Abstract
Context: Exercise in the heat produces cellular conditions that may leave skeletal muscle susceptible to exercise-induced microdamage. Delayed-onset muscle soreness (DOMS) is a clinical model of contraction-induced skeletal muscle injury.
Objective: To determine whether thermoregulation during exercise heat stress adversely affects muscle injury and the accompanying DOMS.
Design: Randomized group test-retest design.
Setting: Laboratory.
Patients or Other Participants: Ten healthy male volunteers were randomly assigned to either the euhydration/hyperthermic or dehydration/hyperthermic group.
Intervention(s): Participants were randomly assigned to treadmill walking in a hot, humid environmental chamber (40°C and 75% relative humidity) with either oral rehydration (euhydration/hyperthermic) or fluid restriction (dehydration/hyperthermic). Immediately after heat exposure and while hyperthermic, participants performed an eccentrically biased downhill run to induce DOMS.
Main Outcome Measure(s): We measured DOMS characteristics pre-exercise and at 0.5, 24, 48, 72, and 96 hours postexercise.
Results: Treadmill exercise and exposure to the hot ambient environment elicited a 0.9% body mass loss for the euhydrated/ hyperthermic (mean rectal temperature after 60 minutes of heat-stress trial = 38.2 ± 0.4°C) and 3.3% body mass loss for the dehydrated/hyperthermic participants (mean rectal temperature after 60 minutes of heat-stress trial = 38.1 ± 0.4°C). Quadriceps perceived pain was significantly higher (F5,40 = 18.717, P ≤ .001) than baseline at 24 and 48 hours postexercise, following the classic pattern of DOMS. Overall lower extremity perceived pain was significantly higher for the dehydration/hyperthermia group than the euhydration/hyperthermia group (F1,8 = 6.713, P = .032). Punctate tenderness of the vastus lateralis for the dehydration/hyperthermic group was 6.9% higher (F5,40 = 4.462, P = .003) than for the euhydration/ hyperthermic group. No clinically important findings were revealed for passive range of motion for knee flexion. For both groups, quadriceps isometric strength (F5,40 = 12.924, P ≤ .001) was 17.5% and 20.0% lower at 0.5 hours postexercise than at 72 and 96 hours postexercise, respectively. Further, quadriceps isometric strength remained 10.5% reduced at 24 hours postexercise compared with 96 hours postexercise.
Conclusions: Skeletal muscle microdamage, indirectly evidenced by DOMS, was exacerbated in hyperthermic participants dehydrated by exercise in a hot ambient environment. Individuals performing novel exercise, particularly with a significant eccentric component, should use caution when training in a hot, humid environment and implement frequent rest and rehydration breaks.
Keywords: heat stress, thermal physiology, isometric strength, pain, downhill running
Training and competing in hot ambient environments produce significant physiologic challenges for athletes. Homeostatic mechanisms are necessary for dissipation of the exercise-induced rise in core body temperature and are influenced by hot environments, particularly when relative humidity increases. As core body temperature rises during strenuous exercise, blood is shunted to the skin and sweat rate increases. Without proper hydration during exercise, particularly in hot environments, hyperthermia and dehydration result as fluid is lost through sweat and insensible respiration.1,2 Dehydration accelerates the rise of core body temperature as effective circulating blood volume is reduced by blood shunted to the skin for thermoregulation and by decreased plasma volume as a result of sweating mechanisms.3–5 As muscle temperature increases, the roles of functional proteins involved in electrolyte distribution across the sarcolemma, calcium release and uptake by the sarcoplasmic reticulum, and actin-myosin interaction are influenced by the hot ambient environment and by the extent of dehydration.2,6 Increasing muscular temperature during eccentric exercise impairs muscle function and induces structural damage to rat skeletal muscle. This temperature-dependent strength loss after eccentric contractions has been attributed to a progressively greater failure of one or more steps in the excitation-contraction coupling pathway and increasing loss of calcium homeostasis with increasing temperature.7 Acute hyperthermia causes significant reductions in muscle strength and endurance in rats as well as in healthy, euhydrated males,2,3,7–10 possibly leaving skeletal muscle vulnerable to contraction-induced injury. The separate and combined effects of hyperthermia and dehydration on eccentric contraction-induced injury, the most common type of muscle injury, remain to be fully elucidated.
Contraction-induced muscle injury resulting from strenuous work or exercise occurs frequently in our daily lives and can have a marked and prolonged effect on our functional capacity. The injury is associated with the performance of eccentric contractions, which are also known as eccentric muscle actions or lengthening or plyometric contractions.7–11 This structural damage is indirectly indicated by delayed-onset muscle soreness (DOMS), the sensation of discomfort or pain that is often combined with muscle tenderness, stiffness, and weakness.12–15 Muscle soreness from DOMS typically commences 24 to 48 hours after activity, peaks between 24 and 72 hours, and resolves within 5 to 7 days.12–16 Pain and discomfort associated with DOMS are usually most severe at the musculotendinous junction. Eccentric activity results in direct strain on the elastic components of the muscle, causing myofibrillar streaming and disruption.15–17 Friden17 reported that DOMS is a common problem in sports medicine and is a deterrent to the attainment of optimal physiologic fitness. The condition may affect athletes who must perform at high levels, particularly in preseason training sessions during the summer months when the environmental stresses may be severe. Because tissue injury can produce an acute inflammatory response18 that may be affected by alterations in thermoregulation, the physiologic responses to muscle injury may be exacerbated during the development of exertional heat illness. No authors, to our knowledge, have examined whether human thermoregulation during exercise in the heat affects the physiologic processes after acute eccentric contraction-induced skeletal muscle injury.
Our purpose was to determine whether thermoregulation during exercise heat stress adversely affects muscle injury and the accompanying DOMS. We hypothesized that muscle injury and the accompanying sequelae would be adversely affected by thermoregulation during eccentric exercise after a heat-stress trial, as evidenced by increased pain, tenderness, and muscle weakness.
METHODS
Participants
The subjects were 10 healthy male volunteers between the ages of 18 and 35 years. No restrictions were placed on participants' heights and masses (Table 1). We studied males to reduce the effects of ovarian hormones on substrate utilization, lipid metabolism, body water fluctuations caused by the effects of sodium and chloride, responses to heat including sweat rate responses, and the protective effects on cell membranes.19–21
Table 1. Age, Height, and Percentage of Body Fat of Euhydrated/Hyperthermic and Dehydrated/Hyperthermic Groups*.
Participants were accepted based on the absence of regular lower body training, running, jogging, or strenuous recreational activities within 6 months of the study. Subjects were instructed to maintain normal activities of daily living and refrain from initiating an exercise program during the study. Participants with a history of lower limb injury or surgery, predisposing cardiovascular and cardiopulmonary disease, or previous heat-related illness were excluded.
Before entering the study, each volunteer completed a health and injury history questionnaire and an informed consent form consistent with the research protocol approved by the Temple University Institutional Review Board. Participants were instructed to refrain from consuming alcohol, caffeine, and tobacco products 24 hours before and during data collection. They were also instructed to avoid attempts to relieve the discomfort of DOMS in the exercised limbs, such as massage, stretching, analgesics, and nonsteroidal anti-inflammatory drugs.
Instruments and Data Collection
Perceived Pain
Perceived pain was measured using a visual analog scale (VAS) consisting of a 10-cm line labeled from left (no soreness) to right (extremely sore). The VAS has been used to reliably quantify perceived pain after eccentric exercise.22–25 The VAS was administered by reading standardized instructions to participants. Participants were asked to assess both quadriceps muscle pain and overall body pain. During each assessment, participants placed a slash mark on the line corresponding with their pain level. The VAS was measured to the nearest 0.1 cm. Each measure of pain (quadriceps and overall) was performed twice on a new, blank scale to avoid bias from previous determinations, and scores were averaged and used for data analysis. The VAS measurement average intratester reliability for the current study was an intraclass correlation coefficient (ICC 2,1) = .99.
Muscle Tenderness
We measured muscle tenderness using a punctate tenderness gauge (PTG) (model 75 force gauge probe; Technical Products Co, North Caldwell, NJ). The PTG has a 2-mm hemispheric probe attached to a force gauge. A 1-cm closed-cell foam stopper covered with low-temperature thermoplastic was attached to the distal end of the probe to simulate digital palpation of the muscle to measure for tenderness.25 Spring-loaded probes similar to the PTG have been used to reliably quantify muscular tenderness.25–27 The PTG has weights traceable to the National Bureau of Standards with a reliability of r = .99, P < .05 (D. C. Meserlian, unpublished data, 1987 to 1991). The PTG measurement intratester reliability for the current study was ICC (2,1) = .76.
Assessment of quadriceps muscle tenderness consisted of reading standardized instructions to each subject before data collection. Each subject was placed in a seated position with the knee fully extended and the hip in 90° of flexion and 0° of abduction and adduction. A custom-made plastic grid with holes at 10 sites spaced approximately 5 cm apart was used to standardize PTG placement over the quadriceps muscle group. The grid was placed over the anterior thigh with the distal corners positioned over the medial and lateral femoral condyles and secured with Velcro straps (Velcro USA, Inc, Manchester, NH). The PTG was placed into each of the grid holes and depressed until the subject reported that the pressure sensation had become painful. The maximum limit of force applied was 6.4 kg; a value of 6.4 kg was considered an absence of muscle tenderness. The force required to elicit pain for each of the sites was averaged for the 3 superficial quadriceps muscles (vastus medialis, vastus lateralis, rectus femoris). The mean value for each muscle was used for data analysis.
Passive Range of Motion
We measured knee-flexion passive range of motion (PROM) bilaterally using a standard clear plastic goniometer (Orthopedic Equipment Co, Bourbon, IN). The goniometer is marked in 1° increments. Knee-flexion PROM assessment consisted of placing the subject prone with the hip in 0° of abduction, flexion, rotation, and extension as described by Norkin and White.28 The femur was stabilized to prevent extraneous movement. The goniometer was aligned with the fulcrum centered over the lateral femoral epicondyle. The proximal arm was placed along the lateral midline of the femur using the greater trochanter as a reference, and the distal arm was placed along the fibula using the lateral malleolus as a reference. The starting position was 0° of knee flexion, and PROM was measured when the examiner moved the knee into flexion and the participant reported pain or soreness. Measurements were performed twice for each leg and averaged for data analysis. Knee-flexion PROM measurement intratester reliability for the current study was ICC (2,1) = .99.
Isometric Muscle Strength
The Biodex B-2000 Isokinetic Dynamometer (Biodex Corp, Shirley, NY), a multijoint testing and rehabilitation system, was used to measure isometric quadriceps strength. Isokinetic dynamometry reliably assesses isometric strength after eccentric exercise.14,25,29,30 The dynamometer was calibrated before each data-collection session with a standard mass on the right knee attachment. Standardized instructions were read to each participant before data collection. Following the manufacturer's guidelines, each subject was seated on the isokinetic dynamometer with the hip flexed to 90° and the knee flexed to 60°. Each participant folded his arms across his chest, and we secured straps over the arms, pelvis, femur, and tibia. The tibial attachment was adjusted proximal to the talocrural joint to allow full ankle motion. The joint line of the knee was aligned with the dynamometer axis. Knee and hip ranges of motion were confirmed goniometrically. Isometric contractions were performed with the dynamometer locked at 60° (midrange) of knee flexion. Participants performed 5 warm-up repetitions of isometric knee extension. After the warm-up, the participants were instructed to apply as much knee-extension pressure as possible against the dynamometer arm for 5 maximal isometric repetitions for each limb. The measurements were averaged and used as the criterion measure for data analysis. Bilateral quadriceps isometric strength measurement intratester reliability for the current study was ICC (2,1) = .95.
Exercise Protocols
Heat-Stress Trial
All heat-stress trials were performed in an environmental chamber (Tenny Engineering, Union, NJ) on a motor-driven treadmill (model Q55; Quinton Instruments, Seattle, WA). The environmental chamber is a stainless steel vault measuring 10 × 6 × 6.5 ft and capable of producing and maintaining temperatures up to 40°C and 85% relative humidity. We used a dry bulb thermometer (Bacharach, Pittsburgh, PA) to monitor ambient temperature and a sling psychrometer (Bacharach) to determine relative humidity. Ambient temperature and relative humidity were recorded every 15 minutes during the heat-stress trials to verify environmental conditions.
All participants were instructed to maintain their normal diets the day before exercise testing. On the morning of the heat-stress trial, volunteers consumed a 4-oz (118-mL) glass of orange juice and either half a bagel or a piece of toast for breakfast. All subjects were weighed and read standard instructions before performing the heat-stress trial, which consisted of treadmill walking in the hot, humid environmental chamber. Temperature and relative humidity were measured frequently to ensure maintenance of a hot, humid environment (40 ± 1.0°C and 75 ± 1.0% relative humidity) for the heat-stress trials. As a safety precaution, participants with a rectal temperature exceeding 39.0°C (model 401 rectal probe and model 43 telethermometer; YSI, Yellow Springs, OH) were removed from the environmental chamber. The warm-up phase consisted of 5 minutes of treadmill walking at 2.5 mi/h (4.02 km/h). The exercise phase consisted of 55 minutes of treadmill walking at 3.0 mi/h (4.83 km/h) for a total of 60 minutes. The treadmill was at 0° grade for all phases of the treadmill walking. Euhydrated participants consumed cool water ad libitum, and dehydrated participants were fluid restricted. A 60-second rest period, during which body mass losses were monitored, was permitted every 15 minutes. In order to ensure hyperthermia, participants were not removed from the environmental chamber until they reached a minimal rectal temperature of 38.0°C. All participants began the downhill run within 15 minutes of completing the heat-stress trial to ensure maintenance of the hyperthermic condition.
Delayed-Onset Muscle Soreness Inducement
In order to induce DOMS, both groups performed an eccentrically biased downhill run (−12° from horizontal) in a thermoneutral environment. Standard instructions were read to each participant. Exercise variables were measured every 15 minutes during the downhill run. After a 5-minute walking warm-up, participants ran downhill for 40 minutes at 5.0 ± 0.2 mi/h (8.05 ± 0.32 km/h) at a 50% heart rate range. The euhydrated participants were allowed water every 15 minutes ad libitum, whereas the dehydration/hyperthermic group continued to be fluid restricted. Upon completion of the exercise session and data collection, dehydrated participants rehydrated to within 1% of pre-exercise body mass before leaving the laboratory.
Procedures
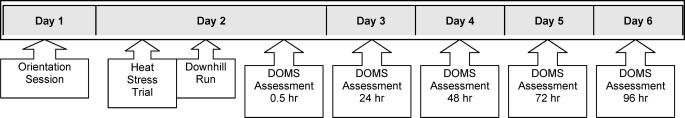
Participants reported to the laboratory for an orientation session (Figure 1) approximately 15 to 20 hours before the experiment for baseline data collection. Each participant was weighed nude in order to determine baseline body mass. Euhydration was defined as ±1% of baseline body mass on the day of data collection. Participants were randomly assigned to 1 of 2 experimental groups (each n = 5): euhydrated/hyperthermic or dehydrated/hyperthermic.
Figure 1. Research protocol flow chart. DOMS indicates delayed-onset muscle soreness.
On the next day, all participants performed the heat-stress trial by walking for 60 minutes in a hot, humid environment (40 ± 2.0°C and 75 ± 10% relative humidity) designed to produce hyperthermia (mean body temperature greater than 38.0°C). The euhydrated/hyperthermic group was permitted water ad libitum during all phases of exercise to maintain a euhydrated body mass. The dehydrated/hyperthermic group was fluid restricted and continued the heat-stress trial (approximately 60 minutes) until a 3.0 ± 1.0% decrease in pre-exercise body mass was achieved.
Immediately after the heat-stress trial and while hyperthermic, participants performed a DOMS-inducing exercise bout consisting of a 45-minute downhill run in a thermoneutral environment (22.0°C and 45% relative humidity). As a safety precaution and to ensure the hyperthermic condition, heart rate, blood pressure, and rectal temperature were monitored every 15 minutes during exercise. Within 60 minutes of the downhill run, participants were assessed bilaterally for DOMS with the following measures: VAS for quadriceps and overall pain, PTG for quadriceps tenderness, PROM for knee flexion, and isometric quadriceps strength. Dependent variables were recorded at 0 hours pre-exercise and at 0.5, 24, 48, 72, and 96 hours postexercise. Body composition was assessed before data collection, and cardiovascular fitness was assessed after data collection was completed.
Data Analysis
The research design was a randomized group test-retest design. We analyzed participant characteristics of age, height, body mass, and percentage of body fat using independent t tests. The DOMS characteristics were analyzed using separate 2 × 6 analyses of variance with repeated measures over time (pre-exercise and 0.5, 24, 48, 72, and 96 hours postexercise). When F ratios indicated a significant interaction in any of the factorial analyses of variance, we used a test of simple main effects to detect differences. Significance was set at P ≤ 0.05 for all statistics. Post hoc pairwise comparisons with Bonferroni adjustments were performed when the univariate tests revealed significance. We used the SPSS statistical program for Windows (version 10.0; SPSS Inc, Chicago, IL) for data analysis.
RESULTS
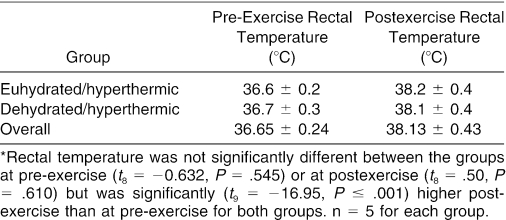
Participants' physical characteristics (see Table 1) and rectal temperature response before and after the heat-stress trial (Table 2) were not significantly different between groups; however, rectal temperature was 3.9% (t9 = −16.95, P ≤ .001) higher postexercise than pre-exercise for both groups. The heat-stress trial (Table 3) resulted in significantly reduced body mass (−3.3% [−2.40 kg], t8 = 9.174, P <.001) for the dehydrated/hyperthermic participants, compared with the euhydrated/hyperthermic participants (−.9% [−0.74 kg]).
Table 2. Rectal Temperature Before and After a 60-Minute Heat-Stress Trial in the Euhydrated/Hyperthermic and Dehydrated/ Hyperthermic Groups and Overall*.
Table 3. Pre-Exercise Body Mass, Postexercise Body Mass, and Percentage of Body Mass Lost in the Euhydrated/Hyperthermic and Dehydrated/Hyperthermic Groups.
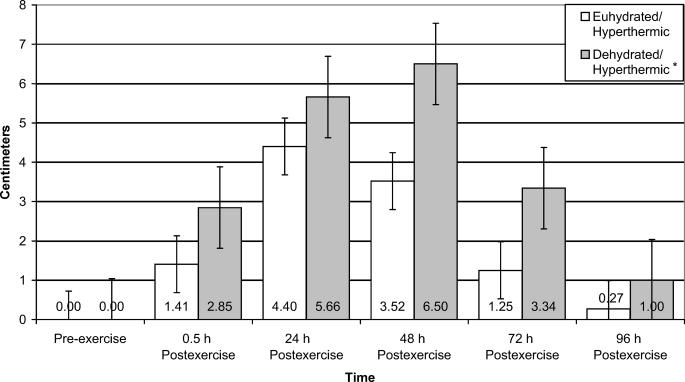
Perceived pain in the quadriceps muscle group was significantly different for both groups over time (F5,40 = 18.717, P ≤ .001, power = 1.000) (Figure 2). Quadriceps perceived pain was increased at 24 and 48 hours postexercise compared with pre-exercise. Quadriceps perceived pain was also increased 54.6% at 48 hours postexercise compared with 0.5 hours postexercise. By 96 hours postexercise, quadriceps perceived pain had decreased by 89.1% and 76.4% from 48 and 72 hours postexercise, respectively. Overall lower extremity perceived pain was 44.0% higher for the dehydration/hyperthermia group than for the euhydration/hyperthermia group (F1,8 = 6.713, P = .032, power = .623) (Figure 3).
Figure 2. Perceived pain of the bilateral quadriceps muscles of euhydrated and dehydrated hyperthermic participants measured pre-exercise and 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly increased pain over pre-exercise for both groups (P < .001). †Significantly more pain than at 0.5 hours postexercise for both groups (P < .001). ‡Significantly less pain than at 48 and 72 hours postexercise for both groups (P < .001).
Figure 3. Perceived overall lower extremity pain of euhydrated and dehydrated hyperthermic participants measured pre-exercise and 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly increased perceived pain compared with the euhydration/hyperthermic group for all time periods (P = .032).
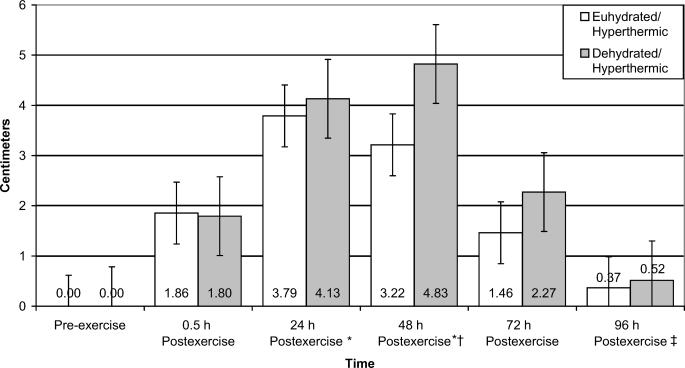
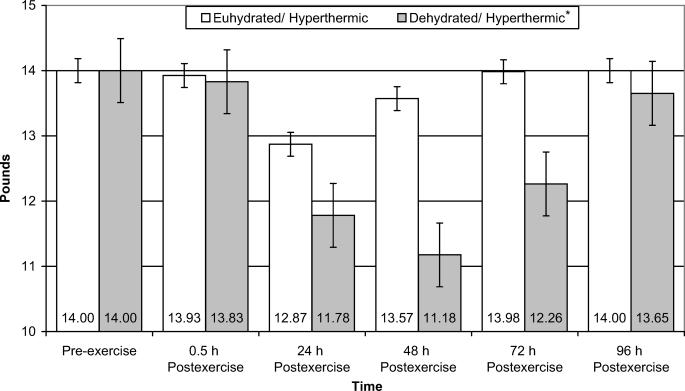
Punctate tenderness of the vastus lateralis data revealed a significant interaction between group and time (F5,40 = 4.462, P = .003, power = .577) (Figure 4). Punctate tenderness of the vastus lateralis for the dehydration/hyperthermic group was 6.9% higher than for the euhydration/hyperthermic group. Punctate tenderness of the vastus medialis revealed a significant main effect for time only (F5,40 = 4.344, P =.003, power = .997); however, we were unable to identify the location of the differences with post hoc testing. No interaction or main effects were found for punctate tenderness of the rectus femoris.
Figure 4. Punctate tenderness of the vastus lateralis muscle of euhydrated and dehydrated hyperthermic participants measured pre-exercise and 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly more punctate tenderness than the euhydration/hyperthermic group for all time periods (P = .040). Note: Punctate tenderness is inversely related to pounds of force applied, ie, the lower the value, the more tenderness.
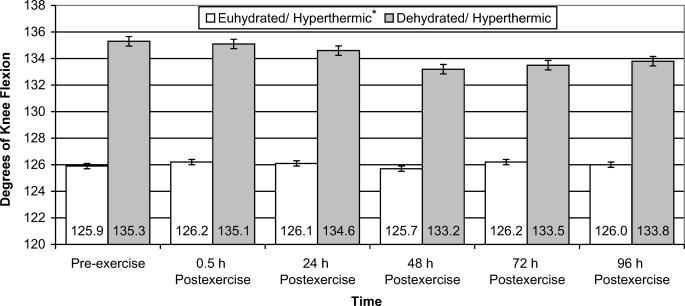
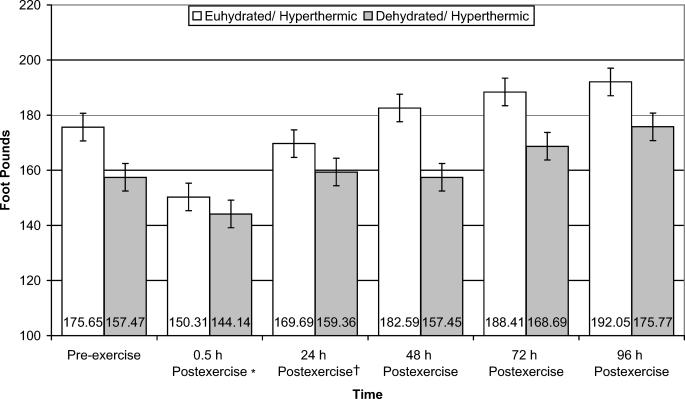
Knee-flexion PROM data revealed that the euhydration/hyperthermic group had 6.1% (F1,8 = 5.542, P = .046, power = .543) less PROM than the dehydration/hyperthermia group, regardless of time (Figure 5). Isometric strength of the quadriceps muscle group was significantly different for both groups over time (F5,40 = 12.924, P ≤ .001, power = 1.000) (Figure 6). Quadriceps isometric strength was 17.5% and 20.0% lower at 0.5 hours postexercise than at 72 and 96 hours postexercise, respectively. Further, quadriceps isometric strength remained 10.5% reduced at 24 hours postexercise compared with 96 hours postexercise.
Figure 5. Knee-flexion passive range of motion of euhydrated and dehydrated hyperthermic participants measured pre-exercise and 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly less passive range of motion than the dehydration/hyperthermia group for all time periods (P = .046).
Figure 6. Isometric quadriceps muscle strength of euhydrated and dehydrated hyperthermic participants measured pre-exercise and 0.5, 24, 48, 72, and 96 hours postexercise. *Significantly decreased strength compared with 72 and 96 hours postexercise for both groups (P < .001). †Significantly decreased strength compared with 96 hours postexercise for both groups (P < .001).
DISCUSSION
We demonstrated that downhill running produces DOMS and that dehydrated/hyperthermic participants have more symptoms of DOMS than do euhydrated/hyperthermic participants. Although the limits of the study do not provide direct evidence of tissue damage, our results suggest a relationship between the degree of physiologic disturbance and the capability of the muscle to generate eccentric force, resulting in microdamage. It is widely accepted and well established that a single bout of novel eccentric exercise causes symptoms of skeletal muscle microdamage, such as strength loss, pain, and muscle tenderness,31 all of which are clinically important in evaluating an athlete's ability to compete. The DOMS occurs regardless of fitness level and can reduce muscular performance because of the lowered inherent capacity of the muscle to produce force.11,12 Mechanical injury after eccentric muscle activity results in damage to the myofiber and the extracellular matrix14,31 and, in extreme cases, leads to rhabdomyolysis. Exertional rhabdomyolysis occurs when muscle damage is severe and is characterized by hypokalemia, intravascular coagulation, hyperuricemia, and lactic acidosis.15 Exertional rhabdomyolysis may occur after an individual performs unaccustomed, exhaustive exercise in the heat and/or may be exacerbated when the individual is dehydrated.6,15
The −12° downhill-running eccentric-exercise perturbation induced DOMS in a small sample of 10 participants, a common sample size in studies requiring numerous and complex physiologic measures. This sample size was large enough to reveal several significant findings, and our nonsignificant findings (for example, the ROM, PTG, and VAS [lower extremity] interaction effects) yielded relatively high power ratings, ranging from .616 to .707. These findings support those of Schwane et al,15 who demonstrated that −10% downhill running resulted in DOMS in 9 subjects. Newham et al32 also noted results similar to ours, demonstrating that participants experienced more quadriceps pain 48 and 72 hours after eccentric stepping-down activity. Noonan and Garrett33 reported that certain muscles are more prone to injury, with a higher risk for the muscles that cross 2 joints because, typically, they are subject to stretch at more than 1 point. This was not the case in our study, and the rectus femoris did not become significantly sorer than the vastus medialis and lateralis muscles. This finding is most likely a result of the hip being placed in a flexed position as the knee is extending while the quadriceps was eccentrically activated during downhill running. With the hip flexed, tension on the rectus femoris muscle is reduced. Because of the anatomical arrangement of the rectus femoris muscle, crossing both the hip and knee joints, the rectus is not as involved in the deceleration of the lower leg during downhill running as are the vasti.34,35 Consequently, the rectus femoris was less sore, which is consistent with the results of quadriceps electromyography during downhill walking conducted by Ciccotti et al.34 These authors found that during stair descent and walking at a −10% grade, the vasti muscles demonstrated greater electromyographic response (medialis more than lateralis) than the rectus femoris. Peak activity of the vasti was exhibited during the terminal swing phase and throughout the mass-bearing phase of the subject's gait. The authors reported that during heel strike, the quadriceps decelerates the leg eccentrically to prevent excessive knee flexion and thereby functions as a shock absorber during initial contact. Newham et al32 noted that the vastus lateralis had greater electric activity after eccentric activity, confirming our results. The vastus lateralis suffered greater soreness than the medialis and rectus femoris muscles.
Dehydration and Hyperthermia Exacerbate Delayed-Onset Muscle Soreness
Within the limits of the current study, we postulate that the condition of dehydration of 3.3% body mass combined with hyperthermia (heat-stress trial resulted in a mean rectal temperature = 38.13 ± 0.43°C) exacerbated the skeletal muscle microdamage that occurs during an eccentric perturbation, as indirectly evidenced by the development of DOMS. Greater soreness of the overall lower body and greater punctate tenderness of at least 1 of the quadriceps muscle group (vastus lateralis) experienced in the dehydration/hyperthermic group than in the euhydration/hyperthermic group indicated that the combination of dehydration and hyperthermia exacerbated the microdamage of skeletal muscle during the eccentric exercise perturbation. Dehydration results in significant increases in core body temperature and muscle temperature during exercise,1–3 potentially contributing to muscle damage, particularly during eccentric activity, because thermoregulation, muscle cell function, and exercise performance are reduced. The magnitude of the increase in core temperature is related to hydration state and ranges from 0.10 to 0.23°C for each 1% of body mass lost.4 We noted a significantly elevated core body temperature.
Eccentric exercise results in higher local temperatures, and during intense physical exertion, deep muscle temperatures can rise to 41°C or greater, resulting in protein destruction proportional to the increased intramuscular temperature.3 Heat shock protein 70 (HSP70) has been demonstrated36,37 to increase after a resistance exercise in which myofibrillar damage is more pronounced than in endurance exercise. The physiologic changes associated with short bouts of eccentric muscle activity suggest that other stressors may be involved in the HSP70 response to such a resistance exercise. It has been proposed36,37 that any environmental condition that increases protein denaturation would be expected to induce a stress response. It is clear that resistance exercise results in protein denaturation and more evidence of damage 48 hours postexercise than endurance exercise. This increased level of myofibrillar damage/repair requires increased cell remodeling and may elicit increased synthesis of stress proteins in general. The molecular chaperone, HSC/HSP70, is important to this process and may explain the 10-fold increase in HSC/HSP70 protein after a single bout of eccentric exercise. High muscle temperature may affect the mechanical properties of muscle, making myofibers more susceptible to damage caused by large mechanical stresses such as eccentric muscle activity and resulting in increased perceived soreness or tenderness.36,37
Dehydration results in a linear increase in core body temperature by as much as 0.40°C for each 1% of body mass lost.4,5 Elevation of core body temperature is greater during exercise in hot, humid environments than in thermoneutral environments,2,4,5 as demonstrated by the 3.9% increase in rectal temperature during the heat-stress trial. The potential for heat dissipation via evaporation is reduced when the body is dehydrated. Dehydration during vigorous exercise leads to elevated core body temperature and intramuscular temperature, often above 40°C.3 Humans tolerate internal temperatures above 41°C for only brief periods of time. The maximum tolerance for body cells is about 45°C, at which point thermal coagulation of intracellular proteins occurs.6 The physiologic mechanisms compensating for heat load are thought to be attributable to the combined effects of plasma hyperosmolarity, dehydration, and hyperthermia. Disruption of intracellular calcium regulation and production of adequate supplies of adenosine triphosphate in a dehydrated state may lead to skeletal muscle microdamage during exercise.6,7 Heat load may result in a higher incidence of cross-bridge “slipping” or other alterations in actin-myosin interactions. Increased muscle temperature may induce structural and functional alterations in various proteins, including HSP70, which is known to be essential to acquiring thermotolerance, or the ability of a cell to survive repeated bouts of heat stress.36,37 Proteins affected most by dehydration and heat load are those involved in electrolyte distribution across the sarcolemma (ie, sodium-potassium and calcium adenosine triphosphatases), calcium release and reuptake by the sarcoplasmic reticulum,7 and components of the mitochondrial respiratory chain.6
Extent of Delayed-Onset Muscle Soreness After Downhill Running
No meaningful differences in PROM of the quadriceps muscle group were observed after the downhill-running eccentric perturbation. In this respect, our results contradict those of Stauber et al13 and Clarkson and Tremblay.38 In both studies, the elbow flexors had a significant decrease in range of motion after eccentric exercise. In comparison, our participants performed a less strenuous aerobic-based eccentric activity with the large quadriceps muscle group than participants in the studies by Stauber et al13 and Clarkson and Tremblay.38 These groups had their subjects perform 70 maximal eccentric repetitions of the elbow flexor muscles. The eccentric activity in our study may not have been strenuous enough or may have been distributed over too large a cross-sectional area to elicit changes in PROM.
Unlike those of Clarkson and Tremblay,38 our participants did not suffer from debilitating muscle soreness after eccentric activity. These researchers demonstrated a significant loss of isometric force generation after eccentric elbow flexion. Similar decreases in isometric strength were reported by other investigators.30,31,39 In the study by Friden et al,31 isometric knee force was measured at 80° of knee flexion, in contrast to our study, in which participants performed isometric activity at 60°. Friden et al31 reported decreases in strength of knee flexion up to 6 days after eccentric activity. Sargeant and Dolan39 found differences in isometric strength at 24 hours postactivity; these differences had recovered by 48 hours. Draganich et al40 demonstrated that during isometric contractions, electromyographic activity was greatest in the quadriceps muscles at 60° and 30° of knee flexion. Newham et al32 reported that isometric quadriceps contractions with the rectus in a shortened position were less painful after a damaging bout of eccentric activity. The rectus femoris is more active during seated isometric-force generation than other quadriceps muscles, and it is possible that isometric strength was not decreased because the rectus was less sore in our study. Deschenes et al41 induced DOMS by isokinetic dynamometry and reported that the rectus femoris was sorer than the vasti muscles because of the anatomic positioning of the rectus femoris. The rectus had an increase in electric activity as measured by electromyography in the postinjury period, whereas the vasti showed no effect of injury on electromyography. These researchers found that during maximal effort, significant decrements in neuromuscular efficiency were detected up to 10 days postexercise, even though peak torque had returned to normal. Because the participants performed an eccentric activity that isolated the rectus femoris and the results of the posttest soreness measures for both groups were the same as for the offending activity, it is likely that the decreased strength reported is a result of the activity and not DOMS.
Our findings may have been influenced by the low-intensity speed and grade of downhill running and the large size of the quadriceps muscle group. In downhill running, the extensor muscles of the lower limb contract eccentrically during each stride to decelerate the center of mass after heel strike. Eccentric muscle contraction during downhill running has been associated with increased mechanical stress and ultrastructural microdamage.14,16,31 Lieber and Friden42 reported that the muscle damage their participants experienced was not a function of peak muscle force but was caused by the magnitude of strain experienced by the muscle during the activity. The extent of the injury increased with the duration of the lengthening action. These authors noted that the duration of stimulation necessary to cause injury decreased with increases in lengthening velocities. This finding implies that slower, more controlled eccentric movements result in less DOMS than faster, less controlled movements. Because we used the experimental protocol of downhill running, which involved relatively slow, controlled movements at low metabolic cost, rather than severely damaging bouts of isokinetic or maximum eccentric activity, muscle soreness in our subjects did not appear to be as severe as muscle soreness reported in other studies.31,42 Running downhill at greater speeds for a shorter time may cause greater soreness and a significant reduction in strength, which was not experienced by our participants.
We demonstrated that, in a hyperthermic condition, dehydration exacerbated the signs and symptoms of DOMS after a downhill-running eccentric-exercise perturbation. We conclude that the combined deleterious effects of dehydration and hyperthermia exacerbate the signs and symptoms of DOMS in healthy males and that these findings have important consequence for athletes training and competing in hot, humid environments. We recommend using caution during novel exercise, particularly with a significant eccentric component, in hot, humid environments and implementing frequent rest breaks for cooling and rehydration to reduce the debilitating effects of DOMS.
REFERENCES
- Gaebelein CJ, Senay LC., Jr. Influence of exercise type, hydration, and heat on plasma volume shifts in men. J Appl Physiol. 1980;49:119–123. doi: 10.1152/jappl.1980.49.1.119. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Mora-Rodriguez R, Below PR, Coyle EF. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol. 1997;82:1229–1236. doi: 10.1152/jappl.1997.82.4.1229. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldi T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Latzka WA, Matott RP, Montain SJ. Hydration effects on temperature regulation. Int J Sports Med. 1998;19:S108–S110. doi: 10.1055/s-2007-971971. (suppl 2) [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Maresh CM, Gabaree CV. Thermal and circulatory responses during exercise: effects of hypohydration, dehydration, and water intake. J Appl Physiol. 1997;82:2028–2035. doi: 10.1152/jappl.1997.82.6.2028. et al. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Febbraio M. Limits to exercise performance in the heat. Int J Sports Med. 1998;19:S115–S116. doi: 10.1055/s-2007-971973. (suppl 2) [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Armstrong RB. Temperature dependency of force loss and Ca(2+) homeostasis in mouse EDL muscle after eccentric contractions. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1122–1132. doi: 10.1152/ajpregu.00671.2001. [DOI] [PubMed] [Google Scholar]
- Hedley AM, Climstein M, Hansen R. The effects of acute heat exposure on muscular strength, muscular endurance, and muscular power in the euhydrated athlete. J Strength Cond Res. 2002;16:353–358. [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Warren GL, Ingalls CP, Lowe DA, Armstrong RB. What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? J Orthop Sports Phys Ther. 2002;32:58–64. doi: 10.2519/jospt.2002.32.2.58. [DOI] [PubMed] [Google Scholar]
- Byrnes WC, Clarkson PM. Delayed onset muscle soreness and training. Clin Sports Med. 1986;5:605–614. [PubMed] [Google Scholar]
- Braun WA, Dutto DJ. The effects of a single bout of downhill running and ensuing delayed onset of muscle soreness on running economy performed 48 h later. Eur J Appl Physiol. 2003;90:29–34. doi: 10.1007/s00421-003-0857-8. [DOI] [PubMed] [Google Scholar]
- Stauber WT, Clarkson PM, Fritz VK, Evans WJ. Extracellular matrix disruption and pain after eccentric muscle action. J Appl Physiol. 1990;69:868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. (11 suppl) [DOI] [PubMed] [Google Scholar]
- Schwane JA, Johnson SR, Vandenakker CB, Armstrong RB. Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc. 1983;15:51–56. [PubMed] [Google Scholar]
- Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990;22:429–435. [PubMed] [Google Scholar]
- Friden J. Muscle soreness after exercise: implications of morphological changes. Int J Sports Med. 1984;5:57–66. doi: 10.1055/s-2008-1025881. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Latzka WA, Sawka MN. Impact of muscle injury and accompanying inflammatory response on thermoregulation during exercise in the heat. J Appl Physiol. 2000;89:1123–1130. doi: 10.1152/jappl.2000.89.3.1123. [DOI] [PubMed] [Google Scholar]
- Rooney TP, Kendrick ZV, Carlson J. Effect of estradiol on the temporal pattern of exercise-induced tissue glycogen depletion in male rats. J Appl Physiol. 1993;75:1502–1506. doi: 10.1152/jappl.1993.75.4.1502. et al. [DOI] [PubMed] [Google Scholar]
- Anderson GS, Ward R, Mekjavic IB. Gender differences in physiological reactions to thermal stress. Eur J Appl Physiol Occup Physiol. 1995;71:95–101. doi: 10.1007/BF00854965. [DOI] [PubMed] [Google Scholar]
- Bar PR, Amelink GJ, Oldenburg B, Blankenstein MA. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci. 1988;42:2677–2681. doi: 10.1016/0024-3205(88)90243-3. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Flandry F, Hunt JP, Terry GC, Houghston JC. Analysis of subjective knee complaints using visual analog scales. Am J Sports Med. 1991;19:112–118. doi: 10.1177/036354659101900204. [DOI] [PubMed] [Google Scholar]
- Mattacola CG, Perrin DH, Gansneder BM, Allen JD, Mickey CA. A comparison of visual analog and graphic rating scales for assessing pain following delayed onset muscle soreness. J Sport Rehabil. 1997;6:38–46. [Google Scholar]
- Gulick DT, Kimura IF, Sitler M, Paolone A, Kelly JD. IV. Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996;31:145–152. [PMC free article] [PubMed] [Google Scholar]
- Cott A, Parkinson W, Bell MJ. Interrater reliability of tender point criterion for fibromyalgia. J Rheumatol. 1992;19:1955–1959. et al. [PubMed] [Google Scholar]
- Edwards RH, Mills KR, Newham DJ. Measurement of severity and distribution of experimental muscle tenderness. J Physiol (Lond) 1981;317:1P–2P. [Google Scholar]
- Norkin CC, White DC. Measurement of Joint Motion: A Guide to Goniometry. Philadelphia, PA: FA Davis; 1985.
- Saxton JM, Donnelly AE. Light concentric exercise during recovery from exercise-induced muscle damage. Int J Sports Med. 1995;16:347–351. doi: 10.1055/s-2007-973018. [DOI] [PubMed] [Google Scholar]
- Golden CL, Dudley GA. Strength after bouts of eccentric or concentric contractions. Med Sci Sports Exerc. 1992;24:926–933. [PubMed] [Google Scholar]
- Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Newham DJ, Mills KR, Quigley BM, Edwards RHT. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 1983;64:55–62. doi: 10.1042/cs0640055. [DOI] [PubMed] [Google Scholar]
- Noonan TJ, Garrett WE., Jr. Injuries at the myotendinous junction. Clin Sports Med. 1992;11:783–806. [PubMed] [Google Scholar]
- Ciccotti MG, Kerlan RK, Perry J, Pink M. An electromyographic analysis of the knee during functional activities, I: the normal profile. Am J Sports Med. 1994;22:645–650. doi: 10.1177/036354659402200512. [DOI] [PubMed] [Google Scholar]
- Mizrahi J, Verbitsky O, Isakov E. Shock accelerations and attenuation in downhill and level running. Clin Biomech (Bristol, Avon) 2000;15:15–20. doi: 10.1016/s0268-0033(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Thompson HS, Scordilis SP, Clarkson PM, Lohrer WA. A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiol Scand. 2001;171:187–193. doi: 10.1046/j.1365-201x.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- Thompson HS, Clarkson PM, Scordilis SP. The repeated bout effect and heat shock proteins: intramuscular HSP27 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol Scand. 2002;174:47–56. doi: 10.1046/j.1365-201x.2002.00922.x. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol. 1988;65:1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ, Dolan P. Human muscle function following prolonged eccentric exercise. Eur J Physiol Occup Physiol. 1987;56:704–711. doi: 10.1007/BF00424814. [DOI] [PubMed] [Google Scholar]
- Draganich LF, Jaeger RJ, Kralj AR. Coactivation of the hamstring and quadriceps during extension of the knee. J Bone Joint Surg Am. 1989;71:1075–1081. [PubMed] [Google Scholar]
- Deschenes MR, Brewer RE, Bush JA, McCoy RW, Volek JS, Kraemer WJ. Neuromuscular disturbance outlasts other symptoms of exercise-induced muscle damage. J Neurol Sci. 2000;174:92–99. doi: 10.1016/s0022-510x(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1983;74:520–526. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]