltd, Christchurch, New Zealand) that was recently calibrated by the manufacturer. In a previous report25 from our laboratory, this temperature measurement setup was noted to be accurate to within ± 0.1°C. We used a manometer (AirCast Inc, Summit, NJ) to measure ice-bag and elastic-wrap compression (42 to 48 mm Hg). The same investigator performed all data measurements.
Procedures
Each subject reported to the Sports Injury Research Laboratory at approximately the same time of day for 3 treatment sessions at least 48 hours apart. During the first session, the subject was familiarized with the study, screened, and assigned to a treatment order according to a balanced Latin square before data collection. The subsequent treatment sessions consisted of data collection only. Familiarization included explanation of the purpose and methods of the study. After providing informed consent, subjects were screened for the inclusion criteria.
Data collection consisted of preparing the subjects, inserting the thermocouples, measuring daily pre-exercise muscle temperatures, administering a treatment condition, and recording changes in muscle temperatures. Subject preparation began 30 minutes before each treatment condition, when a prophylactic antibiotic (500 mg of cephalexin) was administered orally. After ingesting the antibiotic, each subject was positioned supine, the midpoint between the base of the patella and the anterior superior iliac spine was marked, and 3 consecutive vertical skinfold measurements were averaged and divided by 2 to estimate skin and adipose tissue thickness.11 Thereafter, we shaved a 10 × 10-cm area surrounding the skinfold site with a razor and cleansed it with povidone-iodine solution for 30 seconds in a circular pattern.25 Once the area was dry, we used a sterile surgical pen to mark the 2 thermocouple placement sites, 1 cm inferior and superior to the midpoint (Figure 1).
Figure 1. Thermocouple insertion sites.
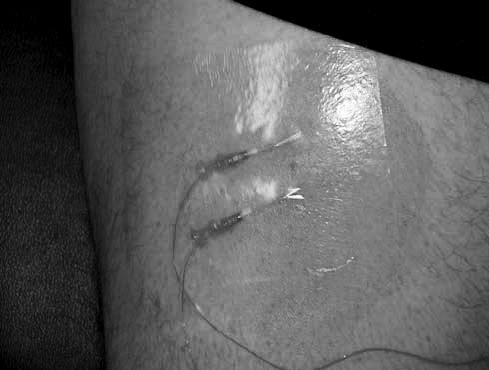
To correctly insert the thermocouples to the needed depth, we added the estimated skin and adipose tissue thickness to our intramuscular target depths on the catheters. The catheters were measured and marked from the tip of the 18-gauge hypodermic needle. Additionally, we used the length of the catheter, including the needle tip, to measure and mark the thermocouple to make sure the thermocouple tip reached the end of the catheter. One needle was inserted 1 cm deep to adipose tissue at the inferior site, whereas the other needle was inserted 2 cm deep to adipose tissue at the superior site. Once the needles were at the correct depth, they were retracted, leaving a flexible Teflon (DuPont, Wilmington, DE) catheter in place.
After feeding the thermocouples into their respective catheters, we secured them to the skin with adhesive tape (Dermiclear, Johnson & Johnson, New Brunswick, NJ) and covered the area with a 10 × 12-cm adhesive dressing (Tegaderm, 3M, St. Paul, MN). The thermocouples were then connected to the data-acquisition device and temperature recording occurred every 15 seconds for 5 minutes before the exercise condition. We used the average of these measurements to determine that day's pre-exercise temperature.
The exercise condition consisted of 30 minutes of either riding a stationary cycle ergometer at 70% to 80% of age-predicted maximum heart rate as calculated by the Karvonen method26 or lying supine on a treatment table. For fear that the thermocouples would break during the stationary cycling, we extracted them and kept them disinfected in a CidexPlus 3.4% glutaldehyde solution (Johnson & Johnson) until needed. At the end of the cycling bout, the thermocouples were rinsed with sterile saline and immediately reinserted into the respective catheters. Once the thermocouples were resecured, temperature recordings were taken every 15 seconds until the end of the study.
The cooling treatment consisted of either applying a 1-kg ice bag held in place with a 15 × 30-cm elastic wrap using 42 to 48 mm Hg of external pressure to the anterior thigh or no treatment, in which the subject lay supine.1 During the Ex/ Ice and No Ex/Ice conditions, temperature recording continued until both intramuscular depths were 10°C less than their respective pre-exercise temperatures. However, during the Ex/ No Ice treatment, temperature recordings ceased once both depths reached their respective pre-exercise temperatures. For the statistical analysis, we used the time for the 2 tissue depths to reach the respective pre-exercise temperatures.
At the end of each data-collection session, we withdrew the thermocouples, cleansed the treatment area with povidone-iodine solution, and applied adhesive bandages with antibiotic ointment over each insertion site. Subjects were then given a second dose of the oral prophylactic antibiotic. The thermocouples were sterilized by immersion in the CidexPlus solution for at least 24 hours. Sterile technique was used during all invasive procedures, which were performed by the same investigator. There was no different in ambient temperature for each condition (22.3 ± 0.4°C for Ex/Ice, 22.5 ± 0.42°C for Ex/No Ice, and 22.4 ± 0.5°C for No Ex/Ice).
Data Analysis
Data were summarized by tissue depth and treatment. Two 2 × 2 factorial repeated-measures analyses of variance (ANOVAs) were performed. The first ANOVA evaluated the effects of ice-bag application and depth on the time for the RF muscle to return to pre-exercise temperatures after exercise. The second ANOVA detected the effect of exercise and depth on the time to cool the RF muscle to 10°C below pre-exercise temperatures using the ice-bag treatment. We used simple main effects testing and dependent t tests to evaluate significant interactions and main effects when present. The level of significance was set a priori at P < .05 for all difference testing. The Statistical Package for the Social Sciences (version 11.0; SPSS Inc, Chicago, IL) was used to analyze all data.
RESULTS
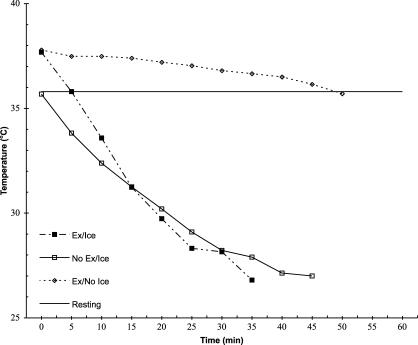
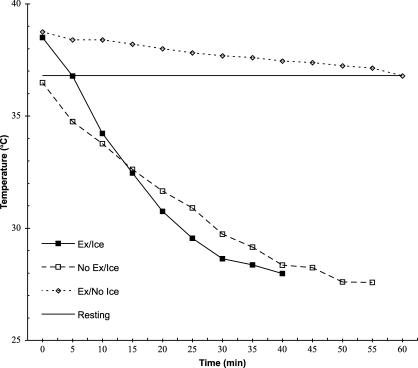
Exercise shortened the time it took to cool both tissue depths to 10°C below pre-exercise resting temperature (F1,5 = 11.6, P = .02, partial η2 = .70, 1 − β = .78) by nearly 13.5 minutes. We found no exercise-by-depth interaction for the reduction in tissue temperature at the 1-cm or 2-cm depths to 10°C below pre-exercise temperatures after rest or exercise (F1,5 = .10, P = .77, partial η2 = .02, 1 − β = .06) (Figures 2 and 3). The 1-cm depth cooled approximately 8 minutes faster than the 2-cm depth, regardless of whether tissues were exercised or not (F1,5 = 261.9, P < .001, partial η2 = .98, 1 − β = 1.0).
Figure 2. Treatment temperature (°C) over time at the 1-cm depth.
Figure 3. Treatment temperature (°C) over time at the 2-cm depth.
The 1-cm and 2-cm depths cooled at different rates, depending on the method used (F1,5 = 44.8, P = .001, partial η2 = .90, 1 − β = 1.0) (Table). Cooling the exercised muscle back to the pre-exercise temperature without using an ice bag took a little more than 35 minutes longer at the 1-cm depth (t5 = 22.0, P < .001) and 47 minutes longer at the 2-cm depth (t5 = 15.2, P < .001). Moreover, the temperature of the 2-cm deep tissue remained elevated longer than the 1-cm deep tissue during that same no–ice-bag treatment condition (t5 = 7.10, P = .001). However, both the 1-cm and 2-cm depths cooled to pre-exercise baseline temperatures within 7 minutes when the ice bag was used (t5 = .97, P = .38).
Time to Target Temperature (min)*.
DISCUSSION
As we investigated the effect of exercise on the cooling efficacy of an ice-bag treatment, both above and below pre-exercise temperature, we were surprised to see that active exercise before cryotherapy application dramatically enhanced the cooling effect in muscular tissues. Even though we speculated that the exercise-related increases in metabolism and temperature in a muscle would negatively affect a cryotherapy treatment, the body's thermoregulatory response to exercise may actually have enhanced the local cooling effect.
During exercise, the body increases blood flow to the skin to remove the heat produced by its energy systems.26,27 This way, the body can use conduction, convection, radiation, and evaporation to lose the heat.27 The quick return to pre-exercise temperatures with the use of an ice bag after exercise can be simply explained by the large temperature gradient between the ice bag and the skin. However, once the temperatures began to decline below pre-exercise temperatures, it would be reasonable to think that the body area would react like nonexercised muscle. But what we observed in this study might be best explained as a whole-body cooling effect enhancing local cooling: after exercise, most of the body is still producing heat, thereby facilitating the body's cooling mechanisms.
One may assume that exercise before cooling enhances the cooling effect; however, when a cryotherapy treatment is administered immediately after an acute injury, this assumption may not be correct. An injury or the responses of the body and tissue to that injury can affect the generation and removal of heat. Therefore, it is difficult to know whether the effect we found occurs after an injury to exercised muscle. Another caution when interpreting the results of this study is that exercising while attempting to cool would seemingly delay cooling effects.
Finally, our observations support the current clinical practice of applying an ice bag for 30 minutes or more to cool large, rested muscle groups at both the 1- and 2-cm depths.28 Our results also support the theory that deeper tissues take longer to cool, regardless of prior activity.24,29 Both effects have additional injury clinical implications, because one purpose of applying cold is to increase the chance for survival of the healthy cells surrounding an injury site that may die because of secondary ischemic or enzymatic causes.20 Hence, tissue depth is to be considered when applying a cold modality, as previously described.28
CONCLUSIONS
Our observations are important for the clinical use of local cryotherapy postexercise. Exercise before cooling reduces the treatment time needed to decrease intramuscular temperature.
Footnotes
Blaine C. Long, MS, ATC; Mitchell L. Cordova, PhD, LAT, ATC, FACSM; Jody B. Brucker, PhD, LAT, ATC; Timothy J. Demchak, PhD, LAT, ATC; and Marcus B. Stone, PhD, LAT, ATC, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article.
Address correspondence to Blaine C. Long, MS, ATC, 106 Smith Field House, Brigham Young University, Provo, UT 84602. Address e-mail to blainelong@byu.edu.
REFERENCES
- Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28:236–245. [PMC free article] [PubMed] [Google Scholar]
- Jutte LS, Merrick MA, Ingersoll CD, Edwards JE. The relationship between intramuscular temperature, skin temperature, and adipose thickness during cryotherapy and rewarming. Arch Phys Med Rehabil. 2001;82:845–850. doi: 10.1053/apmr.2001.23195. [DOI] [PubMed] [Google Scholar]
- Myrer JW, Myrer KA, Measom GJ, Fellingham GW, Evers SL. Muscle temperature is affected by overlying adipose when cryotherapy is administered. J Athl Train. 2001;36:32–36. [PMC free article] [PubMed] [Google Scholar]
- Abramson DI, Chu LS, Tuck ST, Jr, Lee SW, Richardson G, Levin M. Effect of tissue temperatures and blood flow on motor nerve conduction velocity. JAMA. 1966;198:1082–1088. [PubMed] [Google Scholar]
- Hobbs KT. Results of intramuscular temperature changes at various levels after the application of ice. Sports Health. 1983;1:15. [Google Scholar]
- Johnson DJ, Moore S, Moore J, Oliver RA. Effect of cold submersion on intramuscular temperature of the gastrocnemius muscle. Phys Ther. 1979;59:1238–1242. doi: 10.1093/ptj/59.10.1238. [DOI] [PubMed] [Google Scholar]
- Lowden BJ, Moore RJ. Determinants and nature of intramuscular temperature changes during cold therapy. Am J Phys Med. 1975;54:223–233. [PubMed] [Google Scholar]
- Myrer JW, Measom GJ, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33:25–29. [PMC free article] [PubMed] [Google Scholar]
- Waylonis GW. The physiologic effects of ice massage. Arch Phys Med Rehabil. 1967;48:37–42. [PubMed] [Google Scholar]
- Zemke JE, Anderson JC, Guion WK, McMillan J, Joyner AB. Intramuscular temperature responses in the human leg to two forms of cryotherapy: ice massage and ice bag. J Orthop Sports Phys Ther. 1998;27:301–307. doi: 10.2519/jospt.1998.27.4.301. [DOI] [PubMed] [Google Scholar]
- Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28:236–245. [PMC free article] [PubMed]
- Jones DP. Renal metabolism during normoxia, hypoxia, and ischemic injury. Annu Rev Physiol. 1986;48:33–50. doi: 10.1146/annurev.ph.48.030186.000341. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Reffy A, Balint BJ, Jarvinen M, Kvist M. Ischemic-hypoxic changes in muscles with tendon injuries (author's transl) [in Hungarian] Magy Traumatol Orthop Helyreallito Seb. 1981;24:218–226. [PubMed] [Google Scholar]
- Chapman CE. Can the use of physical modalities for pain control be rationalized by the research evidence? Can J Physiol Pharmacol. 1991;69:704–712. doi: 10.1139/y91-105. [DOI] [PubMed] [Google Scholar]
- Knight KL, Rubley MD, Ingersoll CD, Brucker JB. Pain perception is greater during ankle ice immersion than during ice pack application [abstract] J Athl Train. 2000;35:S-45. (suppl) [Google Scholar]
- Basset SW, Lake BM. Use of cold applications in the management of spasticity: report of three cases. Phys Ther Rev. 1958;38:333–334. doi: 10.1093/ptj/38.5.333. [DOI] [PubMed] [Google Scholar]
- Hartviksen K. Ice therapy in spasticity. Acta Neurol Scand Suppl. 1962;3:79–84. [Google Scholar]
- Bennett D. Water at 67° to 69° Fahrenheit to control hemorrhage and swelling encountered in athletic injuries. Athl Train. 1961;1:12–14. [Google Scholar]
- Knight KL, Aquino J, Johannes SM, Urban CD. A reexamination of Lewis' cold-induced vasodilation in the finger and the ankle. Athl Train. 1980; 15:248–250.
- Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31:1516–1521. doi: 10.1097/00005768-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Myrer JW, Measom GJ, Fellingham GW. Exercise after cryotherapy greatly enhances intramuscular rewarming. J Athl Train. 2000;35:412–416. [PMC free article] [PubMed] [Google Scholar]
- Thorsson O, Lilja B, Ahlgren L, Hemdal B, Westlin N. The effect of local cold application on intramuscular blood flow at rest and after running. Med Sci Sports Exerc. 1985;17:710–713. doi: 10.1249/00005768-198512000-00016. [DOI] [PubMed] [Google Scholar]
- Tsang KKW, Buxton BP, Guion WK, Joyner AB, Browder KD. The effects of cryotherapy applied through various barriers. J Sport Rehabil. 1997;6:343–354. [Google Scholar]
- Merrick MA, Jutte LS, Smith ME. Cold modalities with different thermodynamic properties produce different surface and intramuscular temperatures. J Athl Train. 2003;38:28–33. [PMC free article] [PubMed] [Google Scholar]
- Otte JW, Merrick MA, Ingersoll CD, Cordova ML. Subcutaneous adipose tissue thickness alters cooling time during cryotherapy. Arch Phys Med Rehabil. 2002;83:1501–1505. doi: 10.1053/apmr.2002.34833. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Exercise Physiology. 4th ed. Baltimore, MD: Williams & Wilkins; 1996:266.
- Brooks GA, Fahey TD, White TP, Baldwin KM. Exercise Physiology: Human Bioenergetics and Its Application. 3rd ed. Mountain View, CA: Mayfield Publishing; 2000.
- Knight KL, Brucker JB, Stoneman PD, Rubley MD. Muscle injury management with cryotherapy. Athl Ther Today. 2000;5(4):26–30. [Google Scholar]
- Knight KL. Cryotherapy in Sport Injury Management. Champaign, IL: Human Kinetics; 1995.