Abstract
Quality control in the endoplasmic reticulum (ER) prevents the arrival of incorrectly or incompletely folded proteins at their final destinations and targets permanently misfolded proteins for degradation. Such proteins have a high affinity for the ER chaperone BiP and are finally degraded via retrograde translocation from the ER lumen back to the cytosol. This ER-associated protein degradation (ERAD) is currently thought to constitute the main disposal route, but there is growing evidence for a vacuolar role in quality control. We show that BiP is transported to the vacuole in a wortmannin-sensitive manner in tobacco (Nicotiana tabacum) and that it could play an active role in this second disposal route. ER export of BiP occurs via COPII-dependent transport to the Golgi apparatus, where it competes with other HDEL receptor ligands. When HDEL-mediated retrieval from the Golgi fails, BiP is transported to the lytic vacuole via multivesicular bodies, which represent the plant prevacuolar compartment. We also demonstrate that a subset of BiP-ligand complexes is destined to the vacuole and differs from those likely to be disposed of via the ERAD pathway. Vacuolar disposal could act in addition to ERAD to maximize the efficiency of quality control in the secretory pathway.
INTRODUCTION
The endoplasmic reticulum (ER) contains a set of chaperones to promote efficient translocation, synthesis, and folding of proteins transported within the secretory pathway (Vitale and Denecke, 1999; Kleizen and Braakman, 2004). The lumenal binding protein (BiP) is one of the best-characterized ER chaperones. It belongs to the Hsp70 family and is thought to bind to folding and translocation intermediates, misfolded proteins, and peptides displaying hydrophobic regions (Blond-Elguindi et al., 1993; Gething, 1999). The classic view of the biological purpose of this interaction is to prevent aggregation that could lead to permanent misfolding (Gething et al., 1986; Hurtley et al., 1989; Gething and Sambrook, 1992; Hendershot et al., 1996).
Most BiP–protein interaction studies have been performed with synthetic peptides, and only a few model proteins have been analyzed (Vitale et al., 1995; Pedrazzini et al., 1997; Nuttall et al., 2002; Foresti et al., 2003; Mainieri et al., 2004; Randall et al., 2005). BiP-ligands can be coimmunoprecipitated and subsequently released by addition of ATP in vitro, suggesting an energy-dependent release mechanism (Munro and Pelham, 1986; Vitale et al., 1995). Consistent with this assumption, dominant-negative BiP ATPase mutants are able to bind to unfolded proteins but compromise proper folding because they fail to release their ligands (Hendershot et al., 1996). Experiments with the bacterial Hsp70 protein DnaK have demonstrated that this chaperone can actively dissolve protein aggregates formed from misfolded proteins in an ATP-fueled manner (Ben-Zvi et al., 2004). This suggests a more active role beyond merely preventing aggregation, but it remains to be shown if this principle is valid for all members of the Hsp70 family.
When nascent polypeptides emerge in the lumen of the ER during cotranslational translocation, they are still unfolded and interact with BiP (Matlack et al., 1999). Therefore, any secretory protein is a potential BiP-ligand during its life as a folding intermediate. When folding and assembly are complete, hydrophobic regions are no longer displayed and BiP will cease to bind. Permanently misfolded proteins can emerge when physiological conditions are unfavorable or when erroneous proteins are synthesized. In these cases, continuous binding of BiP results eventually in ER-associated protein degradation (ERAD). The current pathway for this event leads via the translocation pore back to the cytosolic proteasome (McCracken and Brodsky, 2003).
Evidence for the ERAD pathway in plants arose from studies with soluble proteins (Di Cola et al., 2001, 2005; Brandizzi et al., 2003) and membrane-spanning proteins (Muller et al., 2005). However, it has become clear that alternative degradation routes in addition to the ERAD pathway exist (Schmitz and Herzog, 2004). Aside from the disposal route, it is currently unknown in any eukaryotic model system how the quality control machinery discriminates between folding intermediates and permanently misfolded proteins.
BiP is constitutively expressed under normal growth conditions, but transcription can be induced by the accumulation of misfolded proteins in the ER (Kozutsumi et al., 1988; Denecke et al., 1991; Fontes et al., 1991; Nuttall et al., 2002). This process has been termed the unfolded protein response (Rutkowski and Kaufman, 2004; Zhang and Kaufman, 2004). In addition, plant BiP can be induced by unfolded protein response–independent signal transduction pathways (Kalinski et al., 1995; Jelitto-Van Dooren et al., 1999; Wang et al., 2005). However, transcriptional induction of BiP seldom leads to increased BiP protein levels, even though mRNA concentrations and pulse labeling demonstrate a higher synthesis rate (Leborgne-Castel et al., 1999). This suggests that under ER stress, BiP turnover is increased, but it remains to be shown where BiP degradation occurs.
We have recently shown that the ER chaperone calreticulin is a constituent of COPI vesicles (Pimpl et al., 2000) and that it leaves the ER in a COPII-dependent manner (Phillipson et al., 2001). This suggests that ER residents are ER export competent and could be degraded in a post-ER compartment such as the vacuole. Evidence for a possible degradation of BiP in the vacuole can also be derived from work in Saccharomyces cerevisiae, where inhibition of COPII-dependent ER export led to the formation of dilated ER cisternae that contained large quantities of BiP together with vacuolar proteins (Nishikawa et al., 1994). Moreover, ER retention defective (ERD2) mutants were shown to contain induced transcription of the BiP gene (Semenza et al., 1990) possibly to compensate for the loss of BiP from the ER when recycling from the Golgi fails. In plants, deletion of the HDEL signal (BiPΔHDEL) does not induce significant BiP secretion, and the truncated molecule is only expressed at low steady state protein levels compared with plants overexpressing wild-type BiP (Crofts et al., 1999; Leborgne-Castel et al., 1999).
Here, we present evidence that BiP is transported to the vacuole rather than being secreted when HDEL-mediated retrieval fails. The chaperone transits via the Golgi apparatus and the multivesicular bodies to its final destination, suggesting an active signal-mediated transport pathway. The possible function in quality control and the disposal of misfolded proteins in a pathway complementary to ERAD is explored.
RESULTS
BiP Secretion Can Be Specifically Induced by the Drug Wortmannin
We hypothesized that the final location for BiP turnover is the lytic vacuole and that this would explain the inefficient secretion of BiPΔHDEL (Munro and Pelham, 1987; Crofts et al., 1999). To obtain evidence for vacuolar sorting, we have used the drug wortmannin because it specifically inhibits vacuolar sorting without affecting anterograde transport (Davidson, 1995). In plants, the drug causes hypersecretion of soluble vacuolar proteins, without affecting the rate of constitutive secretion or the efficiency of HDEL-mediated ER retention (Pimpl et al., 2003). The drug inhibits recycling of the plant vacuolar sorting receptor BP80 from the prevacuolar compartment (PVC) back to the Golgi apparatus, leading to receptor depletion and inefficient vacuolar sorting (daSilva et al., 2005).
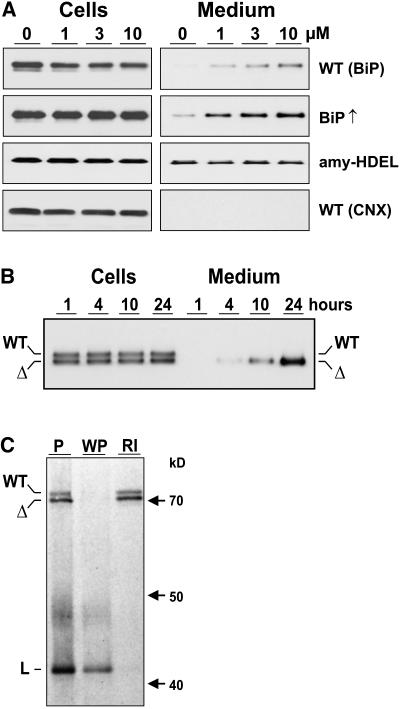
To obtain evidence for vacuolar sorting, protoplasts were isolated from wild-type tobacco (Nicotiana tabacum) leaves and incubated for 24 h with different concentrations of wortmannin. Based on previous observations (Crofts et al., 1999), to detect possible secretion of BiP, the medium was concentrated 10-fold compared with the cell extracts. Figure 1A shows that in the absence of the drug, BiP was not secreted to the culture medium. By contrast, wortmannin drastically induces the secretion of wild-type BiP in a dose-dependent manner (Figure 1A, top panel). This shows that post-ER transport of native BiP occurs in wild-type plants but is normally obscured by vacuolar degradation. Wortmannin-induced BiP secretion was more easily observed when protoplasts from stable BiP-overproducing tobacco lines were analyzed (BiP↑) (Leborgne-Castel et al., 1999).
Figure 1.
Wortmannin Induces BiP Secretion.
(A) Immunodetection of BiP in cells and 10-fold concentrated medium from tobacco wild-type protoplasts (WT BiP) incubated with an increasing concentration of wortmannin given in micromoles above the lanes. The second panel shows the result of the same type of experiment but from protoplasts prepared from stable BiP overproducing transgenic plants (BiP↑) (Leborgne-Castel et al., 1999). The third panel shows results from protoplasts prepared from transgenic plants producing recombinant α-amylase-HDEL (amy-HDEL). The bottom panel shows samples from the top panel but probed with anticalnexin antiserum (WT CNX).
(B) Secretion as a function of time in protoplast suspensions prepared from transgenic plants producing BiP (WT) and BiPΔHDEL (Δ) (Leborgne-Castel et al., 1999) incubated with 10 μM wortmannin. Sampling was performed as in (A) except that equal levels of cells and medium were loaded. Numbers above the lanes refer to time of protoplast incubation until harvesting.
(C) Coimmunoprecipitation and ATP-mediated release of BiP and BiPΔHDEL from a model ligand (L). Protoplasts prepared from a transgenic plant producing both wild-type BiP (WT) and truncated BiPΔHDEL (Δ) were transfected with a plasmid encoding a secreted form of green fluorescent protein (GFP) fused to the P-domain of calreticulin (sGFP-P), previously known to be a strong BiP-ligand (Brandizzi et al., 2003). After 5 h of expression, cells were labeled for 2 h and cell extracts immunoprecipitated with anti-GFP serum. One pellet was washed with ATP release buffer, followed by a reimmunoprecipitation of the released material with anti-BiP serum. Shown is the unwashed pellet (P), the washed pellet (WP), and the reimmunoprecipitation from the washing medium (RI). Molecular mass markers are given in kilodaltons. Note that both BiP and BiPΔHDEL bind to the ligand in an ATP-sensitive manner typical for this chaperone.
Two control experiments were performed to obtain further evidence for vacuolar sorting of BiP. First of all, a neutral HDEL protein derived from the naturally secreted protein α-amylase (amy-HDEL) shows that the drug does not influence amy-HDEL secretion (Figure 1A). This suggests that the observed induction of BiP secretion is neither due to increased ER export nor to inhibited retrieval from the Golgi apparatus. By contrast, the drug reveals that the HDEL-mediated retrieval pathway is leaky for BiP, just as the retention of amy-HDEL is not complete either. The difference between these two HDEL proteins is that the control cargo amy-HDEL has no tendency to enter the vacuolar route and is secreted without wortmannin.
A second control was to rule out nonspecific leakage of BiP from dying cells as a consequence of adverse effects of the drug on cell viability. Calnexin, an ER resident membrane spanning protein, would only be detected in the culture medium due to cell mortality. An anticalnexin serum was thus used to analyze the wild-type samples, in which induced BiP secretion was observed (Figure 1A, top panel). The results show that calnexin was completely retained in the cells regardless of the concentration of the drug (Figure 1A, CNX, bottom panel).
To further rule out nonspecific leakage, a transgenic line expressing equal amounts of BiPΔHDEL compared with endogenous wild-type BiP was used for a protein secretion experiment. This line was previously shown to exhibit very weak secretion of the truncated molecule and required concentration of the medium for detection (Crofts et al., 1999). However, upon treatment with 10 μM wortmannin, it was possible to detect BiPΔHDEL secretion without concentrating the medium (Figure 1B). Total BiPΔHDEL levels increase as a function of time when vacuolar delivery and degradation are prevented by wortmannin treatment, which is easily appreciated when observing the drastically increased BiPΔHDEL levels in the medium and the constant levels in the cells. This illustrates that the normal fate of BiPΔHDEL is rapid degradation in the vacuole, even though defined degradation intermediates cannot be purified from vacuoles.
BiP and BiPΔHDEL differ only by the presence or absence of a retrieval motif that operates from the Golgi apparatus. In the case of nonspecific cell leakage due to mortality, both BiP molecules should have been equally released to the culture medium, but in our experiments, mainly BiPΔHDEL is detected in the medium (Figure 1B). This shows that wortmannin does not cause increased cell mortality under the experimental conditions used.
To rule out that tobacco BiPΔHDEL is exported faster because it is nonfunctional and fails to interact with ligands or other ER residents, it was important to demonstrate that BiPΔHDEL was still acting as a chaperone. We have shown previously that BiPΔHDEL binds as well as wild-type BiP to calreticulin (Crofts et al., 1998). To provide further evidence of functionality, we expressed a known BiP-ligand (Brandizzi et al., 2003) in protoplasts prepared from transgenic plants producing both wild-type BiP as well as BiPΔHDEL. Figure 1C shows that both molecules interact with the ligand equally well and could be released with ATP, followed by reimmunoprecipitation with BiP antiserum. This shows that BiPΔHDEL was still functional as a chaperone. This is consistent with results obtained for the yeast BiP homologue Kar2p, for which it was shown that the C-terminal 10-kD portion of BiP is dispensable for ATP-dependent ligand binding (Tokunaga et al., 1998).
Together, the experiments demonstrate that native BiP is exported from the ER but does not secrete to the medium unless vacuolar sorting is inhibited by wortmannin. This supports our initial hypothesis regarding the apparent lack of secretion when the HDEL motif is deleted.
Wortmannin Deviates BiP Transport from the Lytic Vacuolar Route to the Apoplast in Planta
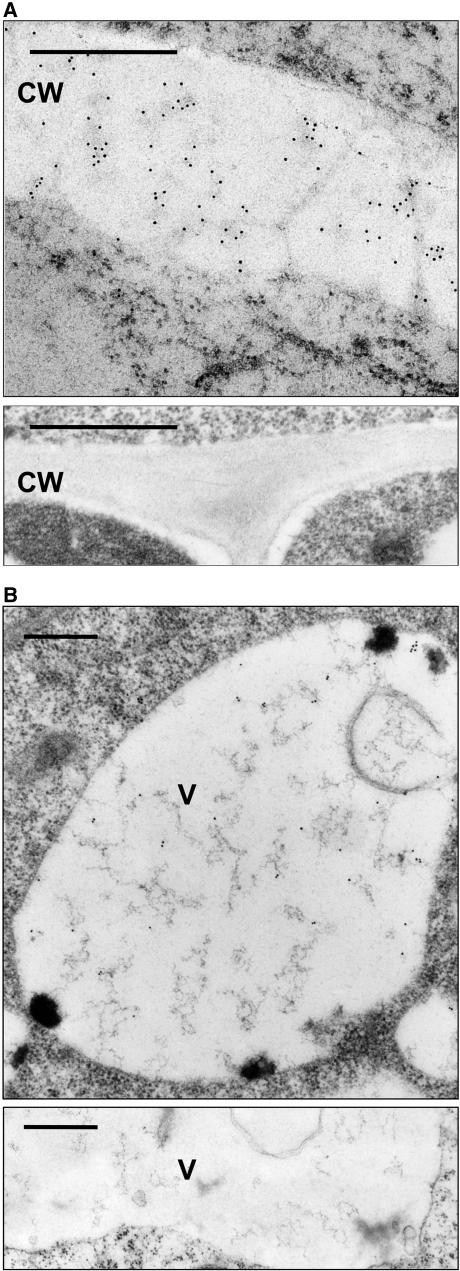
To confirm that native BiP can be secreted in planta, roots from 3-week-old wild-type tobacco seedlings were incubated for 24 h in the presence or absence of wortmannin in Murashige and Skoog (MS) medium. After fixing and embedding, immunogold electron microscopy was performed on plastic sections of the tissue. Figure 2A shows that the drug causes a clear accumulation of BiP in the apoplast (top panel). Cell wall labeling of BiP was never observed without prior incubation with wortmannin (bottom panel). Wortmannin also causes cell wall dilation, possibly due to the apoplastic mis-sorting of vacuolar hydrolases.
Figure 2.
Immunolocalization of BiP.
(A) Wild-type tobacco roots were incubated with 10 μM wortmannin for 24 h, fixed with formaldehyde/glutaraldehyde, and embedded in Lowicryl HM20 at −35°C (top panel). Sections were exposed to BiP antibodies (1:200 dilution) then 10 nm gold-coupled secondary antibodies (Biocell GAR10 1:50). Gold particles are seen throughout the cell wall (CW). The control without wortmannin (bottom panel) does not show immunogold labeling for BiP in the cell wall. Bars = 0.5 μm.
(B) Untreated tobacco cell from a transgenic BiP-overproducing plant (Leborgne-Castel et al., 1999). Immunogold labeling for BiP is observed in the vacuole (V). The control panel below is from a wild-type tobacco plant and does not exhibit labeling of the vacuole. Bars = 0.5 μm.
To obtain further proof of vacuolar sorting of BiP, it was essential to demonstrate its arrival in the vacuoles. Figure 1A shows that only a small portion of native BiP is secreted in the presence of the drug, after redirection from the vacuolar route. To facilitate detection in the vacuoles, roots of BiP-overproducing plants were used (Leborgne-Castel et al., 1999). Figure 2B shows that vacuoles were labeled with anti-BiP serum when roots were analyzed from BiP-overproducing plants grown under normal culture conditions without wortmannin. This provides direct evidence for the vacuole as the end location for BiP in the secretory pathway. However, untransformed wild-type tobacco showed no BiP labeling in the vacuoles (Figure 2B, bottom panel).
BiP Leaves the ER in COPII Vesicles and Is Transported via the Golgi Apparatus
The capability of BiP to reach the lytic vacuole (Figure 2B) or to secrete to the apoplast in the presence of wortmannin (Figures 1 and 2A) suggests that BiP leaves the ER, reaches the Golgi, and is transported via the PVC to the central lytic vacuole. This hypothesis is based on the finding that wortmannin specifically redirects soluble vacuolar cargo from the Golgi to the cell surface (Pimpl et al., 2003; daSilva et al., 2005). However, a recent proposal for a constitutive Golgi-independent route for ER residents to the vacuole (Tamura et al., 2004) opens the question regarding its transport pathway.
Export of soluble proteins from the ER occurs via COPII vesicles, resulting in delivery of their cargo to the Golgi apparatus. In plants, a COPII-independent ER export route has been described for a subset of molecules that bypass the Golgi apparatus while in transit to the storage vacuole (Hara-Nishimura et al., 1998; Toyooka et al., 2000). More recent evidence suggests that soluble proteins can also be transported to the cell surface when COPII-mediated ER export is inhibited (Törmäkangas et al., 2001).
COPII-mediated transport can be specifically inhibited in vivo via overexpression of the Sar1p-specific guanine nucleotide exchange factor Sec12p, which inhibits COPII vesicle budding (Phillipson et al., 2001). Similarly, coexpression of dominant-negative mutant forms of the GTPase Sar1p also blocks ER-to-Golgi transport: Sar1(T31N) does not bind to GTP and inhibits vesicle budding, whereas Sar1(H74L) has low GTP hydrolysis activity and leads to inefficient vesicle uncoating and thus reduces vesicle fusion with the Golgi apparatus (Takeuchi et al., 2000; Phillipson et al., 2001; Pimpl et al., 2003). To test if BiP transport occurs via the Golgi apparatus, we have implemented the above approaches to dissect the ER export pathway of BiP.
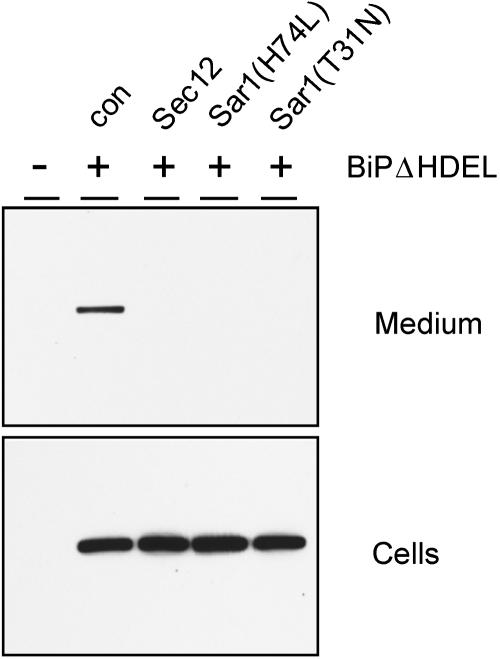
Tobacco protoplasts were transfected with plasmids encoding myc-tagged BiP lacking its ER retention motif (BiPΔHDEL), followed by incubation with wortmannin to prevent vacuolar degradation and permit secretion as seen in Figure 1B. Inhibition of ER export was achieved by cotransfection of plasmids encoding either Sec12p, Sar1(T31N), or Sar1(H74L). Transfected cells were incubated for 48 h and cells and medium analyzed by protein gel blots. Figure 3 shows that BiPΔHDEL is slowly secreted to the culture medium in the absence of effectors (con). Inhibition of COPII-mediated transport via cotransfection with any of the effector molecules strongly reduces the secretion of BiPΔHDEL. This result demonstrates that COPII-mediated transport between the ER and the Golgi apparatus is required for the post-ER delivery of BiP, which can be visualized when vacuolar sorting and rapid degradation are prevented by wortmannin.
Figure 3.
Inhibition of BiPΔHDEL Secretion by Blocking COPII-Mediated ER-to-Golgi Traffic.
Protoplasts were transfected with 30 μg of BiPΔHDEL-encoding plasmid (+) alone (con) or with plasmids (30 μg) encoding Sec12p, Sar1(H74L), or Sar1(T31N) and incubated for 48 h in the presence of wortmannin (10 μM), followed by separation of cells and medium. Mock transfected cells (−) were used as a negative control to illustrate the specificity of the anti-C-myc serum. Note the clear presence of secreted BiPΔHDEL in the transfected cells only when effector molecules were absent.
BiP Transport to the Golgi Can Saturate the HDEL Receptor
It has been suggested that selective import of secretory cargo into COPII vesicles occurs at higher efficiency compared with the ER resident BiP (Barlowe et al., 1994) or an HDEL-tagged bulk flow marker (Malkus et al., 2002). Figures 1 to 3 suggest that BiP is ER export competent and that export occurs at a sufficient rate in plants to allow biochemical detection. In addition, we have previously shown that calreticulin exhibits a 10-fold higher apparent secretion rate compared with BiP when the HDEL motif is deleted from either molecule (Crofts et al., 1999). We now wanted to compare ER export of BiP and calreticulin with that of a naturally secreted protein using an in vivo transport assay.
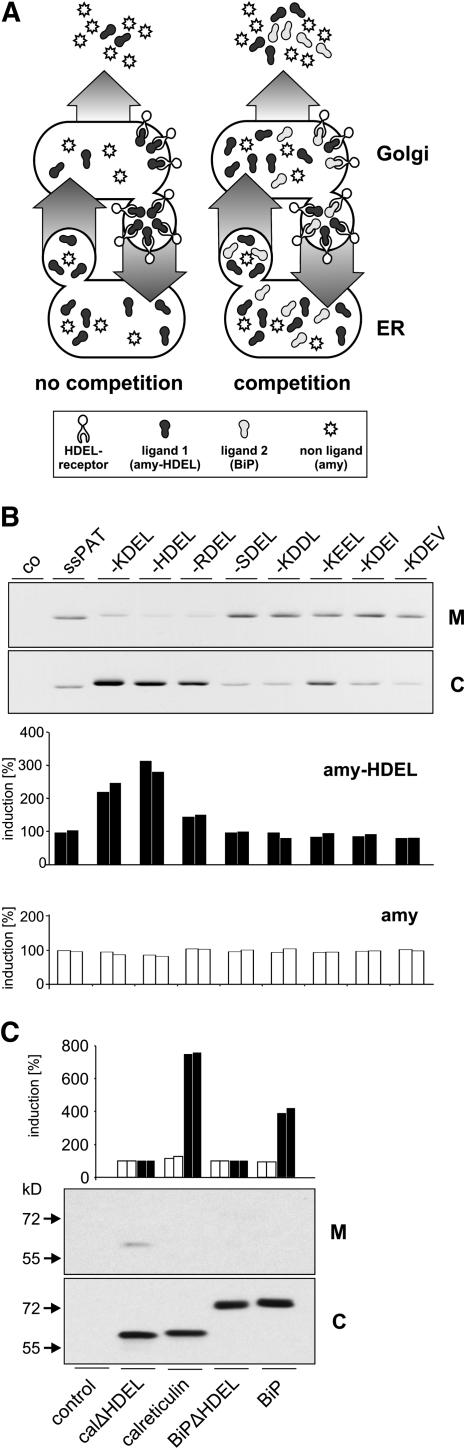
We took advantage of the properties of α-amylase (amy) and a derivative carrying the C-terminal ER retention motif HDEL (amy-HDEL) as reporter proteins (Phillipson et al., 2001). Both molecules are stable within the secretory pathway and in the extracellular matrix, permitting quantitative transport analysis via their enzymatic activity. α-Amylase is a naturally secreted protein and leaves the ER effectively in a COPII-dependent manner (Phillipson et al., 2001). amy-HDEL exits the ER in the same way but returns via COPI-mediated transport back to the ER (Pimpl et al., 2000). ER retention of amy-HDEL is dependent on expression levels. At low levels, retention is effective and most of the cargo molecule is retained in the cell. Higher concentrations lead to a dose-dependent secretion of amy-HDEL through progressive saturation of the HDEL receptor (Phillipson et al., 2001). The model in Figure 4A illustrates how amy-HDEL expressed at low levels could be used as an indicator for receptor competition by a second HDEL protein. This in vivo cargo competition assay could serve as a positive method to detect arrival at the Golgi apparatus.
Figure 4.
Cargo Competition Assay for the HDEL Receptor.
(A) The principle of the cargo competition assay in the absence (no competition) or presence (competition) of competing ligands for binding to the HDEL receptor in the Golgi apparatus. The model illustrates how a second ligand will lead to increased secretion of itself and the monitoring reporter amy-HDEL and has no effect on the nonligand (amy).
(B) Cotransfection of tobacco protoplasts with 5 μg plasmid DNA encoding either amy or amy-HDEL with 30 μg plasmid DNA encoding PAT derivatives. The top panel shows the immunodetection of PAT derivatives, using anti-PAT serum in culture medium (M) and cellular fractions (C). The constructs were described previously (Denecke et al., 1992) and represent chimeric genes composed of a signal peptide fused to phosphinothricine acetyl transferase (ssPAT) and additionally fused tetrapeptides to the C terminus, indicated in single-letter codes above the lanes. As a control (co) for protein gel blots and amy assays, protoplasts were mock transfected. The bar graphs below show the increase in the secretion index in percent for amy-HDEL (closed bars) and amy (open bars). The secretion index is defined as the ratio between amy activity in the culture medium compared with the activity in the cellular fraction (Phillipson et al., 2001). To facilitate comparison, the secretion index was set to 100% for the samples in which secretory PAT (ssPAT) was cotransfected.
(C) Cotransfection of tobacco protoplasts with 5 μg plasmid DNA encoding for either amy or amy-HDEL with 30 μg plasmid DNA encoding for myc-tagged calreticulinΔHDEL, wild-type calreticulin, BiPΔHDEL, and wild-type BiP. The bar graph shows the increase in the secretion index in percent for amy (open bars) and amy-HDEL (closed bars). The bottom panel shows the immunodetection of myc-tagged ER chaperones in the medium (M) and the cells (C). Molecular mass markers are indicated at the left hand side of the panel. Note that only calreticulinΔHDEL is detected in the medium but that both chaperones compete with amy-HDEL for the HDEL receptor.
To establish this assay, phosphinothricine acetyl transferase (PAT) was used as a model competitor. This prokaryotic cytosolic protein was introduced into the secretory pathway via signal peptide fusion to test the bulk flow principle in plants (Denecke et al., 1990). Competition with amy-HDEL for the HDEL receptor was analyzed using secreted PAT as well as derivatives fused to the tetrapeptides KDEL, HDEL, RDEL, SDEL, KDDL, KEEL, KDEI, and KDEV as competitors. A constant amount of plasmid encoding amy-HDEL was cotransfected with either of the various PAT encoding plasmids.
Figure 4B shows that PAT derivatives were partitioned as expected from previous findings (Denecke et al., 1992) and that the tetrapeptides HDEL, KDEL, and RDEL confer ER retention. The secretion index of amy-HDEL (closed bars) is increased for these three cases. By contrast, secreted PAT or the tagged derivatives with tetrapeptides that do not confer efficient retention had no effect on amy-HDEL secretion. The results show that bulk flow of PAT must be sufficient to yield competing concentrations at the Golgi apparatus to affect amy-HDEL recycling. A crucial control was the repetition of the entire experiment with the control nonligand amy (Figure 4B, open bars). Coexpression of PAT derivatives did not reveal any measurable effect on the secretion index, which illustrates the specificity of the cargo competition assay.
We now wanted to use this competition assay to analyze the ER export efficiency of the resident proteins calreticulin and BiP. A cargo competition experiment was therefore designed in which myc-tagged calreticulin, calreticulinΔHDEL, BiP, and BiPΔHDEL were coexpressed with a constant amount of either amy-HDEL or amy. Figure 4C shows that all competitors were retained in the cells except for truncated calreticulin. However, both chaperones cause an increase in the secretion of amy-HDEL compared with the truncated proteins. By contrast, the control cargo amy was not affected by the competitors. This demonstrates that these ER residents exit the ER sufficiently to compete with amy-HDEL for the receptor in the Golgi apparatus, as already shown for the bulk flow marker PAT.
BiP Is Transported via Multivesicular Bodies to the Vacuole
Having shown that BiP is exported to the Golgi apparatus, its route to the vacuole had to be established. Export from the Golgi to the lytic vacuole is thought to occur via the PVC (Sanderfoot et al., 1998). This organelle has recently been identified as multivesicular bodies in plants, which can be easily recognized in electron micrographs of high pressure frozen tissues due to their unique morphology (Tse et al., 2004). Similar to the rescuing function of the Golgi apparatus for essential ER components, multivesicular bodies have been proposed to serve as a compartment from which vacuolar sorting receptors can be rescued to return to the Golgi apparatus (reviewed in Seaman, 2005). We hypothesized that BiP would pass from the Golgi apparatus via this compartment to the vacuole.
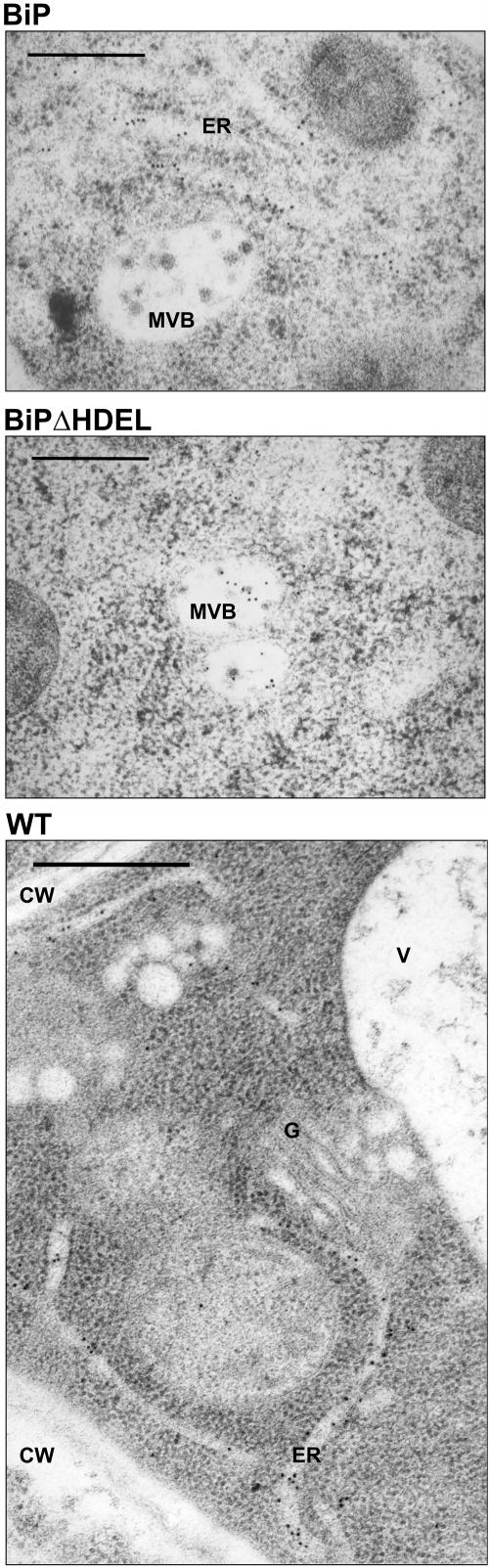
We thus compared wild-type plants with transgenic plants overproducing either wild-type BiP or ER retention–deficient BiPΔHDEL. Roots from these plants were frozen at high pressure for optimal preservation of ultrastructures (Tse et al., 2004). Figure 5 shows that labeling of multivesicular bodies with BiP antiserum was only observed in roots of BiPΔHDEL producing plants. This indicates that BiP transits this compartment on its way to the vacuole. Furthermore, the detection of BiPΔHDEL but not BiP in multivesicular bodies underlines that recycling from the Golgi apparatus reduces vacuolar transport and demonstrates the Golgi dependence of the entire pathway. Compared with wild-type BiP, which can recycle via the HDEL receptor back to the ER, detectable quantities of BiPΔHDEL enter the vacuolar transport route on reaching the Golgi apparatus.
Figure 5.
Specific Localization of BiPΔHDEL to Multivesicular Bodies.
Roots from transgenic plants overproducing wild-type BiP or BiPΔHDEL were analyzed by high-pressure freezing followed by freeze substitution and Lowicryl HM20 embedding. Thin sections were stained with anti-BiP serum and secondary antibodies linked to 10-nm gold particles. Note the 10-nm gold labeling in the ER of BiP-overproducing plants and the absence in the multivesicular body (MVB). Out of 25 multivesicular bodies, none exhibited labeling in BiP-overproducing plants. By contrast, sections from BiPΔHDEL-overproducing plants revealed an average multivesicular body labeling density of 5.2 out of a total of 25 multivesicular bodies analyzed. The control panel from untransformed wild-type plants shows an overview to illustrate the specificity of the antiserum. Note that only the ER is labeled in this section, and no labeling was seen in the vacuole (V), the cell wall (CW), and the Golgi apparatus (G). Bars = 20 μm.
Evidence for Two Populations of BiP Ligands, One Destined for the Lytic Vacuole
The puzzling question remains as to why BiP should be transported to the lytic vacuole. Allowing a chaperone or a complex containing a misfolded protein to accumulate in the secretory pathway or to reach the apoplast could be detrimental for the cells. When BiP and BiP complexes escape from the HDEL receptor, vacuolar sorting followed by degradation would solve this problem. However, it is tempting to assume that vacuolar sorting of BiP plays a more active role in quality control. Due to rapid degradation in the vacuoles, BiP-ligands may not have been detected in this organelle. To test if BiP-ligand complexes exit the ER and are transported to the vacuoles, we analyzed if they could be revealed when vacuolar sorting is redirected to the culture medium by wortmannin where proteolytic activities are lower.
Although all nascent secretory proteins are initially associated with BiP at the translocation step (Matlack et al., 1999), the interaction with folding intermediates is transient and difficult to monitor. To detect permanently misfolded proteins, cells were incubated with tunicamycin, a drug that inhibits N-linked glycosylation thereby causing permanent misfolding (Kozutsumi et al., 1988). Under these conditions, BiP-ligands can be detected by coimmunoprecipitation with BiP antiserum and can be specifically released with magnesium and ATP (Munro and Pelham, 1986; Vitale et al., 1995).
Protoplasts from wild-type tobacco leaves were isolated and thoroughly washed to minimize proteolytic activities in the surrounding medium. Protoplasts were continuously labeled in the presence of wortmannin and tunicamycin for 20 h. Cells and medium were separated and analyzed by immunoprecipitation using anti-BiP serum. To avoid nonspecific precipitation, it was crucial to clear antigen antibody solutions by high-speed centrifugation, followed by protein-A Sepharose binding and subsequent low-speed sedimentation. The latter washing step was repeated four times to remove any nonspecifically bound proteins. The BiP-ligand population was then released from the washed sediments using BiP release buffer supplemented with ATP. The cleared supernatant was analyzed by SDS-PAGE and phosphorimaging. This elaborate sequence of steps was found to be essential to allow comparison of BiP-ligand populations.
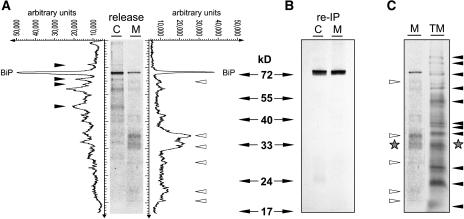
Figure 6A shows a comparison between intracellular BiP-ligands and extracellular BiP-ligands that have been specifically released from the chaperone BiP using ATP. Interestingly, the population of ligands differs significantly between cells and culture medium. Specific polypeptides are predominantly found in the cellular fractions (closed arrowheads), while other polypeptides are enriched in the medium fraction (open arrowheads).
Figure 6.
Demonstration of BiP-Ligand Complexes Exiting the ER.
(A) Immunoprecipitation of BiP-ligands from cell extracts (C) and the culture medium (M) of wortmannin-treated wild-type cells followed by ATP release and subsequent analysis by SDS-PAGE and autoradiography. Line scanning data obtained with AIDA software (Raytest) are given in arbitrary units. Arrowheads indicate specific BiP-ligands exclusively found either in cell extracts (closed) or the culture medium (open).
(B) Reimmunoprecipitation (re-IP) of ATP-released polypeptides with anti-BiP serum from (A). Compared with the quantities loaded in (A), fivefold higher amounts were used for the reimmunoprecipitation in order to test the presence of lower molecular weight BiP degradation products. Note that none of the bands identified in (A) (closed and open arrowheads) are precipitated with anti-BiP serum.
(C) Comparison between the total labeled secreted fraction in the medium and the ATP-released polypeptides from secreted BiP in (A). Note the different patterns in the two lanes except for one band (stars).
Both populations contain BiP itself originating from BiP multimers that can be disrupted with ATP as well (C.J. Snowden and J. Denecke, unpublished observations). This represents only a minor fraction of the initial precipitate from which the ATP release was performed. To demonstrate the identity of BiP in the released material, fivefold higher quantities of ATP-released molecules loaded in Figure 6A were reimmunoprecipitated with BiP antiserum. Only the full-length BiP itself was recovered (Figure 6B). This control experiment verifies the identity of BiP and demonstrates that the released polypeptides in Figure 6A are unlikely to represent BiP degradation products.
To test if BiP could randomly associate with secreted proteins, we analyzed the total population of secreted proteins in the cell suspension from this experiment. Figure 6C shows a comparison of labeled secreted molecules in the culture medium with the polypeptides released with ATP from secreted BiP. A large number of abundant bands of the total medium sample were not represented in the specific ATP-released proteins from immunoprecipitated BiP (closed arrows). Likewise, most bands released from secreted BiP (open arrows) did not match up with polypeptides in the total medium fraction. While on one occasion polypeptides appeared to be present in both fractions (stars), the otherwise differential pattern shows that abundance alone is not a prerequisite for BiP binding and suggests that the ATP release reveals a specific subset of proteins with a high affinity for BiP.
We finally wanted to test if a possible ERAD substrate can be partially transported to the vacuole. The BiP-ligand used to test the functionality of BiPΔHDEL (Figure 1C) is a fusion of secreted green fluorescent protein with the P-domain of calreticulin (sGFP-P) and was shown to enter the ER, undergo signal peptide processing and BiP interaction, followed by retrograde translocation to the cytosol and ultimately the nucleoplasm (Brandizzi et al., 2003). If vacuolar disposal complements the ERAD pathway, then it should be possible to reveal eventual vacuolar sorting by incubation with wortmannin.
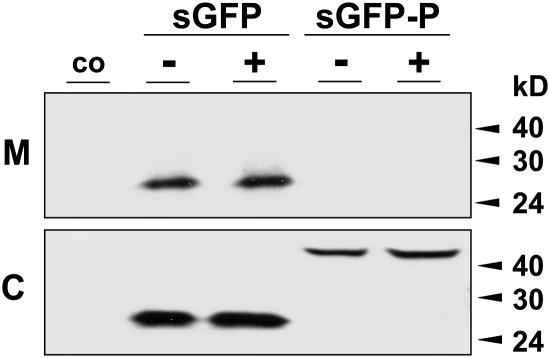
We thus expressed secreted GFP and sGFP-P in protoplasts incubated with and without wortmannin. Figure 7 shows that secreted GFP is transported to the medium, and it is not influenced by the drug. By contrast, sGFP-P is fully retained in the cells as shown before (Brandizzi et al., 2003), and wortmannin does not reveal any secretion either. This suggests that the fusion protein is not transported to the vacuole for degradation in addition to its proposed ERAD route. Consistent with this, Figure 6A showed several ATP-released bands released from cellular BiP that were not found in the medium.
Figure 7.
Transient Expression Experiment to Measure Wortmannin-Induced Secretion.
Wild-type tobacco protoplasts were either mock-transfected (co) or transfected with secreted GFP (sGFP) or sGFP-P. After incubation in the presence (+) and absence (−) of wortmannin (10 μM), cells (C) and medium (M) were harvested 24 h after transfection, and equal quantities were compared by protein gel blots. Molecular mass markers are given in kilodaltons on the right side of the panel. Note the complete lack of secretion of sGFP-P regardless of drug treatment.
The combined results demonstrate that specific BiP precipitation and subsequent ATP release reveal two subsets of BiP-ligand complexes. One of these is possibly capable of leaving the ER to reach the vacuole. The use of wortmannin facilitates detection of the latter as it prevents the normal vacuolar sorting and rapid proteolysis. These observations may lead to strategies to identify a typical BiP-ligand that is disposed of by the vacuole.
DISCUSSION
The Vacuolar Transport Route of BiP
A common principle of biological systems is that nothing is perfect but little is left to chance. Quality control at multiple checkpoints is often the key for the survival of the fittest in nature, and the secretory pathway appears to be no exception to this rule. When de novo–synthesized proteins enter the ER, nascent chains are immediately assisted by several chaperones, and BiP is probably first in line (Matlack et al., 1999). Chaperones facilitate protein folding and reduce aggregation by masking exposed hydrophobic regions (Hartl, 1996; Kleizen and Braakman, 2004). If folding fails, the ER quality control machinery will target the affected proteins for degradation (Ellgaard and Helenius, 2003; Goldberg, 2003). Currently, the ERAD pathway, leading back through the Sec61 pore for disposal by the cytosolic proteasome, has received most of the attention in the field (Ward et al., 1995; Hiller et al., 1996; Kleizen and Braakman, 2004; Schmitz and Herzog, 2004). However, the vacuole has been considered as an alternative location for disposal (Hong et al., 1996; Spear and Ng, 2003).
In this work, we have shown that BiP is transported in a wortmannin-sensitive manner to the lytic vacuole. Wortmannin induced the secretion of native BiP in wild-type tobacco protoplasts and to a greater extent in BiP-overproducing plants (Figure 1). Control experiments showed that this was not due to cell mortality or to the effect of wortmannin on HDEL retention but as a result of preventing Golgi-to-vacuole transport. In addition, native BiP could be redirected to the apoplast in wild-type tobacco roots upon treatment with the drug (Figure 2).
We have also shown that BiP traffics through the Golgi apparatus. Inhibition of COPII-dependent ER-to-Golgi traffic prevented wortmannin-induced secretion (Figure 3). Furthermore, increased traffic of BiP through multivesicular bodies was detected when BiPΔHDEL-producing plants were analyzed but not with wild-type BiP-overproducing plants (Figure 5). Although detection of transport intermediates is always difficult, deletion of the HDEL motif led to sufficient cargo flow to allow a localization of BiP in multivesicular bodies. This strongly suggests that BiP has to overcome the hurdle imposed by the HDEL receptor–mediated retrieval before it can be transported to the PVC and further on to the vacuole. Together with the demonstrated dependence on COPII transport, these results show that BiP exits the ER, reaches the Golgi, and can either be retrieved back to the ER or proceed to the vacuole.
It has recently been suggested that there is a constitutive signal-independent pathway leading from the ER to the vacuole by which ER residents and bulk flow markers reach the vacuole without transiting the Golgi apparatus (Tamura et al., 2004). This pathway is completely distinct from the route described above, but the two findings do not exclude each other. Tamura et al. (2004) argue that 9- to 11-d-old stationary Arabidopsis thaliana suspension cells are likely to experience glucose and nitrogen starvation and would therefore be prone to autophagic transport to vacuoles.
This evolutionarily conserved process is distinct from the COPII-mediated ER-to-Golgi transport. Autophagy delivers entire organelles to the vacuole, including plastids, peroxisomes, and ER fragments, and is thought to provide energy and amino acids for survival under starvation (Kuma et al., 2004; Niwa et al., 2004; Yoshimori, 2004; Hamasaki et al., 2005). Therefore, autophagy may occur in addition to the Golgi-dependent vacuolar sorting of BiP. However, it is unlikely that such nondiscriminative vacuolar delivery of entire ER fragments contributes to specific quality control mechanisms ensuring degradation of misfolded proteins while rescuing folding intermediates and correctly folded proteins.
Receptor Competition as a Tool to Monitor ER Export
We have suggested before that a bulk-flow mechanism for ER export may be underestimated because proteins can be transported to the vacuole for degradation instead of being secreted (Phillipson et al., 2001). Evidence that bulk flow of ER residents occurs in COPII vesicles was obtained by inhibition of this pathway, which revealed accumulation of ER resident proteins (Nishikawa et al., 1994; Phillipson et al., 2001). The most dramatic observation was made in yeast, where BiP accumulates at high levels, resulting in dilations of the ER, termed BiP bodies (Nishikawa et al., 1994). Figure 3 shows that wortmannin-induced secretion of BiP is COPII dependent, which corresponds to these previous findings. However, this approach does not monitor cargo arrival at the Golgi apparatus but illustrates the consequences of preventing export from the ER.
To develop a more direct method to detect arrival in the Golgi apparatus, we have used an in vivo cargo competition assay that we have suggested recently (Pimpl and Denecke, 2002). We could show that an HDEL-tagged bulk flow marker (PAT) and the two ER resident proteins, calreticulin and BiP, can effectively compete with amy-HDEL for the HDEL receptor at the level of the Golgi apparatus (Figure 4). The indicator molecule amy-HDEL is an artificial ligand created from the naturally secreted protein amy, which was fused to the tetrapeptide HDEL. This molecule is highly ER export competent, is exported to the Golgi apparatus via COPII-mediated transport, and can recycle via the HDEL receptor back to the ER. Competition for the HDEL receptor can be monitored via an induced secretion of amy-HDEL (Figure 4).
If ER residents such as BiP and calreticulin are excluded from ER export, they could not reach the Golgi apparatus to compete for the HDEL receptor. However, our direct competition assay demonstrates arrival at the Golgi apparatus for both proteins (Figure 4C). The experiments were performed in a system that was not perturbed with drugs and demonstrate that BiP can reach the Golgi apparatus. When devoid of its HDEL motif, only calreticulin is secreted, consistent with previous findings (Crofts et al., 1999). We suggest that vacuolar sorting of BiP masks ER export and thus explains the discrepancy.
Finally, we have demonstrated that BiPΔHDEL maintains its chaperone function as monitored by ATP-dependent binding to a known ligand (Figure 1C). The difference between BiP and BiPΔHDEL should thus be the presence or absence of a signal allowing retrieval from the Golgi apparatus. Figure 1B clearly shows that with wortmannin, the truncated molecule is preferentially secreted to the medium. The data show that in plants, BiP is not excluded from ER export and depends on its HDEL motif to accumulate in the ER. Also in yeast, defective HDEL-mediated recycling from the Golgi apparatus leads to depletion of ER residents and is lethal (Townsley et al., 1994). This would not occur if ER export of residents were not a frequent event.
A Possible Role for Vacuolar Sorting in Quality Control?
It is possible that vacuolar sorting of BiP is merely a safeguard against secretion of BiP and BiP-ligand complexes to the apoplast, where they could be harmful. Furthermore, vacuolar sorting of BiP that escapes the HDEL receptor may be a way to recover valuable amino acids. However, the results from our work raise the possibility of a more active role for this process.
Although a significant number of misfolded proteins are thought to be degraded through the ERAD pathway, a BiP-ligand has been shown to exit the ER and to return via COPI-mediated vesicle transport (Yamamoto et al., 2001). Furthermore, a number of ERAD substrates, including mutant carboxypeptidase Y (CPY*), require transport between the ER and Golgi for their degradation (Plemper et al., 1997; Caldwell et al., 2001; Vashist et al., 2001; Jarosch et al., 2002; Taxis et al., 2002). Interestingly, when CPY* is expressed in cell lines mutated in the ERAD pathway, its degradation becomes dependent on vacuolar proteases (Taxis et al., 2002; Spear and Ng, 2003). Vacuolar disposal could thus be an alternative or possibly complementary degradation route.
Our results suggest that a subpopulation of BiP-ligands may be destined for degradation in the lytic vacuole even when ERAD is available (Figure 6). This population was isolated from the culture medium through redirection of the vacuolar transport route by wortmannin. Control experiments revealed no evidence for the presence of BiP degradation fragments in the ATP-released portion, nor did the pattern correspond to the total fraction of secreted polypeptides in the medium. The current definition of BiP-ligands is that they can be coimmunoprecipitated with BiP and subsequently released from the pellet by addition of ATP in vitro (Munro and Pelham, 1986; Vitale et al., 1995). Since no cross-linking agents were used, the results indicate that BiP-ligand complexes are stable enough to be directed to the vacuole by BiP.
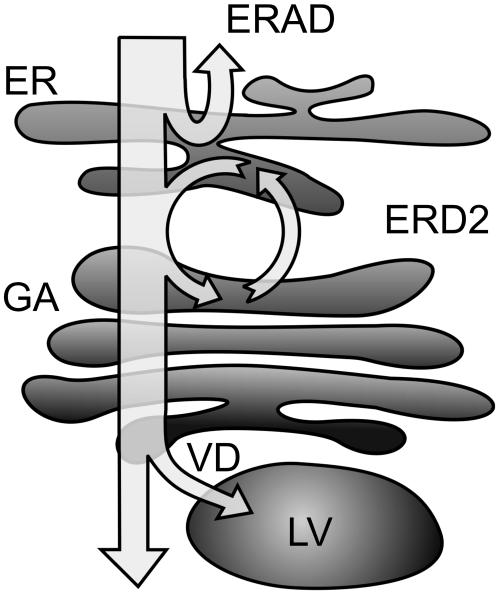
Therefore, we raise the possibility of multiple quality control mechanisms in the secretory pathway (Figure 8). The first step is performed via the ERAD pathway, which is probably crucial for the clearance of translocation pores as well as to relieve the ER lumen of harmful complexes. However, a potentially misfolded protein can be exported from the ER, but it will generally be rescued together with associated BiP via the HDEL-mediated route. This would be of advantage to folding intermediates because they could continue to fold in the ER, which is an ideal environment for folding assisted by a vast range of helper molecules.
Figure 8.
Model Describing the Possible Fate of Newly Synthesized Proteins within the Secretory Pathway.
A first quality control step occurs via ERAD back through the Sec61 pore into the cytosol for disposal by the proteasome. The second mechanism is based on the action of ER chaperones such as BiP. HDEL-mediated recycling from the Golgi apparatus (GA) via the HDEL receptor (ERD2) will either permit further folding, disposal via ERAD, or renewed ER export. If the HDEL retrieval checkpoint is overcome as well, the final quality control leads to vacuolar disposal (VD) in the lytic vacuole (LV).
Unlike folding intermediates, proteins can also be permanently misfolded, either through mutation, incorrect synthesis, or adverse physiological conditions. Both types of ligands will display hydrophobic regions on the surface, but the latter category will continuously reassociate to BiP. Therefore, such proteins will complete many more cycles through the HDEL system compared with the average folding intermediate. Even though the probability of escaping from the HDEL receptor is low at any one attempt, the overall probability increases with the number of attempts. If a BiP-ligand complex escapes from the HDEL system, vacuolar sorting from the Golgi apparatus could then lead to disposal.
There is precedence for VPS10-dependent and -independent vacuolar disposal of misfolded proteins in S. cerevisiae (Hong et al., 1996; Holkeri and Makarow, 1998; Zhang et al., 2001; Coughlan et al., 2004). The first of these reports describes how correctly folded invertase carrying a small misfolded portion was found to be targeted to the vacuole in a VPS10-mediated fashion (Hong et al., 1996). Perhaps in this case, vacuolar disposal is preferable over ERAD because the latter would have to involve unfolding of the invertase moiety. VPS10 has the same topology as the plant vacuolar sorting receptor BP80 and was proposed to recognize hydrophobic portions of misfolded proteins (Jorgensen et al., 1999). By analogy with VPS10 in yeast, the plant vacuolar sorting receptor BP80 could also bind misfolded proteins in addition to its normal role in recognizing specific vacuolar sorting signals. When BiP-ligand complexes reach the trans-Golgi apparatus, BP80 could deliver the complexes to the vacuole and BiP would then be degraded as collateral casualty. Alternatively, BiP itself could carry sorting information to reach the vacuole.
Two arguments favor the second model. First of all, BiP is expected to mask exposed hydrophobic regions on misfolded proteins, which would then be unavailable for interaction with the vacuolar sorting receptor. Secondly, overexpression of BiP and in particular BiPΔHDEL increases the amount of BiP in transit to vacuoles without the requirement of additional ER stress (Figures 1, 2, and 5). Therefore, we predict that BiP carries a vacuolar sorting signal that enables the chaperone to act as an adaptor between ill-defined hydrophobic regions of misfolded proteins with specific sorting machinery.
METHODS
Plasmids for Transient Expression of Cargo Molecules
Previously established plasmids were used encoding the reporters amy, its derivative amy-HDEL (Crofts et al., 1999), plasmids encoding PAT derivatives (Denecke et al., 1992) and myc-epitope–tagged calreticulin with and without HDEL (Phillipson et al., 2001) for HDEL receptor competition studies, secreted GFP (sGFP) and sGFP-P (Brandizzi et al., 2003), and the effector molecules Sec12p and Sar1(H74L) for the inhibition of COPII-mediated ER export (Phillipson et al., 2001).
For new recombinant plasmids, all DNA manipulations were done according to established procedures. The Escherichia coli MC1061 strain (Casadaban and Cohen, 1980) was used for the amplification of all plasmids. Furthermore, Sar1(T34N) was mutagenized using the following pair of oligonucleotides: Sar1T34Nsense (5′-ATAATGCTGGCAAAAATACATTACTTCACAT-3′) and Sar1T34N anti (5′-ATGTGAAGTAATGTATTTTTGCCAGCATTAT-3′). Myc-tagged BiP constructs for HDEL receptor competition experiments were generated by PCR amplification of the BiP coding region pDE800 using the oligonucleotide combinations of the 35S sense (5′-AGGAAGGTGGCTCCTACA-3′) with a hybrid antisense oligonucleotide (5′-TGCTTCGGATCCTCTAGCTAGCCAAGTCCTCTTCAGAAATAAGCTTTTGCTCCCTAGCACCTGATTCTCCTCCTGGTGC-3′), followed by restriction digest with NcoI and BamHI and cloning into pAmy that was also cut with NcoI and BamHI and dephosphorylated, yielding myc-epitope–tagged full-length BiP devoid of its retention motif (pJLH9). The DNA fragment carrying the retention motif was obtained from pDE800 as an NheI-SphI fragment, which was inserted into pJLH9, previously cut with NheI and SphI, followed by dephosphorylation, yielding myc-epitope–tagged full-length BiP carrying the retention motif (pJLH8).
Plant Material for Transport Experiments
Tobacco plants (Nicotiana tabacum cv Petit Havana) (Maliga et al., 1973) were grown in MS medium (Murashige and Skoog, 1962) supplemented with 2% sucrose in a controlled room at 22°C with a 16-h daylength at the light irradiance of 200 μE m−2 s under sterile conditions. Transport experiments with protoplasts from wild-type plants and stable transgenic lines (Figure 1) have been performed with tobacco leaf protoplasts isolated essentially as described (Denecke and Vitale, 1995) except that TEX medium was used for five consecutive washing steps to ensure protein-free culture medium at the beginning of the incubation. Cell suspensions were incubated for 24 h with an increasing concentration of wortmannin, after which cells and medium were harvested as described (Denecke and Vitale, 1995) and protein extracts analyzed by protein gel blots and immunodetection (see below). Medium was either analyzed as such or 10-fold concentrated.
Drugs
Wortmannin was dissolved as a 33 mM stock solution in DMSO, and the final concentrations used are given in the figure legends. For tunicamycin treatments, 20 mg/mL stock solutions in DMSO were used to supplement TEX medium to reach a final drug concentration of 20 μg/mL.
Transient Expression with Tobacco Protoplasts
Tobacco leaf protoplasts were prepared using buffers and procedures as described (Denecke and Vitale, 1995). Prior to electroporation, protoplasts were resuspended in electroporation buffer at a concentration of 5 × 106 protoplasts/mL. Five hundred microliters of the obtained protoplast mix were pipetted into a disposable 1-mL plastic cuvette and mixed with an appropriate amount of plasmid DNA or mixtures of plasmids previously dissolved in 100 μL of electroporation buffer. The protoplast suspensions were then incubated for 5 min, followed by electroporation with stainless steel electrodes at a distance of 3.5 mm, using a complete exponential discharge of a 1000-μF capacitor charged at 160 V. After 15 min of rest, electroporated protoplasts were removed from the cuvettes by washing in 1 mL TEX buffer twice and placed in 5-cm Petri dishes for incubation. Plasmid concentrations used are given in the figure legends.
Further analysis depended on the experiment. For in vivo labeling and BiP-ligand interaction studies (Figures 1C and 6), cells were concentrated after 5 h of incubation and treated as described below. For protein transport studies and inhibition of COPII-mediated transport (Figures 3 and 4), harvesting of cells and medium was initiated by 5 min of centrifugation, which causes flotation of cells. One milliliter of the underlying medium was manually removed with a fine Pasteur pipette, and the remaining suspension was diluted 10-fold with 250 mM NaCl. A further 5-min centrifugation allowed complete recovery of washed cells in the form of a tight pellet, and the supernatant was completely removed with a peristaltic pump.
Protein Extraction and Electrophoresis
Protein analysis of protoplast culture medium was performed after concentration with aqueous ammonium sulfate solution (60% final) in the presence of BSA as carrier (200 μg per 600 μL of medium), resuspending the protein precipitate in TE 50/2 (50 mM Tris-HCl and 2 mM EDTA, pH 8.0) supplemented with protease inhibitor cocktail (Complete Mini; Roche Diagnostics) according to the manufacturer's instructions. Using this method, culture medium samples were 10-fold concentrated. Protein extraction of cells was conducted after sonication of the protoplasts in TE 50/2 with supplements as above in a 10-fold lower volume as the cell suspension.
Protein cell extracts or concentrated medium were mixed with an equal volume of freshly prepared 2× Xtreme loading dye: 900 μL of sample buffer mix (0.1% bromophenol blue, 5 mM EDTA, 200 mM Tris-HCl, pH 8.8, and 1 M sucrose) are mixed with 300 μL of 10% SDS and 20 μL of 1 M DTT. Samples were incubated at 95°C for 5 min and loaded on SDS-PAGE.
In order to separate BiP from BiPΔHDEL, Xtreme SDS-PAGE was performed containing sucrose-supplemented stacking gels (5% Protogel [30% acrylamide, 0.8% bisacrylamide; supplied by National Diagnostics], 15% sucrose, 66 mM Tris-HCl, pH 6.8, 0.1% SDS, 0.2% N,N,N′,N′-tetramethylethylenediamine, and 0.033% ammonium persulfate). Separation gels contained Protogel at either 8% for BiP or 12% for PAT separation on gels and otherwise contained 420 mM Tris-HCl, pH 8.8, 0.1% SDS, 0.055% N,N,N′,N′-tetramethylethylenediamine, and 0.033% ammonium persulfate. All percentages are given in w/v ratios. Gels were run in running buffer (6 g/L Tris, 28.8 g/L glycine, and 1 g/L SDS).
Immunodetection of Protein Gel Blots
After electroblotting on nitrocellulase membranes, immunodetection of proteins was performed using rabbit polyclonal antisera raised against either BiP (1:15000) (Denecke et al., 1991), PAT (1:5000) (Denecke et al., 1990), or the c-myc epitope (1:5000, C-myc A-14; Santa Cruz Biotechnology). The secondary antibody was peroxidase-labeled anti-rabbit IgG (Amersham Biosciences UK) used according to manufacturer's instructions. Immunodetection was performed using enhanced chemiluminescence with freshly prepared solution 1 (1 mL 1 M Tris-HCl, pH 8.5, 100 μL 250 mM luminol, and 44 μL 90 mM p-coumaric acid, brought to a final volume of 10 mL with distilled water) and solution 2 (1 mL 1 M Tris-HCl, pH 8.5, and 6 μL 30% H202, brought to a final volume of 10 mL with distilled water). Stock solutions of luminol (3-aminophthalhydrazide; Fluka) were prepared by dissolving 0.44 g into 10 mL DMSO and stored at −20°C. Stock solutions of p-coumaric acid (Sigma-Aldrich) were prepared by dissolving 0.15 g into 10 mL DMSO and stored at −20°C. Solutions 1 and 2 were mixed and used immediately for incubation with nitrocellulose membranes, followed by exposure to x-ray film (MXB; Kodak).
Enzymatic Assays
Extraction of amy, the subsequent amy assays, and calculation of the secretion index were done as described (Crofts et al., 1999; Phillipson et al., 2001). For direct comparisons in the HDEL-receptor competition experiments, we have set the secretion index in the presence of noncompetitor to 100% for the cargo molecules PAT, calreticulin, and BiP.
Immunolocalization of BiP in Plant Tissues
Wild-type tobacco roots were incubated with 10 μM wortmannin for 24 h, after which the tips were excised and fixed with formaldehyde/glutaraldehyde and embedded in Lowicryl HM20 at −35°C. For vacuolar localization, untreated tobacco roots from a transgenic BiP overproducer were used (Leborgne-Castel et al., 1999). Fixation and embedding procedures have been described previously (Crofts et al., 1999).
For high-pressure freezing, root tips were excised from BiP- and BiPΔHDEL-overproducing plants (Crofts et al., 1999) and briefly washed in MS medium. Following a subsequent washing step in hexadecene, tips were immediately frozen in a high-pressure freezer (HPF010; Bal-Tec). Freeze substitutions were performed in an AFS freeze substitution unit (Leica) in dry acetone supplemented with 0.1% uranyl acetate at −85°C for 3 d before slowly being warmed to −35°C for a period of 18 h. Samples were embedded in Lowycryl HM20 using gelatin capsules, and polymerization of the resin occurred with constant UV light for 2 d at −35°C and an additional 3 d at 22°C.
Thin sections were incubated with anti-BiP antiserum at a primary dilution of 1:200, followed by incubation with 10 nm gold-coupled secondary antibodies (Biocell GAR10 1:50) at a dilution of 1:30. Aqueous uranyl acetate/lead citrate poststained sections were examined in a Philips CM10 transmission electron microscope operating at 80 kV.
BiP–Model Ligand Interaction Studies
Pulse labeling of protoplast suspensions were conducted with 5 × 106 protoplasts/mL in TEX medium containing Amersham 35S Promix (100 μCi/mL). For Figure 1C, tobacco leaf protoplasts from stable transgenic plants expressing BiPΔHDEL (Leborgne-Castel et al., 1999) were transfected with a plasmid encoding sGFP-P (Brandizzi et al., 2003) using the transient expression procedure described above. Cell suspensions were incubated for 5 h, followed by washing and subsequent concentration in a final volume of 1 mL TEX supplemented with Amersham 35S Promix (100 μCi/mL). Labeling was performed for 2 h, and cells were subsequently harvested. Cell pellets were extracted in 1 mL of protoplast homogenization buffer (Crofts et al., 1998), and the extracts were immunoprecipitated with anti-GFP serum (daSilva et al., 2005). One pellet was washed with BiP release buffer supplemented with 3 mM ATP (Crofts et al., 1998), followed by reimmunoprecipitation of the released material with anti-BiP serum.
Coimmunoprecipitation and ATP-Mediated Release of BiP-Ligand Populations
Wild-type tobacco protoplasts were prepared in TEX medium, and cell suspensions were preincubated for 5 h, followed by one further washing step with TEX medium. Protoplasts (2 × 107) were finally resuspended in a total volume of 5 mL TEX medium and incubated with the drugs tunicamycin (20 μg/mL) and wortmannin (10 μM) in the presence of Amersham 35S Promix (100 μCi/mL). Continuous metabolic labeling was performed for 20 h, followed by gentle separation of cells and culture medium without a chase, except that cells were washed with a 20-fold excess of 250 mM NaCl followed by sedimentation at 100 g for 3 min to remove contaminating medium from the labeling period.
Cellular BiP and BiP complexes were extracted from the cells in protein homogenization buffer as described previously (Crofts et al., 1998) but in the presence of protease inhibitor cocktail (Complete Mini) according to manufacturer's instructions. BiP in cell extracts and directly taken from the culture medium was specifically precipitated with anti-BiP serum in Net-Gel buffer as described (daSilva et al., 2005) but in the presence of protease inhibitor cocktail (Complete Mini) according to manufacturer's instructions. Sedimentation was followed by incubation with BiP release buffer supplemented with 3 mM ATP (Crofts et al., 1998). BiP-ligands were released in a minimal total volume of 50 μL and centrifuged twice to remove any possible protein-A Sepharose. The cleared supernatant was diluted with an equal volume of SDS sample buffer, and polypeptides were separated via molecular weight by SDS-PAGE, followed by electroblotting on nitrocellulose membranes, subsequent autoradiography by phosphorimaging, and quantification via AIDA software (Raytest).
Reimmunoprecipitation of released BiP-ligands from cells and medium was done with anti-BiP serum on 250 μL of cleared supernatant, obtained by repeating the procedure describe above fivefold. Total secreted and labeled proteins were analyzed directly using the harvested culture medium without additional concentration.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Q03684 (BiP) and CAA59694 (calreticulin).
Acknowledgments
This work was partially supported by the Biotechnology and Biological Sciences Research Council, the German Research Council, and the European Union (HPRN-CT-2002-00262; BioInteractions). J.P.T. is indebted to the British Council for supporting the bench-exchange between Leeds and Heidelberg. C.J.S. is grateful for the Biotechnology and Biological Sciences Research Council QUOTA studentship.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jurgen Denecke (j.denecke@leeds.ac.uk).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.036665.
References
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77 895–907. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi, A., De Los Rios, P., Dietler, G., and Goloubinoff, P. (2004). Active solubilization and refolding of stable protein aggregates by cooperative unfolding action of individual hsp70 chaperones. J. Biol. Chem. 279 37298–37303. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi, S., Cwirla, S.E., Dower, W.J., Lipshutz, R.J., Sprang, S.R., Sambrook, J.F., and Gething, M.J. (1993). Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75 717–728. [DOI] [PubMed] [Google Scholar]
- Brandizzi, F., Hanton, S., DaSilva, L.L., Boevink, P., Evans, D., Oparka, K., Denecke, J., and Hawes, C. (2003). ER quality control can lead to retrograde transport from the ER lumen to the cytosol and the nucleoplasm in plants. Plant J. 34 269–281. [DOI] [PubMed] [Google Scholar]
- Caldwell, S.R., Hill, K.J., and Cooper, A.A. (2001). Degradation of endoplasmic reticulum (ER) quality control substrates requires transport between the ER and Golgi. J. Biol. Chem. 276 23296–23303. [DOI] [PubMed] [Google Scholar]
- Casadaban, M.J., and Cohen, S.N. (1980). Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138 179–207. [DOI] [PubMed] [Google Scholar]
- Coughlan, C.M., Walker, J.L., Cochran, J.C., Wittrup, K.D., and Brodsky, J.L. (2004). Degradation of mutated bovine pancreatic trypsin inhibitor in the yeast vacuole suggests post-endoplasmic reticulum protein quality control. J. Biol. Chem. 279 15289–15297. [DOI] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Hillmer, S., Robinson, D.G., Phillipson, B., Carlsson, L.E., Ashford, D.A., and Denecke, J. (1999). Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11 2233–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Pesca, M., Vitale, A., and Denecke, J. (1998). BiP and calreticulin form an abundant complex that is independent of endoplasmic reticulum stress. Plant Cell 10 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva, L.L., Taylor, J.P., Hadlington, J.L., Hanton, S.L., Snowden, C.J., Fox, S.J., Foresti, O., Brandizzi, F., and Denecke, J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, H.W. (1995). Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol. 130 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., Botterman, J., and Deblaere, R. (1990). Protein secretion in plant cells can occur via a default pathway. Plant Cell 2 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., De Rycke, R., and Botterman, J. (1992). Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., Goldman, M.H., Demolder, J., Seurinck, J., and Botterman, J. (1991). The tobacco luminal binding protein is encoded by a multigene family. Plant Cell 3 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., and Vitale, A. (1995). The use of protoplasts to study protein synthesis and transport by the plant endomembrane system. Methods Cell Biol. 50 335–348. [DOI] [PubMed] [Google Scholar]
- Di Cola, A., Frigerio, L., Lord, J.M., Ceriotti, A., and Roberts, L.M. (2001). Ricin A chain without its partner B chain is degraded after retrotranslocation from the endoplasmic reticulum to the cytosol in plant cells. Proc. Natl. Acad. Sci. USA 98 14726–14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cola, A., Frigerio, L., Lord, J.M., Roberts, L.M., and Ceriotti, A. (2005). Endoplasmic reticulum-associated degradation of ricin a chain has unique and plant-specific features. Plant Physiol. 137 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard, L., and Helenius, A. (2003). Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4 181–191. [DOI] [PubMed] [Google Scholar]
- Fontes, E.B., Shank, B.B., Wrobel, R.L., Moose, S.P., O'Brian, G.R., Wurtzel, E.T., and Boston, R.S. (1991). Characterization of an immunoglobulin binding protein homolog in the maize floury-2 endosperm mutant. Plant Cell 3 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti, O., Frigerio, L., Holkeri, H., de Virgilio, M., Vavassori, S., and Vitale, A. (2003). A phaseolin domain involved directly in trimer assembly is a determinant for binding by the chaperone BiP. Plant Cell 15 2464–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething, M.J. (1999). Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10 465–472. [DOI] [PubMed] [Google Scholar]
- Gething, M.J., McCammon, K., and Sambrook, J. (1986). Expression of wild-type and mutant forms of influenza hemagglutinin: The role of folding in intracellular transport. Cell 46 939–950. [DOI] [PubMed] [Google Scholar]
- Gething, M.J., and Sambrook, J. (1992). Protein folding in the cell. Nature 355 33–45. [DOI] [PubMed] [Google Scholar]
- Goldberg, A.L. (2003). Protein degradation and protection against misfolded or damaged proteins. Nature 426 895–899. [DOI] [PubMed] [Google Scholar]
- Hamasaki, M., Noda, T., Baba, M., and Ohsumi, Y. (2005). Starvation triggers the delivery of the endoplasmic reticulum to the vacuole via autophagy in yeast. Traffic 6 56–65. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hatano, K., Takeuchi, Y., and Nishimura, M. (1998). Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl, F.U. (1996). Molecular chaperones in cellular protein folding. Nature 381 571–579. [DOI] [PubMed] [Google Scholar]
- Hendershot, L., Wei, J., Gaut, J., Melnick, J., Aviel, S., and Argon, Y. (1996). Inhibition of immunoglobulin folding and secretion by dominant negative BiP ATPase mutants. Proc. Natl. Acad. Sci. USA 93 5269–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller, M.M., Finger, A., Schweiger, M., and Wolf, D.H. (1996). ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273 1725–1728. [DOI] [PubMed] [Google Scholar]
- Holkeri, H., and Makarow, M. (1998). Different degradation pathways for heterologous glycoproteins in yeast. FEBS Lett. 429 162–166. [DOI] [PubMed] [Google Scholar]
- Hong, E., Davidson, A.R., and Kaiser, C.A. (1996). A pathway for targeting soluble misfolded proteins to the yeast vacuole. J. Cell Biol. 135 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley, S.M., Bole, D.G., Hoover-Litty, H., Helenius, A., and Copeland, C.S. (1989). Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J. Cell Biol. 108 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosch, E., Taxis, C., Volkwein, C., Bordallo, J., Finley, D., Wolf, D.H., and Sommer, T. (2002). Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat. Cell Biol. 4 134–139. [DOI] [PubMed] [Google Scholar]
- Jelitto-Van Dooren, E.P., Vidal, S., and Denecke, J. (1999). Anticipating endoplasmic reticulum stress. A novel early response before pathogenesis-related gene induction. Plant Cell 11 1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, M.U., Emr, S.D., and Winther, J.R. (1999). Ligand recognition and domain structure of Vps10p, a vacuolar protein sorting receptor in Saccharomyces cerevisiae. Eur. J. Biochem. 260 461–469. [DOI] [PubMed] [Google Scholar]
- Kalinski, A., Rowley, D.L., Loer, D.S., Foley, C., Buta, G., and Herman, E.M. (1995). Binding-protein expression is subject to temporal, developmental and stress-induced regulation in terminally differentiated soybean organs. Planta 195 611–621. [DOI] [PubMed] [Google Scholar]
- Kleizen, B., and Braakman, I. (2004). Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 16 343–349. [DOI] [PubMed] [Google Scholar]
- Kozutsumi, Y., Segal, M., Normington, K., Gething, M.J., and Sambrook, J. (1988). The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332 462–464. [DOI] [PubMed] [Google Scholar]
- Kuma, A., Hatano, M., Matsui, M., Yamamoto, A., Nakaya, H., Yoshimori, T., Ohsumi, Y., Tokuhisa, T., and Mizushima, N. (2004). The role of autophagy during the early neonatal starvation period. Nature 432 1032–1036. [DOI] [PubMed] [Google Scholar]
- Leborgne-Castel, N., Jelitto-Van Dooren, E.P., Crofts, A.J., and Denecke, J. (1999). Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell 11 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainieri, D., Rossi, M., Archinti, M., Bellucci, M., De Marchis, F., Vavassori, S., Pompa, A., Arcioni, S., and Vitale, A. (2004). Zeolin. A new recombinant storage protein constructed using maize gamma-zein and bean phaseolin. Plant Physiol. 136 3447–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliga, P., Sz-Breznovits, A., and Marton, L. (1973). Streptomycin-resistant plants from callus culture of haploid tobacco. Nat. New Biol. 244 29–30. [DOI] [PubMed] [Google Scholar]
- Malkus, P., Jiang, F., and Schekman, R. (2002). Concentrative sorting of secretory cargo proteins into COPII-coated vesicles. J. Cell Biol. 159 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack, K.E., Misselwitz, B., Plath, K., and Rapoport, T.A. (1999). BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell 97 553–564. [DOI] [PubMed] [Google Scholar]
- McCracken, A.A., and Brodsky, J.L. (2003). Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD). Bioessays 25 868–877. [DOI] [PubMed] [Google Scholar]
- Muller, J., Piffanelli, P., Devoto, A., Miklis, M., Elliott, C., Ortmann, B., Schulze-Lefert, P., and Panstruga, R. (2005). Conserved ERAD-like quality control of a plant polytopic membrane protein. Plant Cell 17 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S., and Pelham, H.R. (1986). An Hsp70-like protein in the ER: Identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46 291–300. [DOI] [PubMed] [Google Scholar]
- Munro, S., and Pelham, H.R. (1987). A C-terminal signal prevents secretion of luminal ER proteins. Cell 48 899–907. [DOI] [PubMed] [Google Scholar]
- Murashige, R., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15 473–497. [Google Scholar]
- Nishikawa, S., Hirata, A., and Nakano, A. (1994). Inhibition of endoplasmic reticulum (ER)-to-Golgi transport induces relocalization of binding protein (BiP) within the ER to form the BiP bodies. Mol. Biol. Cell 5 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, Y., Kato, T., Tabata, S., Seki, M., Kobayashi, M., Shinozaki, K., and Moriyasu, Y. (2004). Disposal of chloroplasts with abnormal function into the vacuole in Arabidopsis thaliana cotyledon cells. Protoplasma 223 229–232. [DOI] [PubMed] [Google Scholar]
- Nuttall, J., Vine, N., Hadlington, J.L., Drake, P., Frigerio, L., and Ma, J.K. (2002). ER-resident chaperone interactions with recombinant antibodies in transgenic plants. Eur. J. Biochem. 269 6042–6051. [DOI] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bielli, A., de Virgilio, M., Frigerio, L., Pesca, M., Faoro, F., Bollini, R., Ceriotti, A., and Vitale, A. (1997). Protein quality control along the route to the plant vacuole. Plant Cell 9 1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson, B.A., Pimpl, P., daSilva, L.L., Crofts, A.J., Taylor, J.P., Movafeghi, A., Robinson, D.G., and Denecke, J. (2001). Secretory bulk flow of soluble proteins is COPII dependent. Plant Cell 13 2005–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl, P., and Denecke, J. (2002). Protein-protein interactions in the secretory pathway, a growing demand for experimental approaches in vivo. Plant Mol. Biol. 50 887–902. [DOI] [PubMed] [Google Scholar]
- Pimpl, P., Hanton, S.L., Taylor, J.P., Pinto-DaSilva, L.L., and Denecke, J. (2003). The GTPase ARF1p controls the sequence-specific vacuolar sorting route to the lytic vacuole. Plant Cell 15 1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl, P., Movafeghi, A., Coughlan, S., Denecke, J., Hillmer, S., and Robinson, D.G. (2000). In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12 2219–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemper, R.K., Bohmler, S., Bordallo, J., Sommer, T., and Wolf, D.H. (1997). Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature 388 891–895. [DOI] [PubMed] [Google Scholar]
- Randall, J.J., Sutton, D.W., Hanson, S.F., and Kemp, J.D. (2005). BiP and zein binding domains within the delta zein protein. Planta 221 656–666. [DOI] [PubMed] [Google Scholar]
- Rutkowski, D.T., and Kaufman, R.J. (2004). A trip to the ER: Coping with stress. Trends Cell Biol. 14 20–28. [DOI] [PubMed] [Google Scholar]
- Sanderfoot, A.A., Ahmed, S.U., Marty-Mazars, D., Rapoport, I., Kirchhausen, T., Marty, F., and Raikhel, N.V. (1998). A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 95 9920–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, A., and Herzog, V. (2004). Endoplasmic reticulum-associated degradation: Exceptions to the rule. Eur. J. Cell Biol. 83 501–509. [DOI] [PubMed] [Google Scholar]
- Seaman, M.N. (2005). Recycle your receptors with retromer. Trends Cell Biol. 15 68–75. [DOI] [PubMed] [Google Scholar]
- Semenza, J.C., Hardwick, K.G., Dean, N., and Pelham, H.R. (1990). ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61 1349–1357. [DOI] [PubMed] [Google Scholar]
- Spear, E.D., and Ng, D.T. (2003). Stress tolerance of misfolded carboxypeptidase Y requires maintenance of protein trafficking and degradative pathways. Mol. Biol. Cell 14 2756–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, M., Ueda, T., Sato, K., Abe, H., Nagata, T., and Nakano, A. (2000). A dominant negative mutant of Sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 23 517–525. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Yamada, K., Shimada, T., and Hara-Nishimura, I. (2004). Endoplasmic reticulum-resident proteins are constitutively transported to vacuoles for degradation. Plant J. 39 393–402. [DOI] [PubMed] [Google Scholar]
- Taxis, C., Vogel, F., and Wolf, D.H. (2002). ER-golgi traffic is a prerequisite for efficient ER degradation. Mol. Biol. Cell 13 1806–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga, M., Kato, S., Kawamura-Watabe, A., Tanaka, R., and Tokunaga, H. (1998). Characterization of deletion mutations in the carboxy-terminal peptide-binding domain of the Kar2 protein in Saccharomyces cerevisiae. Yeast 14 1285–1295. [DOI] [PubMed] [Google Scholar]
- Törmäkangas, K., Hadlington, J.L., Pimpl, P., Hillmer, S., Brandizzi, F., Teeri, T.H., and Denecke, J. (2001). A vacuolar sorting domain may also influence the way in which proteins leave the endoplasmic reticulum. Plant Cell 13 2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley, F.M., Frigerio, G., and Pelham, H.R. (1994). Retrieval of HDEL proteins is required for growth of yeast cells. J. Cell Biol. 127 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka, K., Okamoto, T., and Minamikawa, T. (2000). Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J. Cell Biol. 148 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, Y.C., Mo, B., Hillmer, S., Zhao, M., Lo, S.W., Robinson, D.G., and Jiang, L. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16 672–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist, S., Kim, W., Belden, W.J., Spear, E.D., Barlowe, C., and Ng, D.T. (2001). Distinct retrieval and retention mechanisms are required for the quality control of endoplasmic reticulum protein folding. J. Cell Biol. 155 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., Bielli, A., and Ceriotti, A. (1995). The binding protein associates with monomeric phaseolin. Plant Physiol. 107 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Denecke, J. (1999). The endoplasmic reticulum-gateway of the secretory pathway. Plant Cell 11 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., Weaver, N.D., Kesarwani, M., and Dong, X. (2005). Induction of protein secretory pathway is required for systemic acquired resistance. Science 308 1036–1040. [DOI] [PubMed] [Google Scholar]
- Ward, C.L., Omura, S., and Kopito, R.R. (1995). Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83 121–127. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K., Fujii, R., Toyofuku, Y., Saito, T., Koseki, H., Hsu, V.W., and Aoe, T. (2001). The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J. 20 3082–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori, T. (2004). Autophagy: A regulated bulk degradation process inside cells. Biochem. Biophys. Res. Commun. 313 453–458. [DOI] [PubMed] [Google Scholar]
- Zhang, B., Chang, A., Kjeldsen, T.B., and Arvan, P. (2001). Intracellular retention of newly synthesized insulin in yeast is caused by endoproteolytic processing in the Golgi complex. J. Cell Biol. 153 1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K., and Kaufman, R.J. (2004). Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 279 25935–25938. [DOI] [PubMed] [Google Scholar]