Abstract
Context: Ice bags “to go” are a common practice in athletic training.
Objective: To determine the effect of submaximal exercise on tissue temperatures during a common ice-bag application.
Design: 2 X 5 fully repeated-measures design with treatment (cooling while resting, cooling while walking) and time (pretreatment, immediately after ice application, and at 10, 20, and 30 minutes during treatment) as the independent variables.
Setting: Laboratory setting.
Patients or Other Participants: Sixteen healthy, physically active volunteers (age = 21.63 ± 2.63 yrs, height = 68.97 ± 4.00 cm, mass = 80.97 ± 18.18 kg, calf skinfold = 21.1 ± 9.3 mm).
Main Outcome Measure(s): Left triceps surae intramuscular and skin temperatures, as measured by thermocouples to the nearest 0.1°C, served as dependent measures.
Intervention(s): After collecting baseline temperatures, we secured a 1.0-kg ice bag to the calf using plastic wrap before the subject either rested prone or walked on a treadmill at 4.5 km/h for 30 minutes.
Results: Treatment did not (P < 0.10) affect the ∼15°C (P < 0.0001) surface temperature decrease, which remained depressed immediately upon ice-bag application (P < 0.05). Conversely, intramuscular temperature continually cooled (34 to 28°C), while subjects rested (P < 0.0001), whereas no change took place during walking (P = 0.49). Moreover, at the 20- and 30-minute treatment intervals, the resting intramuscular temperatures were, respectively, 3.9°C and 5.4°C cooler than the walking intramuscular temperatures (P < 0.01).
Conclusions: The current trend of wrapping “to go” ice bags to the leg is not likely to achieve deep tissue cooling despite surface temperature decreases.
Keywords: cold, cooling, compression, cryotherapy, exercise, lower leg, thermocouple, modalities
Cryotherapy is often used for its cooling effects on both superficial and intramuscular tissues, which lead to further physiologic changes.1–6 Among these changes are vasoconstriction and decreased metabolism, spasm and edema formation, and pain sensation.2 Although the true cellular events affected by cryotherapy are not fully understood,7,8 cryotherapy is considered to be the most beneficial treatment for rapidly reducing cellular metabolism, regardless of prior treatment activity level.9 Thus, some clinicians believe that ice bags should be applied to avoid overuse injuries. Moreover, if pain reduction is the main goal of the cryotherapy treatment, such as cryokinetics, then exercising while cooling may be of great benefit. However, the effect of exercise on tissue temperature while cooling has not been investigated.
Additionally, because compression enhances the intramuscular and surface cooling of a cold modality10 and time is often of the essence, ice bags are commonly applied “to go,” using an elastic or plastic wrap. This satisfies both the athlete's need to continue with daily time commitments and the athletic trainer's need to attend to other responsibilities. Although these intentions are good, it is not known if this to-go ice pack is effectively cooling the underlying tissues, especially if the target muscle tissue being cooled is submaximally active, such as the calf during walking.
To date, authors investigating the effects of exercise on cryotherapy treatments have looked at exercise before11 and after an ice treatment.5,12,13 Exercise before an ice treatment enhances cooling,11 whereas exercise after an ice treatment increases the rate of rewarming.5,12,13 What is lacking from the literature is information regarding the effect of exercise on a cryotherapy treatment when both are applied simultaneously. Therefore, our purpose was to determine the effect of walking on a common triceps surae ice-bag treatment when applied to previously rested and uninjured legs.
METHODS
Design
A 2 by 5 factorial design with repeated measures on both factors guided this study. The independent variables were treatment (ice while resting or ice while walking) and time (pretreatment, immediately after ice application, and at 10, 20, and 30 minutes during treatment). Superficial and intramuscular (1.5 cm plus half the skinfold measure) temperatures of the left triceps surae were the dependent variables.
Subjects
Sixteen healthy, physically active volunteers (9 males and 7 females; mean age, 21.63 ± 2.63 years; mean height, 68.97 ± 4.00 cm; mean mass, 80.97 ± 18.18 kg; mean calf skinfold size, 21.1 ± 5.5 mm) consented to participate. Subjects who reported a history of vascular abnormalities, recent illness, acute lower extremity injury within the last 3 months, or allergies to cold application or antibiotics were excluded. Furthermore, to control for the effect of adipose tissue on the removal of heat,14,15 subjects with a measured posterior calf skinfold outside the 15- to 30-mm range were also excluded. This study was approved by the institutional review board.
Instruments
We used a skinfold caliper (Lange C-130; Beta Technology Inc, Cambridge, MD) to determine the superficial tissue thickness of the posterior calf at the greatest girth. Superficial temperature was measured using a superficial thermocouple (TX-29; Columbus Instruments, Columbus, OH). Intramuscular temperature was measured using a fine-wire, implantable type T thermocouple (TX-23-18; Columbus Instruments), which was inserted into the triceps surae through an 18-gauge by 4.76-cm intravenous catheter (Alliance Medical, Russellville, MO). Both thermocouples were connected to a portable temperature data acquisition device (Datalogger MMS 3000-T6V4; Commtest Instruments Ltd, Christchurch, New Zealand). The manufacturer recently calibrated the data acquisition device, and we verified that recordings were equal to a National Institute of Standards and Technology-traceable mercury in glass thermometer before data collection. The data from this device were electronically transferred to a desktop computer for statistical analysis.
Procedures
Dressed in gym attire, subjects reported to the Exercise Physiology Laboratory for 2 treatment sessions at the same time of day approximately 48 hours apart. The first session consisted of familiarizing and screening the subjects, obtaining informed consent, selecting treatment order according to a balanced order design, and data collection. The second session consisted of data collection only. Data collection involved preparing the subjects, inserting thermocouples, and collecting temperature data before and during the respective treatments.
Approximately 15 minutes before data collection began, subjects were placed in the prone position on a treatment table. A surgical pen mark was made on the posterior aspect of the lateral head of the gastrocnemius at the greatest girth to identify the thermocouple insertion site. The mean of 3 consecutive vertical skinfold measurements at this site was recorded and divided by 2 to estimate superficial tissue thickness and added to 1.5 cm to ensure the thermocouple was located at the target depth.
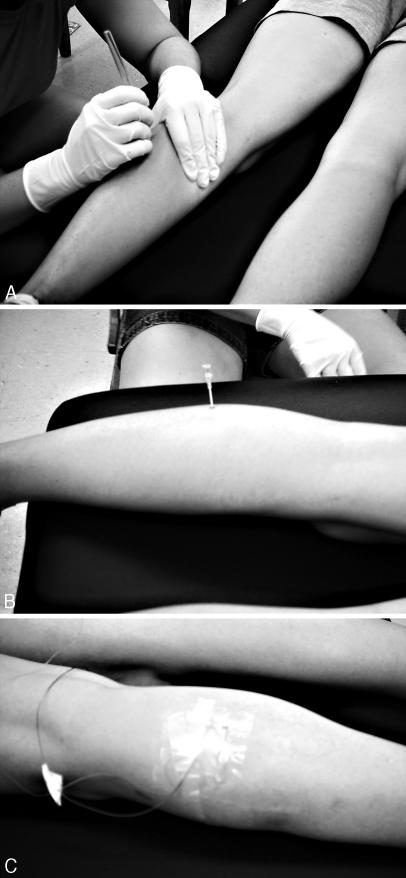
A 25-cm2 area at the insertion site was shaved, cleansed with povidone-iodine, and then swabbed with an alcohol preparation pad. Using the calculated target-depth measure, we measured and marked the intravenous catheter from the tip of the needle to ensure correct insertion depth. Similarly, the intramuscular thermocouple was marked 10 cm from its tip to ensure the correct insertion depth. The intravenous needle with catheter was implanted straight posterior to anterior at the specified target depth (Figure 1A), and the needle was retracted, leaving the flexible catheter embedded in the tissue (Figure 1B). The type T thermocouple was threaded into the catheter to the target depth. The thermocouple and catheter were secured to the skin with Dermiclear adhesive tape (Johnson & Johnson, New Brunswick, NJ) and Bioclusive dressing (Johnson & Johnson, Arlington, TX) (Figure 1C). Once the thermocouples were secured and connected to the data acquisition device, we began temperature recording. The room temperature in the laboratory was 22°C. Baseline temperature was established by averaging the 5-minute temperature recordings immediately pretreatment once the readings on the Datalogger remained unchanged (±0.1°C). This average temperature was used in the statistical analysis as the pretreatment measure. The temperatures were then recorded every minute for the duration of the treatment session. A plastic ice bag that measured 45.7 by 25.4 cm (Cramer Products, Inc, Gardner, KS) filled with 1.0 kg of ice nuggets (NME954WS-32A ice machine; Scotsman, Vernon Hills, IL) was secured to the calf using plastic wrap (GoodWrappers, Baltimore, MD). To maintain consistency of the ice-bag compression, the same certified athletic trainer applied all treatments. Additionally, a manometer (AirCast Inc, Summit, NJ) was used to quantify the compression of 10 pilot-tested ice treatments. The average of the compression produced by this type of ice application was 62.0 ± 6.8 mm Hg.
Figure 1. A, Insertion of catheter. B, Catheter after insertion. C, Thermocouples secured with tape and Bioclusive dressing.
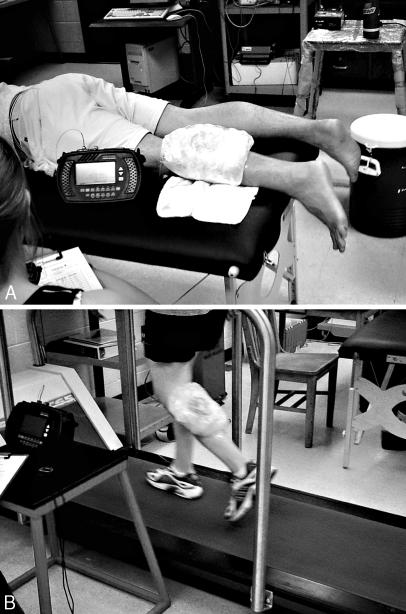
Once the ice bag was applied, the subject either remained prone (Figure 2A) or walked on a treadmill (MedTrack Controller ST65; Quinton, Inc, Bothell, WA) at a speed of 4.5 km/h for 30 minutes (Figure 2B). Thereafter, the thermocouple was removed and disinfected in a CidexPlus (Johnson & Johnson, New Brunswick, NJ) 3.4% glutaldehyde solution for at least 20 minutes. The treatment area was cleansed with saline solution, and the insertion wound was covered with antibiotic ointment and an adhesive bandage. Information listing the signs and symptoms of infection and proper wound management was provided and discussed with each subject. This end-of-study wound care was applied after each session, and no subject reported any complications.
Figure 2. A, Subject resting prone. B, Subject walking on treadmill with ice bag secured to triceps surae muscle.
Statistical Analysis
We calculated two 2 by 5 factorial repeated-measures analyses of variance with the Scheffé multiple comparison tests to analyze the effect of treatment and time on the triceps surae interface and intramuscular temperatures separately. Correction for violations of sphericity was performed using the Geisser-Greenhouse ε procedure. The level of significance was set at P ≤ .05 for all tests.
RESULTS
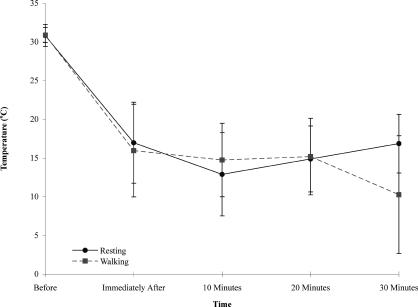
The intramuscular temperatures were affected by the treatment and time (F4,60 = 23.51, P = .005, η2 = 0.14, 1 − β = 0.90) (Table 1, Figure 3); the Geisser-Greenhouse ε correction was used for all F tests except for one, as noted. The 2 subsequent 1 by 5 analyses of variance revealed that the intramuscular temperature decreased over time in the resting group (F4,60 = 95.68, P < .001, η2 = 0.64, 1 − β = 1.00). However, temperature did not change in the walking group (F4,60 = 0.85, P = .50, η2 = 0.02, 1 − β = 0.17). Additionally, 3 of the 5 post hoc, dependent, 2-tailed t tests showed that the resting and walking groups started with the musculature at the same temperature (t30 = 0.070, P = .94, 1 − β = 0.05), were similar at the time the ice pack was applied (t30 = 0.034, P = .97, 1 − β = 0.05), and had not varied much by the 10-minute mark (t30 = 0.55, P = .57, 1 − β = 0.08). The last 2 post hoc t tests indicated that the resting group's intramuscular temperatures were less than those of the walking group at 20 (t30 = 5.03, P < .0001, 1 − β = 1.00) and 30 (t30 = 6.63, P < .00001, 1 − β = 1.00) minutes of treatment.
Table 1. Intramuscular Temperatures Across Time.
Figure 3. Intramuscular temperatures across time (before and after ice application) for both treatments.
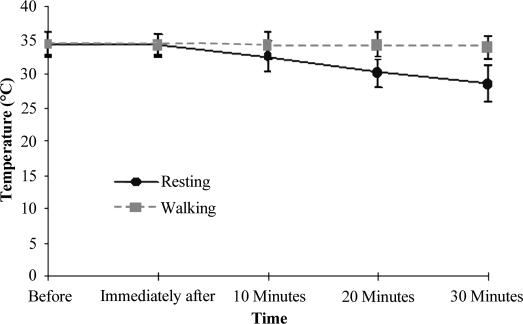
Different from the intramuscular results, the superficial temperatures decreased over time (F4,60 = 95.29, P < .0001, η2 = 0.64, 1 − β = 1.00), without an influence of the treatment (F1,15 = 3.04, P = .10, η2 = 0.004, 1 − β = 0.37; the Geisser-Greenhouse ε correction was not used for this F test) (Table 2, Figure 4). Post hoc multiple comparison analyses indicated that only the pretreatment temperature was higher than the other time measures.
Table 2. Superficial Temperatures Across Time.
Figure 4. Superficial temperatures across time (before and after ice application) for both treatments.
DISCUSSION
Our results support previous findings that ice packs are effective in decreasing intramuscular temperature at rest.10,12,16 We found no change in intramuscular temperature during the ice and walking treatment, despite superficial recordings that showed skin surface cooling. Thus, exercising the muscle during cooling limits the cooling effect of an ice bag, despite skin surface cooling.
Several physiologic factors are likely responsible for limiting the cooling effect of the ice bag on intramuscular temperature during exercise: muscle metabolism, blood flow, and affinity for thermal exchange. Active muscles produce heat as a by-product of the chemical reactions required for manufacturing and using adenosine triphosphate in the working muscles. One group17 showed that with 15 minutes of exercise, intramuscular temperature increased by an average of 2.20°C ± 0.63°C. Another group11 reported an increase in quadriceps muscle temperature of approximately 2°C with 30 minutes of cycling at 70% of age-predicted maximum heart rate. The heat produced in the muscle is transferred to the surrounding tissues by conduction. Excessive thermal energy (heat) is shunted to the skin through blood flow for removal by radiation and evaporation if the sweat glands are activated. Blood flow to the working muscles increases during exercise to deliver needed nutrients and oxygen, while also removing waste from the muscle.18 Consequently, blood flow also increases to the tissues directly above the active muscle,17 which should help to dissipate the heat that forms in the muscle. However, blood flow during an ice-bag application decreases, according to previous studies.13,19 Knight and Londeree19 found no differences in postexercise blood flow rates during heat, cold, and control treatments. They suggested that the exercise effects could have been so great as to negate the temperature effects. Our results concur with this finding for intramuscular temperature; however, this does not seem to be true for superficial temperature.
When an ice bag is applied to the skin, cooling depends on the affinity for thermal exchange by the skin and the ice bag. An increased temperature gradient between the active muscle and the ice bag16 could increase thermal exchange. We speculate this is one possible reason why cooling was observed at the skin surface and not in the muscle during the ice and walking treatment. Other factors include a constant production of heat from the working muscle, as discussed earlier, and the application technique. We suspect that the use of plastic wrap to keep the ice bag in place provides both compression and insulation for maximal thermal exchange with the ice bag. However, this may also have inhibited the body's natural mechanism for thermoregulation by not allowing heat to escape from the working muscle by either reduced cellular perfusion from the compression or radiation and evaporation exchanges by surrounding the area with plastic wrap and the small dressing.
The approach of applying an ice bag to the calf and letting the athlete walk is not effective for cooling deep muscle tissue. However, we do not know if the same effect would be seen if ice is placed on less active weight-bearing or upper extremity muscles. We also do not know what effect a to-go ice bag has on athletes after physical activity, which is something we have begun investigating.
CONCLUSIONS
Wrapping a to-go ice bag over active lower leg muscles does not induce temperature decreases in deeper tissues, despite skin cooling, as does cooling while resting. Therefore, we suggest that cooling be performed while resting the underlying tissues to maximize thermal exchange of the deeper tissues. With regard to pain control from the to-go ice bag, based on the superficial temperature changes, it seems that the activity level of the underlying tissues may not affect this positive outcome. Moreover, it suggests that pain control may still occur rapidly; however, these areas need further investigation.
Acknowledgments
We thank the School of Graduate Studies at Indiana State University, Terre Haute, IN, for the research funds that were granted to support this study.
Footnotes
Andrea L. Bender, MS, ATC, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Erin E. Kramer, MS, ATC, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting and final approval of the article. Jody B. Brucker, PhD, LAT, ATC, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Timothy J. Demchak, PhD, LAT, ATC, and Mitchell L. Cordova, PhD, ATC, FACSM, contributed to conception and design; analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Marcus B. Stone, PhD, LAT, ATC, contributed to conception and design; acquisition of the data; and drafting, critical revision, and final approval of the article.
Address correspondence to Jody B. Brucker, PhD, LAT, ATC, C-11 Arena Building, Indiana State University, Terre Haute, IN 47809. Address e-mail to jody_brucker@indstate.edu.
REFERENCES
- Hardy M, Woodall W. Therapeutic effects of heat, cold, and stretch on connective tissue. J Hand Ther. 1998;11:148–156. doi: 10.1016/s0894-1130(98)80013-6. [DOI] [PubMed] [Google Scholar]
- Knight KL. Cryotherapy in Sport Injury Management. Champaign, IL: Human Kinetics; 1995.
- Kellett J. Acute soft tissue injuries: a review of the literature. Med Sci Sports Exerc. 1986;18:489–500. [PubMed] [Google Scholar]
- McMaster WC. A literary review on ice therapy in injuries. Am J Sports Med. 1977;5:124–126. doi: 10.1177/036354657700500305. [DOI] [PubMed] [Google Scholar]
- Myrer JW, Measom G, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33:25–29. [PMC free article] [PubMed] [Google Scholar]
- Swenson C, Sward L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996;6:193–200. doi: 10.1111/j.1600-0838.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train. 2002;37:209–217. [PMC free article] [PubMed] [Google Scholar]
- Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31:1516–1521. doi: 10.1097/00005768-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Knight KL, Brucker J, Stoneman PD, Rubley MD. Muscle injury management with cryotherapy. Athl Ther Today. 2000;5(4):26–30. [Google Scholar]
- Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28:239–245. [PMC free article] [PubMed] [Google Scholar]
- Long BC, Cordova ML, Brucker JB, Demchak TJ, Stone MB. Effect of exercise on quadriceps muscle cooling time. J Athl Train. 2005;40:260– 263. [PMC free article] [PubMed]
- Myrer JW, Measom G, Fellingham GW. Exercise after cryotherapy greatly enhances intramuscular rewarming. J Athl Train. 2000;35:412–416. [PMC free article] [PubMed] [Google Scholar]
- Thorsson O, Lilja B, Ahgren L, Hemdal B, Westlin N. The effect of local cold application on intramuscular blood flow at rest and after running. Med Sci Sports Exerc. 1985;17:710–713. doi: 10.1249/00005768-198512000-00016. [DOI] [PubMed] [Google Scholar]
- Myrer WJ, Myrer KA, Measom GJ, Fellingham GW, Evers SL. Muscle temperature is affected by overlying adipose when cryotherapy is administered. J Athl Train. 2001;36:32–36. [PMC free article] [PubMed] [Google Scholar]
- Otte JW, Merrick MA, Ingersoll CD, Cordova ML. Subcutaneous adipose tissue thickness alters cooling time during cryotherapy. Arch Phys Med Rehabil. 2002;83:1501–1505. doi: 10.1053/apmr.2002.34833. [DOI] [PubMed] [Google Scholar]
- Zemke JE, Andersen JC, Guion WK, McMillan J, Joyner AB. Intramuscular temperature responses in the human leg to two forms of cryotherapy: ice massage and ice bag. J Orthop Sports Phys Ther. 1998;27:301–307. doi: 10.2519/jospt.1998.27.4.301. [DOI] [PubMed] [Google Scholar]
- Wirth NJ, Van Lunen BL, Mistry D, Saliba E, McCue FC. Temperature changes in deep muscles of humans during upper and lower extremity exercise. J Athl Train. 1998;33:211–215. [PMC free article] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 4th ed. Baltimore, MD: Williams & Wilkins; 1996.
- Knight KL, Londeree BR. Comparison of blood flow in the ankle of uninjured subjects during therapeutic applications of heat, cold, and exercise. Med Sci Sports Exerc. 1980;12:76–80. doi: 10.1249/00005768-198021000-00015. [DOI] [PubMed] [Google Scholar]