Abstract
Background: Planned care of patients with chronic diseases in primary care depends on being able to identify them. A recorded label of asthma does not necessarily mean that the patient is currently symptomatic, and failure to record the diagnosis may influence future care.
Aim: To determine the degree of under- and over-reporting of the diagnosis of asthma for patients aged 16–55 years inclusive in one large general practice.
Design: A questionnaire validated for the detection of bronchial hyper-reactivity was sent to all patients recorded as having asthma and their matched controls. Patients with a diagnosis of asthma and symptomatic bronchial hyper-reactivity were considered to have asthma. Evidence of asthma in the written and computer records was sought for two groups: patients with asthma and without symptoms of bronchial hyper-reactivity, and controls with symptoms of bronchial hyper-reactivity.
Setting: A semi-rural group practice with 14 830 patients.
Method: Questionnaires were sent to 833 patients and 831 controls matched by age and sex.
Results: Response rates were 79.1% (659/833) for patients with asthma and 70.6% (587/831) for control patients. Of the patients with asthma who replied, 60.5% (399/659) had symptomatic bronchial hyper-reactivity. Of those with asthma and a negative bronchial hyper-reactivity status (based on the questionnaire), 190/260 (73.1%) were considered to have had asthma when diagnosed, on review of their primary care records. There was no evidence to suggest asthma in 45 (17.3%) of the 260 patients who had a negative bronchial hyper-reactivity status. Of the control patients, 41 (7.0%) of the 587 responders had symptomatic bronchial hyper-reactivity and nine of these may have asthma. By extrapolation, we estimate that there were possibly another 99 patients with symptoms of asthma, who had not been labelled as having asthma, and 362 patients with symptoms of bronchial hyper-reactivity who had not reported them to their doctors or had not had them recognised.
Conclusions: There is an 89.4% chance that a patient recorded as having asthma has, or has had, asthma.
Keywords: asthma, diagnosis, questionnaires, primary health care
Introduction
ASTHMA is the most common chronic disease affecting all age groups in Britain today; there are over 3 million people suffering from asthma during the course of any one year in the United Kingdom (UK). It is mainly diagnosed and treated in general practice, with the majority of those with symptoms consulting their general practitioner (GP) at least once during that year.1
The prevalence of asthma and chronic pulmonary disease in the general population has been compared to an iceberg.2 The visible part of the iceberg represents patients known to their GP, the submerged part represents patients not known to the healthcare system. These patients may have minimal or episodic symptoms, have adapted to their symptoms, or be afraid of their symptoms and fail to bring them to their doctor. In some cases, asthma may be detected during screening surveys.
In clinical practice, asthma is diagnosed based on the history, clinical findings, and the results of peak flow rate measurements in addition to response to medication. It is axiomatic that patients suffering from chronic diseases can be confidently identified from practice databases, however the proportion of patients correctly identified on practice morbidity registers varies greatly.3,4
It was suggested that there was reluctance to diagnose asthma in childhood in the 1970s and 1980s,5,6 and that it took many consultations for the condition to be recognised, even in practices with a particular interest in the condition.7 Others, however, suggested that there was a tendency to overdiagnose and treat.8 Only 71.3% of those currently taking medication and 41% of those having been previously prescribed antiasthma medication had a formal diagnosis of asthma in a review of the medical records of all 10 685 children registered in Tayside for ‘key items’ associated with asthma.9 Diagnoses made in childhood will be carried over into adult records.
The recording of consultations on computers was found to be more complete than those recorded in paper case records while both methods were being used in four Leeds general practices between 1990 and 1995.10 There were, not unexpectedly, differences in the completeness of recording consultations between practices, doctors and patients. The Honiton practice has used computer records only since 1990. The accuracy of the information derived from the database and used in this study can be attributed in part to ‘spring-cleaning’ in the mid-1990s with regard to recording the diagnosis of asthma over the years: old codes and abbreviations were sought and recoded. There were no specific interventions aimed at clinicians to improve their diagnosis and recording of asthma.
HOW THIS FITS IN
What do we know?
Exercises to validate clinical databases have often relied on comparison with paper records.
What does this paper add?
It is important to recognise the variability in the expression of diseases, and that there should always be consideration of symptomatology and evidence for a diagnosis. This study uses a questionnaire to detect symptoms of bronchal hyper-reactivity, and a record search to look for evidence of past asthma in those patients not reporting them. In this well organised practice with a mature computer environment, there is an 89.4% chance that a patient recorded as having asthma has, or has had, asthma.
The aim of this study was to determine the degree of possible over- and under-recording of the diagnosis of asthma in our clinical database, based on scrutinising patient records and questionnaire-reported symptoms utilising a set of questions validated by Venables et al.11 These questions related to wheeze and difficulty breathing in defined circumstances, such as during exercise and sleep, in order to detect bronchial hyper-reactivity and asthma in research; subjects had been given histamine by inhalation.
Bronchial hyper-reactivity is defined as the development of a specific decrease in the forced expiratory volume in the first second (FEV1) after exposure to methacholine or histamine.12 Although it is not routinely measured in the UK for diagnostic purposes, questionnaires validated against its presence are widely used for epidemiological purposes.13,14 As there is no ‘gold standard’ for defining asthma for such purposes, ‘current asthma’ has been defined as bronchial hyper-reactivity plus wheeze in the previous 12 months.15
Setting
This study was conducted in a semi-rural group practice in East Devon with 14 830 patients, and 8.5 whole-time equivalent GPs and one practice nurse specialising in asthma care. The practice had been fully computerised since 1990 with clinical records only being made on computer. Read coding of an existing diagnosis of asthma and new asthma diagnoses commenced in 1995.
Ethical approval was obtained from the local medical research ethics committee in Exeter.
Method
In an attempt to identify all patients who could be considered to have asthma based on their records, the general enquiry system of the Exeter general practice computer system (Protechnic-Exeter) was used to produce seven populations of currently registered patients aged 16–55 years inclusive on 1 October 1997, who satisfied one or more of the search criteria:
Read coded ‘asthma’ diagnosis in medical summary, H33.
Specific attendances recorded on the asthma care computer screen.
An intervention for asthma recorded on the asthma care computer screen.
A textual entry containing ‘asthma’ or ‘wheez’ on the medical history summary screen. These terms included wheezy bronchitis and related to a diagnostic label rather than merely a symptom or sign.
Inhaled steroids being available on the repeat medication screen.
Inhaled bronchodilators being available on the repeat medication screen.
Cromolyns being available on the repeat medication screen.
The higher group searches were more specifically related to a diagnosis of asthma, but the other searches were included to ensure as wide a pick-up as possible.
Those patients who fell into more than one group, for example, because of having a Read Code diagnosis and a prescription for bronchodilators and/or inhaled steroids, were represented in more than one population, but were subsequently labelled as belonging to the highest group in which they were found in the hierarchy. The sample was limited to those aged 16–55 years to minimise the effects of diagnostic uncertainty in childhood and confusion with chronic obstructive pulmonary disease (COPD) in older age groups.
A group of control patients matched by age and sex was also identified from the database. A questionnaire survey of both groups was conducted using validated questions for the detection of bronchial hyper-reactivity, taken from Venables et al11 (Box 1).
Box 1. Questions used to determine symptomatic bronchial hyper-reactivity (taken from Venables et al11).
-
If you run or climb stairs, do you ever: Cough?
Wheeze?
Get tight in the chest?
-
Is your sleep ever broken by: Wheeze?
Difficulty breathing?
-
Do you ever wake with: Wheeze?
Difficulty breathing?
-
Do you ever wheeze in: Smoky room?
Dusty place?
Non-responders in both groups were sent a follow-up letter and a further questionnaire after a month. At the beginning of 1998, a ‘one in five’ random sample of non-responders was selected using random number tables. Telephone surveys were conducted for these patients if possible.
Patients responding to the postal questionnaire or telephone interview were divided into two groups: those who had a positive bronchial hyper-reactivity status, and those who had a negative bronchial hyper-reactivity status, according to their responses as validated by Venables et al.11 They were not subjected to laboratory tests for bronchial hyper-reactivity.
Patients were considered to have had a positive bronchial hyper-reactivity status in the year prior to the survey if they answered ‘yes’ to three or more of the nine parts of the four questions in the study questionnaire that had been validated by Venables et al;11 a sensitivity of 69% and specificity of 94% was found by Venables et al at this level. The high specificity of their findings enabled us to be confident that those patients classified by the questionnaire as positive for bronchial hyper-reactivity would have been positive if tested in a laboratory.
Patients identified in the broad searches for asthma who scored levels suggestive of current bronchial hyper-reactivity were considered likely to have asthma. The group of patients with a negative bronchial hyper-reactivity status was of particular interest as these patients had been labelled as having asthma at some stage or had had asthma medication set up on their computer screens for them to request. In an attempt to investigate whether there was evidence in their records to support their diagnoses of asthma, the GP clinical records of these patients were scrutinised.
To determine the degree of underdiagnosis of asthma, the notes of those control patients with a positive bronchial hyper-reactivity status were also reviewed. Patients were considered to have evidence of asthma in their records if there were entries relating to the peak flow rates, response to medication, and histories suggestive of asthma based on the opinion of a GP assessor.
Results were analysed using SPSS 7.5.1 for Windows. The significance of differences in the distribution of responses to the questions between the groups were analysed using χ2 tests, unless the expected frequency within a cell was low. In such cases, Fisher's exact test was used.
Results
The computer search strategies produced data regarding the number of patients considered as having a diagnosis of asthma, as shown in Table 1. The majority of patients were identified based on Read Codes or asthma care activity; only 51 (6.1%) were found as a result of extending the searches to include those patients identifiable only as a result of being listed on the computer as having repeat antiasthma medication.
Table 1.
Number of patients considered to have a diagnosis of asthma as produced by the hierarchical computer search strategy.
| Search strategy | Number of patients |
|---|---|
| Read coded asthma | 670 |
| Asthma screen attendance | 39 |
| Asthma screen intervention record | 0 |
| Textual disease label entry containing ‘asthma’ ‘wheez’ | 73 |
| Inhaled steroids available on a repeat basis | 27 |
| Inhaled bronchodilators on a repeat basis | 24 |
| Mast cell stabilisers (cromolyns) on a repeat basis | 0 |
Figure 1 provides a summary of the main study findings. The response rates to the questionnaire survey were 659/833 (79.1%) for patients with asthma and 587/831 (70.6%) for the controls. The mean age of the responders in the group with asthma was 34.3 years (range 16.1–55.9 years) with 266 (40.4%) men and 393 (59.6%) women. The mean age of the control group responders was 34.7 years (range 16.1–55.9 years) with 265 (45.1%) men.
Figure 1.
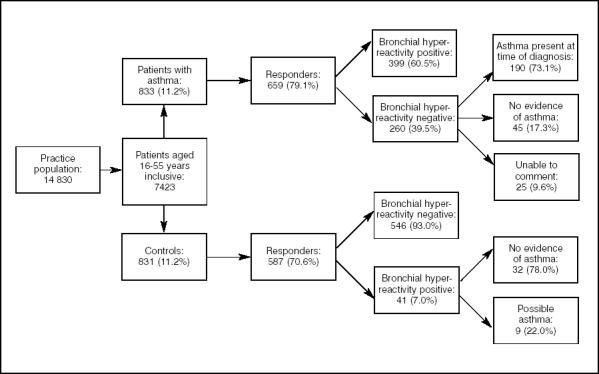
Summary of main findings.
In total, 399 out of 659 patients with asthma (60.5%), and 41 of 587 controls (7.0%) were classed as having symptomatic bronchial hyper-reactivity. The answers to additional questions about sleep disturbance, waking symptoms, and symptoms associated with exercise in the study questionnaire sent to the patients with asthma were analysed according to bronchial hyper-reactivity status. There were statistically significant differences in the answers to all these questions between the groups that had either a positive or negative bronchial hyper-reactivity status (P<0.001); those patients with a positive status of bronchial hyper-reactivity reported more symptoms. However, of patients with a bronchial hyper-reactivity negative status, 41/260 (15.8%) reported waking at least once a month, 46/260 (17.7%) reported symptoms on waking, and 79/260 (30.4%) reported symptoms on exercise.
Patients with asthma with negative bronchial hyper-reactivity
Of the 260 patients, 190 (73.1%) were considered to have evidence of asthma at the time of diagnosis: of these, 84 (44.2%) had no recorded symptoms or treatment for 2 years. A total of 44/190 (23.2%) were using regular preventative medication, the likely reason being that they didn't report symptoms suggestive of bronchial hyper-reactivity. The remaining 62/190 (32.6%) were using occasional bronchodilators.
There was no evidence to support a diagnosis of asthma in the records of 45/260 (17.3%) of the patients with a negative bronchial hyper-reactivity status. For 25/260 (9.6%) of patients, it was not possible to comment as there might have been a record of antiasthma medication having been prescribed, but no record of the response or patient's return for follow-up. In other cases, the practice no longer held the old paper records.
Control patients with positive bronchial hyper-reactivity
Of the control group responders (n = 587), 41 (7.0%) were found to have a positive bronchial hyper-reactivity status. Review of the medical records revealed that nine patients had asthma-related entries in their notes without the diagnostic term ‘asthma’ or an entry containing ‘wheez’ in the summary, or antiasthma medication being available on a repeat basis. There was no evidence of asthma in 32/41 (78.0%) of the control patients with a positive bronchial hyper-reactivity status.
Discussion
Summary of main findings
This study used a questionnaire survey of current symptoms predictive of asthma, and a notes review to validate the security of a diagnosis of asthma on the computer database. Using the validated bronchial hyper-reactivity questions, 60.5% of the patients with asthma who replied had symptomatic bronchial hyper-reactivity. Of the 39.5% patients who were asymptomatic, the asthma diagnosis was confirmed by notes searching in 73.0% of this group. This resulted in a total of 589 (89.4%) recorded patients with asthma having a secure diagnosis of asthma either before, or at the time of, the study. Of the 260 patients with asymptomatic bronchial hyper-reactivity, there was no evidence of asthma in 70 (26.9%) of them (in 25 [9.6%] it was not possible to comment), or 10.6% of the total group of patients with asthma.
Within the controls with a positive bronchial hyper-reactivity status, nine of the 41 (22.0%) were thought to have possible asthma; these represent nine out of 587 of all the controls who replied, a false negative rate of 1.5% in the control group. Applying this to all the patients without asthma in the 16–55-year age group (n = 6590), there are possibly another 99 patients with current symptoms of non-specific hyper-reactivity at the time of the original questionnaire, who have not been labelled as having asthma in the practice. By extrapolation, a prevalence of 5.5% of symptomatic control patients existed in the population aged 16–55 years that was not known to have asthma. This translates to some 362 people who may have symptoms of bronchial hyper-reactivity, but who are not bringing them to the attention of their doctors, or that having presented them they are not being recognised and recorded.
Of the 659 patients with asthma, who were identified using the combined search strategies in the study and who replied to the questionnaire, 399 were considered to have a positive status of bronchial hyper-reactivity. Together with the 190 who had, or had had, asthma, it then becomes clear that 589 of 659 responders (89.4%) could be considered as having asthma. In other words, in the practice database there is an 89.4% chance that a patient recorded as having asthma or one of its proxies has, or has had, asthma.
Analysis of the replies in the telephone sample of those who failed to respond to the postal questionnaire suggested that, in terms of prevalence of bronchial hyper-reactivity, presence of symptoms, or use of medication, there were no significant differences between this group and those returning the questionnaires by post. This suggests that the non-responders were probably not dissimilar to the responders and failure to know of their likely replies to the questionnaire would not have affected the results significantly.
There is both over- and under-recording of asthma in the general practice computer system database. Accurate prevalence can only be reported if information is recorded in a timely and accurate manner and should be reported in terms of ‘current’ asthma or a previous diagnosis of asthma.
The meaning of the study is that, based on the findings in this practice, GPs can be confident that around 90% of patients recorded as having asthma, do indeed have, or have had, asthma. The use of the bronchial hyper-reactivity questions can be an indication of poor symptom control in a patient with asthma, or can be used to screen a population not known to have asthma.
In our practice, searches for asthma and its proxies initially gave a prevalence of 11.2% (833/7423) in the age group of 16–55 years; using the 90% accuracy figure found in the study, this would suggest a prevalence of 10% of having, or ever having had, asthma, which had been presented, recognised, and recorded in this age group.
A survey of the validity and utility of electronic records in a general practice by Hassey et al16 aimed to measure whether the practice's patient records were a true record of the health events associated with the patients of the practice. Read Codes in the records were considered to equate with the true presence or absence of the associated conditions. The method was based on using Read coded entries over the previous 5 years, but with regard to asthma and ischaemic heart disease, considered only the previous year. The prevalence of asthma was 5.3% with a sensitivity of 87.3% and positive predictive value of 86.3%. No external checks such as patient questionnaires or examinations were used in this study and by only including data from the previous year they would be assessing ‘current’ asthma only.
Hassey et al's study16 looked at the accuracy of computer records compared with paper-based records for the same patients, rather than the accuracy of the actual diagnosis. However, a group in Sweden attempted to assess how often the diagnosis of bronchial asthma is correct.17 The patients (all aged above 18 years) had all visited a doctor in 1994 or 1995 and had a diagnosis of bronchial asthma recorded for that consultation, or requested antiasthma drugs and had a diagnosis of bronchial asthma in their records in the past. They were subsequently examined by a specialist in allergies, and if the diagnosis was still considered uncertain after a meeting between the GP, nurse, and the specialist, they were evaluated by further spirometry after a course of oral prednisolone. Of these patients, 34% were found to have no asthmatic disease.17 The authors commented that many studies report on the underdiagnosis of asthma, but few on its overdiagnosis. This is in contrast to our study where 89.4% of patients recorded as having asthma or one of its proxies was considered to have, or have had, asthma at the time of diagnosis.
Strengths and limitations of this study
The strengths of the study centre on the high response rates in the groups of patients with asthma and their controls (79.1% and 70.6% respectively), the existence of a fully computerised database for over 7 years, and a relatively stable population with little missing data. Although the use of self-report data is always prone to bias of recall, we used a validated set of questions to determine bronchial hyper-reactivity.
Limitations of the study relate to it only being conducted in one general practice population which, although representative of an ‘average’ Devon rural town with similar Townsend and Jarman scores of deprivation to others in the county,18 is not going to necessarily reflect the situation in inner cities, industrial areas, and areas with high multiethnic representation. Although the prevalence of treated asthma varies by deprivation indices and regions nationally, one study19 showed that there is little variation in the management of asthma across deprivation groupings or regions. Other studies, however, have shown that although there was little evidence of a strong relationship between service provision and population need in provision of asthma care in general practice, there was geographical variation, and methods of delivery are highly variable.20,21 It is, therefore, likely that the data recorded by practices will vary according to the way care is organised.
Implications for future research or clinical practice
The importance of recognising asthma and recording it using specific clinical codes will facilitate beneficial planned care of patients with the condition. Read Codes are now available to use before the diagnosis is actually confirmed to reflect the degree of uncertainty that exists in many cases.
This study shows that patients with specific conditions can be identified by the use of different search strategies. this has now become a well known practice for ‘data cleansing’ in general practice computer systems. It is important to recognise the variability of expression of asthma in terms of clinical status and it would be useful to be able to recognise this in a clinical summary for each patient. Using a validated bronchial hyper-reactivity questionnaire would be a means of screening patients not known to have asthma and to attempt to assess control in those patients on treatment.
Acknowledgments
We would like to acknowledge the helpful advice from Professor Sir Denis Pereira Gray during the course of this study. We would like to thank members of Honiton Group Practice and East Devon Respiratory Research Group for their valuable input throughout the project. This work was supported by a grant from The Northcott Devon Medical Foundation.
References
- 1.Levy M, Hilton S. Asthma in practice. 4th edn. London: The Royal College of General Practitioners; 1999. [Google Scholar]
- 2.Tirimanna PR, van Schayck CP, den Otter JJ, et al. Prevalence of asthma and COPD in general practice in 1992: has it changed since 1977? Br J Gen Pract. 1996;46(406):277–281. [PMC free article] [PubMed] [Google Scholar]
- 3.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moher M, Yudkin P, Turner R, et al. An assessment of morbidity registers for coronary heart disease in primary care. ASSIST (ASSessment of Implementation STrategy) trial collaborative group. Br J Gen Pract. 2000;50(458):706–709. [PMC free article] [PubMed] [Google Scholar]
- 5.Speight AN. Is childhood asthma being underdiagnosed and undertreated? BMJ. 1978;2(6133):331–332. doi: 10.1136/bmj.2.6133.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speight AN, Lee DA, Hey EN. Underdiagnosis and under- treatment of asthma in childhood. BMJ (Clin Res Ed) 1983;286(6373):1253–1256. doi: 10.1136/bmj.286.6373.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy M, Bell L. General practice audit of asthma in childhood. BMJ (Clin Res Ed) 1984;289(6452):1115–1116. doi: 10.1136/bmj.289.6452.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverman M. Out of the mouths of babes and sucklings: lessons from early childhood asthma. Thorax. 1993;48(12):1200–1204. doi: 10.1136/thx.48.12.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neville RG, Bryce FP, Robertson FM, et al. Diagnosis and treatment of asthma in children: usefulness of a review of medical records. Br J Gen Pract. 1992;42(365):501–503. [PMC free article] [PubMed] [Google Scholar]
- 10.Neal RD, Heywood PL, Morley S. Real world data-retrieval and validation of consultation data from four general practices. Fam Pract. 1996;13(5):455–461. doi: 10.1093/fampra/13.5.455. [DOI] [PubMed] [Google Scholar]
- 11.Venables KM, Farrer N, Sharp L, et al. Respiratory symptoms questionnaire for asthma epidemiology: validity and reproducibility. Thorax. 1993;48(3):214–219. doi: 10.1136/thx.48.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977;7(3):235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 13.Burr ML. Diagnosing asthma by questionnaire in epidemiological surveys. Clin Exp Allergy. 1992;22(5):509–510. doi: 10.1111/j.1365-2222.1992.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins MA, Clarke JR, Carlin JB, et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25(3):609–616. doi: 10.1093/ije/25.3.609. [DOI] [PubMed] [Google Scholar]
- 15.Toelle BG, Peat JK, Salome CM, et al. Toward a definition of asthma for epidemiology. Am Rev Respir Dis. 1992;146(3):633–637. doi: 10.1164/ajrccm/146.3.633. [DOI] [PubMed] [Google Scholar]
- 16.Hassey A, Gerrett D, Wilson A. A survey of validity and utility of electronic patient records in a general practice. BMJ. 2001;322(7299):1401–1405. doi: 10.1136/bmj.322.7299.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marklund B, Tunsater A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16(2):112–116. doi: 10.1093/fampra/16.2.112. [DOI] [PubMed] [Google Scholar]
- 18.Devon County Council. Life in Devon homepage. 2002. http://www.devon.gov.uk/dris/commstat/csta_mnu.html (accessed 6 September 2004)
- 19.Hoare J, Bruce M, Majeed A. Prevalence of treated asthma and its management in general practice in England and Wales, 1994–1998. Health Statistics Quarterly 2003. 27 February 2003;No 17 [Google Scholar]
- 20.Baker D, Hann M. General practitioner services in primary care groups in England: is there inequity between service availabilty and population need? Health Place. 2001;7(2):67–74. doi: 10.1016/s1353-8292(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 21.Baker D, Middleton E, Campbell S. The impact of chronic disease management in primary care on inequality in asthma severity. J Pub Health Med. 2003;25(3):258–60. doi: 10.1093/pubmed/fdg048. [DOI] [PubMed] [Google Scholar]


