Prevalence and Burden in the Medically Ill
Maurizio Fava, M.D., stated that depression is the second most common chronic condition (after hypertension) treated in general medical practice,1 making the prevalence and burden of depression in the medically ill a substantial clinical problem. Evidence suggests that 1 of 10 primary care patients experiences major depressive disorder (MDD), but many cases are unidentified or improperly treated.1 This confusion could be related to the fact that some of the symptoms of depression may overlap with those of the concomitant medical illnesses.
Diagnosis of Depression With Comorbid Medical Illness
Dr. Fava related that it is often difficult to distinguish depression as a primary mood disorder in a patient with a general medical condition because it may be viewed as secondary to the medical illness itself. Even when depression appears to be secondary, symptoms may not resolve after the medical condition is treated.
Further complicating diagnosis is the concept of depression as a reactive condition, i.e., depression is the psychological consequence of the medical illness and, therefore, viewed as less severe and more transient in nature than a true depressive disorder. However, some clinicians have questioned this theory and proposed that depression in the context of a medical illness is not a psychological consequence but, instead, the medical illness may expose an existing, underlying mood disorder or may trigger a recurrence of a preexisting mood disorder.
Dr. Fava stressed that accurate diagnosis can also be a challenge because of the overlap of symptoms, such as weight loss and fatigue, which occur in depression as well as in many medical illnesses such as diabetes, cancer, and thyroid disease. The common psychological, behavioral, and physical symptoms associated with depression include, but are not limited to, anxiety, low self-esteem, reduced concentration, lack of interest or pleasure, reduced productivity, social withdrawal, headaches, gastrointestinal problems, and heart palpitations (Table 1).2
Table 1.
Common Psychological, Behavioral, and Somatic Symptoms of Unipolar Depressiona

Many roadblocks hinder the diagnosis and treatment of depression in the medically ill, one being that patients may have certain thoughts or assumptions about their depression that may cause them to minimize the symptoms or not seek treatment for them. For example, patients may think it is normal to feel sad after developing a specific medical condition (i.e., “Of course you are depressed after you are diagnosed with a tumor.”). Similarly, clinicians may focus more on the medical illness and view it as the only cause of the depression. Therefore, clinicians may not provide treatment for the depression (i.e., “Treat only the medical illness since it caused the depression.”).
Prevalence of MDD in Patients With General Medical Illness
Dr. Fava asserted that there is a higher prevalence of MDD3,4 in medically ill subjects than in the normal population.5 Conversely, medical illness is a risk factor for MDD.6,7 Also, the presence of depression is associated with lower recovery rates and poorer function in individuals with medical illnesses.8
A study conducted by Wells et al.5 in 1988 showed that both the 6-month prevalence and lifetime prevalence of affective disorders in patients with any medical illness were almost twice that of individuals without medical illness. Another study, by Koike et al.,9 corroborates this evidence. Patients with depression and comorbid medical illness were more likely to still have depression at 6- and 12-month follow-up than those with only depression and no medical illness.
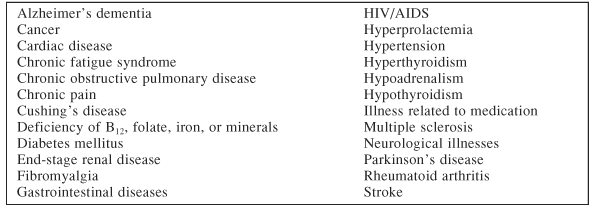
Table 2 illustrates the medical conditions associated with depression. Dr. Fava highlighted the prevalence of depression in individuals with cardiac disease, especially after myocardial infarction (32%),10 after the first documentation of coronary artery disease by coronary angiography (18%),11 in the first year after heart transplant (17%),12 and in those with congestive heart failure (50%).13 Similarly, patients with type I and type II diabetes mellitus may have a 20% to 30% prevalence of MDD, which is 2 or 3 times that of the general population.14 Studies also indicate that there is a clear association between depression and hyperglycemia,15 which may be related to complications in diabetes itself.16
Table 2.
Medical Conditions Associated With a High Rate of Depression
Findings from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D),17 the largest study ever conducted on depression, showed that a comorbid medical condition was present in 52.8% of the 1500 patients with MDD. This comorbidity was associated with older age, lower income, unemployment, limited education, longer duration of index depressive episode, and an absence of self-reported family history of depression. From a clinical standpoint, Dr. Fava acknowledged that somatic symptoms of depression appear to be reported at a higher rate in patients with depression who have general medical conditions than in those without such conditions.
The Burden of MDD
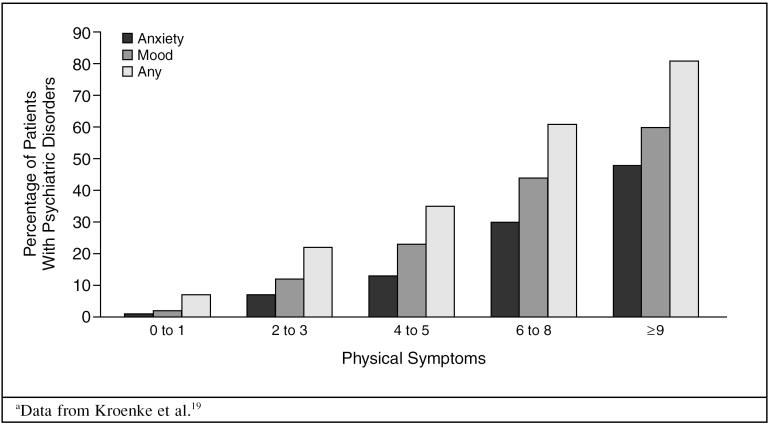
The presence of depression, Dr. Fava reinforced, has clear psychological, behavioral, and physical effects on patients. Psychological symptoms may affect one's ability to cope effectively with the medical illness; behavioral symptoms may interfere with treatment adherence, nutrition, and self-care; and somatic symptoms may complicate the clinical picture and further reduce physical functioning. According to the Medical Outcomes Study,18 physical functioning in individuals with depressive symptoms was better than in those with heart disease but was worse than in those with back problems, arthritis, diabetes, and hypertension. In all patients, a higher number of physical symptoms increased the likelihood of having poor physical functioning and mood and/or anxiety disorders (Figure 1).19
Figure 1.
Number of Physical Symptoms Associated With Psychiatric Disordersa
According to the Global Burden of Disease Study,20 major depression is the fourth highest cause of burden worldwide and is projected to be second highest by the year 2020. Dr. Fava stated that the key contributors to the burden of depression are increased morbidity and mortality, decreased productivity, increased suffering by friends and family, impaired personal relationships, increased risk of violence toward significant others, and a greater use of medical resources.
The morbidity of hospitalized patients reflects a direct relationship between depression and health status.21 In a study22 of acutely ill hospitalized older patients with depression, the patients with 6 or more depressive symptoms were more likely to decline in health and less likely to improve during and after hospitalization than the patients with fewer symptoms of depression.
In a 16-year prospective study23 of a general population sample, an increased risk of death has also been associated with the effects of depression. This increase is especially evident in elderly individuals with MDD, with 23.1% to 59.3% greater mortality risks than in men and women (respectively) without MDD.24
The Medical Outcomes Study18 showed that patients who meet the criteria for MDD, dysthymic disorder, or both function more poorly than other primary care patients in that they have greater limitations in physical activities, social activities, and occupational roles. Dr. Fava noted that disability may be more severe in patients with depression and comorbid conditions.
Depressive disorders affect not only patients but also their friends, family members, and employers. Depressive symptoms may hinder relationships by creating apathy, social withdrawal, and libido changes and may impair a patient's ability to raise children or fulfill other roles within the family structure. Dr. Fava also recognized the economic burden on society, such as the diminished ability to support oneself and others. The social effects of depression may lead to shame and stigmatization for the patient and his or her family.
From the health care cost perspective, primary care patients with depression have almost twice the annual health care costs of patients without the disorder.25 Furthermore, a study26 conducted in the California Medicaid population revealed that in patients with treatment-resistant MDD, the health care costs were approximately $1,000 more than in those patients who did not experience resistance.
Conclusion
Dr. Fava emphasized that depression is a common condition among the medically ill. Those who suffer from this debilitating disorder in addition to a medical condition are often underdiagnosed and under-treated. Patients who suffer from depression have not only psychological symptoms but also behavioral and physical symptoms. These symptoms lead to increased morbidity and mortality rates, decreased productivity and psychosocial functioning, decreased ability to function normally with family and friends, and an increased use of medical resources. The burden of depression is especially great in patients with comorbid illness, and conversely, the addition of medical comorbidities in individuals with depression may lead to more somatic symptoms and more persistent depressive symptoms.
REFERENCES
- Whooley MA, Simon GE.. Managing depression in medical outpatients. N Engl J Med. 2000;343:1942–1950. doi: 10.1056/NEJM200012283432607. [DOI] [PubMed] [Google Scholar]
- Cassano P, Fava M.. Depression and public health: an overview. J Psychosom Res. 2002;53:849–857. doi: 10.1016/s0022-3999(02)00304-5. [DOI] [PubMed] [Google Scholar]
- Patten SB.. Long-term medical conditions and major depression in the Canadian population. Can J Psychiatry. 1999;44:151–157. doi: 10.1177/070674379904400205. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Schneiderman N, and Blumenthal JA. et al. Cognitive and somatic symptoms of depression are associated with medical comorbidity in patients after acute myocardial infarction. Am Heart J. 2003 146:48–54. [DOI] [PubMed] [Google Scholar]
- Wells KB, Golding JM, Burnam MA.. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am J Psych. 1988;145:976–981. doi: 10.1176/ajp.145.8.976. [DOI] [PubMed] [Google Scholar]
- Koenig HG, Meador KG, and Cohen HJ. et al. Depression in elderly hospitalized patients with medical illness. Arch Intern Med. 1988 148:1929–1936. [PubMed] [Google Scholar]
- Koenig K, Meador KG, and Shelp F. et al. Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991 39:881–890. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Ryan CE, and Miller IV. et al. 12-Month outcome of patients with major depression and comorbid psychiatric or medical illness. Am J Psychiatry. 1991 148:345–350. [DOI] [PubMed] [Google Scholar]
- Koike AK, Unutzer J, Wells KB.. Improving the care for depression in patients with comorbid medical disease. Am J Psychiatry. 2002;159:1738–1745. doi: 10.1176/appi.ajp.159.10.1738. [DOI] [PubMed] [Google Scholar]
- Lesperence F, Frasure-Smith N, Talajic M.. Major depression before and after myocardial infarction: its nature and consequences. Psychosom. 1996;58:99–110. doi: 10.1097/00006842-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Carney RM, Rich MW, and Tevelde A. et al. Major depressive disorder in coronary artery disease. Am J Cardiol. 1987 60:1273–1275. [DOI] [PubMed] [Google Scholar]
- Dew MA, Roth LH, Schulberg RG.. Prevalence and predictors of depression and anxiety related disorders during the year after heart transplantation. Gen Hosp Psychiatry. 1996;18(suppl 6):48S–61S. doi: 10.1016/s0163-8343(96)00077-1. [DOI] [PubMed] [Google Scholar]
- Lainscak M, Keber I.. Patient's view of heart failure: from the understanding to the quality of life. Eur J Cardiovasc Nurs. 2003;2:275–281. doi: 10.1016/S1474-5151(03)00064-1. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, and Clouse RE. et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001 24:1069–1078. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, and Freedland KE. et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000 23:934–942. [DOI] [PubMed] [Google Scholar]
- de Groot M, Anderson R, and Freedland KE. et al. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001 63:619–630. [DOI] [PubMed] [Google Scholar]
- Yates WR, Mitchell J, and Rush AJ. et al. Clinical features of depressed outpatients with and without co-occuring general medical conditions in STAR*D. Gen Hosp Psychiatry. 2004 26:421–429. [DOI] [PubMed] [Google Scholar]
- Wells KB, Stewart A, and Hays RD. et al. The functioning and well-being of depressed patients: results from the Medical Outcomes Study. JAMA. 1989 262:914–919. [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, and Williams JB. et al. Physical symptoms in primary care. predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994 3:774–779. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD.. Evidence-based health policy: lessons from the Global Burden of Disease Study. Science. 1996;274:1593–1594. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Fawcett J.. The morbidity and mortality of clinical depression. Int Clin Psychopharmacol. 1993;8:217–220. doi: 10.1097/00004850-199300840-00002. [DOI] [PubMed] [Google Scholar]
- Covinsky KE, Fortinsky RH, and Palmer RM. et al. Relation between symptoms of depression and health status outcomes in acutely ill hospitalized older persons. Ann Intern Med. 1997 126:417–425. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Monson RR, and Olivier DC. et al. Affective disorders and mortality: a general population study. Arch Gen Psychiatry. 1987 44:473–480. [DOI] [PubMed] [Google Scholar]
- Schoevers RA, Geerlings MI, and Beekman AT. et al. Association of depression and gender with mortality in old age: results from the Amsterdam Study of the Elderly (AMSTEL). Br J Psychiatry. 2000 177:336–342. [DOI] [PubMed] [Google Scholar]
- Simon GE, VonKorff M, Barlow W.. Health care costs of primary care patients with recognized depression. Arch Gen Psychiatry. 1995;52:850–856. doi: 10.1001/archpsyc.1995.03950220060012. [DOI] [PubMed] [Google Scholar]
- McCombs JS, Nichol MB, and Stimmel GL. et al. The cost of antidepressant drug failure: a study of antidepressant use patterns in a Medicaid population. J Clin Psychiatry. 1990 51(6, suppl):60–69. [PubMed] [Google Scholar]
Identifying, Evaluating, and Monitoring Depression in Patients With Medical Conditions
Larry Culpepper, M.D., M.P.H., said that not only is depression common among medically ill patients, but it can be a risk factor for medical illness. One of the most consistent indicators of depression is the development of a serious medical condition. For example, an individual who experiences depression as a young adult is twice as likely to develop cardiac disease in late adulthood.1 Likewise, the presence of depression is also likely to increase the risk for diabetes,2 Parkinson's disease, and rheumatoid arthritis; the likelihood of a first stroke with the onset of cancer; and an overall vulnerability to chronic medical illnesses in general.3 Dr. Culpepper emphasized that patients with a comorbid medical condition and major depressive disorder are at a high risk of rapid progression of the medical illness resulting in a severe outcome. Therefore, patients with medical illnesses should be screened for depression. Physicians should also consider several other patient characteristics besides the medical illness that may alert them to a risk for developing depression.
Risk Factors for Depression Besides Medical Illness
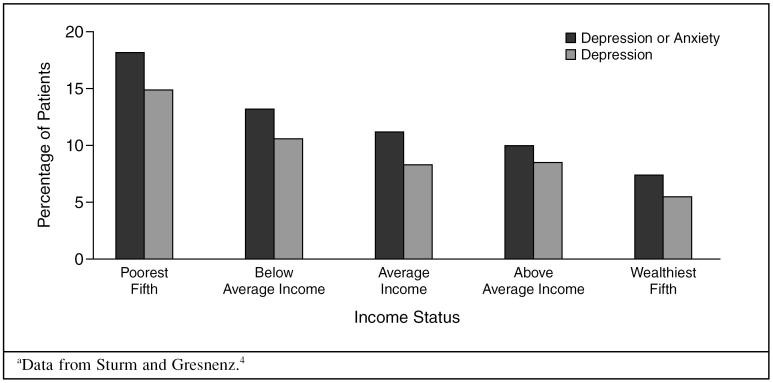
A potential risk factor for depression is an individual's or family's low income. There is a consistent relationship between low income and increased risk for major depressive episode in communities around the world. In a study4 of 60 metropolitan or economic areas of the United States, the depression rate in the poorest fifth of the population (14.9%) was more than twice that of the wealthiest fifth (5.5%) (Figure 2).
Figure 2.
Prevalence of Depression and Anxiety According to Incomea
Family history is also an important contributor to the risk of developing depression. Dr. Culpepper stated that the risk of developing major depression, anxiety, or conduct disorders in early adulthood is 3 to 8 times higher in people with a parent or grandparent with depression than in those without such a family history.5 Similarly, the early onset of a parent's major depression (at less than 30 years old) increases a child's odds of developing the same disorder either as a child or in early adulthood by 7 to 13 times.5
Married individuals seem to have twice the risk of developing major depression if their spouse is depressed than if their spouse is not depressed.6 This effect has been consistently demonstrated.6–8
Abuse, physical and/or sexual, is a high risk factor for depression in women. Dr. Culpepper noted that there is a gradient across abuse history. Women who have had no abuse are at the lowest risk for developing major depression; women with abuse in either childhood or adulthood have a slightly higher risk; and women who have suffered abuse both as a child and as an adult are at the most serious risk for major depression across the course of their adult life.9
Indicators of Depression
The presence of physical symptoms is an indicator of possible depression. Kroenke et al.10 conducted a landmark 3-year survey of 1000 primary care patients in the Midwest. The results show an increased risk of mood disorders being the underlying source of symptomatology in patients with a high number of physical symptoms. For example, patients with 0 or 1 physical symptom have a 2% risk and patients with 9 or more symptoms have a 60% risk.
Evaluating Depression
Some variables must be taken into consideration to accurately evaluate the level of depression in the patient and also to distinguish it from other disorders that may be present. One variable in the differential diagnosis is treatment for a preexisting medical condition. For example, individuals with cancer or chronic obstructive pulmonary disease (COPD) may suffer from fatigue or insomnia due to certain treatments. Because these symptoms are also related to depression, a correct identification of the cause may be difficult.
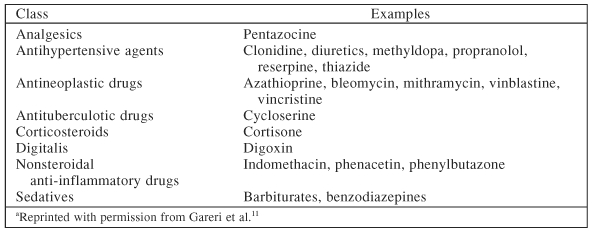
Another variable to consider in the differential diagnosis is hypothyroidism. Dr. Culpepper recommended that primary care doctors should have a low threshold for testing for hypothyroidism. In addition, the diagnosis of depression may be complicated by medications the patient is taking for the medical illness, some of which may induce symptoms of depression (Table 3).11
Table 3.
Medications That May Induce Depressiona
Other psychiatric disorders also should be considered in the differential diagnosis. A family history of bipolar disorder or a personal history of cyclothymia may indicate that the patient has bipolar disorder rather than unipolar major depression. Also, although anxiety symptoms are generally associated with depression, physicians should consider if the symptoms may indicate a specific disorder such as a social anxiety disorder or posttraumatic stress disorder.
Finally, bereavement can accompany and often mimic major depression, especially in older individuals with a history of loss.12 However, Dr. Culpepper stressed that these are two different processes; bereavement is a stage of life, usually over the duration of 2 months, which most people work through. When individuals continue grieving over a prolonged period of time, they may have major depression characterized by decreased function, continual sadness, unlikely remarriage (in spousal loss), and an overall decline in their quality of life and an increased morbidity/mortality when evaluated at 2 years. This depression can be reversed with treatment.
Although Dr. Culpepper said that cognitive, affective, behavioral, and physical symptoms can indicate depression, the presence of cognitive symptoms (e.g., lack of concentration, negative thinking) is the most reliable indicator. Dr. Culpepper advised that any symptoms should be counted toward the diagnosis of major depression, even though there may be alternate medical explanations. A diagnostic dilemma arises when there are severe cognitive impairments, as is the case in pseudodementia, which can result from depression. Psuedodementia tends to develop over a period of weeks, unlike the development of dementia which usually evolves more slowly. Patients with pseudodementia are often concerned about their cognitive ability in contrast to the indifference shown by those with true dementia.12 Dr. Culpepper recommended treating individuals with apparent pseudodementia over a month or 2 with a selective serotonin reuptake inhibitor or serotonin norepinephrine re-uptake inhibitor to evaluate whether the dementia is reversible once their depression improves.
Screening for Depression
Screening for depression—when coupled with active management—can reliably lead to better response rates. The United States Preventive Services Task Force13 now advises practitioners to begin screening for major depression by asking 2 questions: (1) Over the past 2 weeks, have you felt down, depressed, or hopeless? and (2) Over the past 2 weeks, have you felt little interest or pleasure in doing things? If either of these questions is answered positively, then a second, more comprehensive evaluation is warranted to identify depression in the patient. Dr. Culpepper stated that this simple 2-question screen greatly decreases the number of potential patients that would need further assessment.
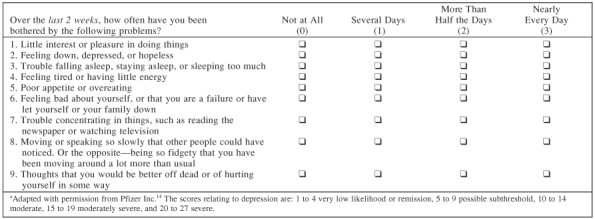
The Patient Health Questionnaire-9 (PHQ-9) (Table 4), a diagnostic tool for further evaluation after the initial 2 questions, is an internationally used exam that consists of 9 self-report questions.14 This test is reliable not only for the initial diagnosis of depression but also to monitor patients as they move through the stages of treatment.15 For patients who score a 10 or more, further assessment is necessary to confirm the diagnosis of major depression and also to establish any potential comorbidities.14
Table 4.
Patient Health Questionnaire-9 (PHQ-9) Symptom Checklista
Monitoring Treatment of Depression
Once the diagnosis of MDD has been established, the treatment process can begin. The first phase of this process is moving the patient from full symptomatology into response (usually lasting at least 2 to 3 months) and then into remission. Repeated assessment during this treatment phase to identify nonresponsive or plateaued patients and rectify their course of treatment is crucial.16 Once the patient is in remission, the clinician should help the patient maintain that progress by developing the patient's skill at self-monitoring to recognize relapse or to detect early signs of recurrence in order to seek intervention appropriately. Finally, in patients with a serious or nontransient condition, psychotherapy added to drug therapy is recommended to attain the best outcome.17 During the treatment process, Dr. Culpepper noted that it may be helpful to create a flowchart of the patient's most functionally impairing symptoms along with the side effects from medication or therapy in order to maximize the treatment effort.
Conclusion
Several patient characteristics besides medical illness, such as low income, family history of depression, spousal depression, and abuse, should be recognized in order to fully understand the patients' risk for depression. The presence of a large number of physical symptoms, rapid onset of pseudodementia, and the development of medical illnesses may indicate depression. Clinicians also should distinguish MDD from side effects of treatment for medical illnesses, hypothyroidism, other psychiatric disorders, and bereavement. To accurately identify depression in patients, an appropriate method of assessment should be used, e.g., the PHQ-9 self-rated questionnaire. Once the disorder is identified, clinicians should monitor and evaluate their patients regularly in order to provide effective treatment.
REFERENCES
- Musselman DL, Evans DL, Nemeroff CB.. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Betan E, and Larsen H. et al. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003 54:317–329. [DOI] [PubMed] [Google Scholar]
- Cohen-Cole SA, Kaufmen KG.. Major depression in physical illness: diagnosis, prevalence, and antidepressant treatment (a ten-year review: 1982–1992) Depression. 1993;1:181–204. [Google Scholar]
- Sturm R, Gresnenz CR.. Relations of income inequality and family income to chronic medical conditions and mental health disorders: national survey. BMJ. 2002;324:20–23. doi: 10.1136/bmj.324.7328.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramaratne PJ, Weissman MM.. Onset of psychopathology in offspring by developmental phase and parental depression. J Am Acad Child Adolesc Psychiatry. 1998;37:933–942. doi: 10.1097/00004583-199809000-00013. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C, and Pringle M. et al. Married couples' risk of same disease: cross sectional study. BMJ. 2002 325:636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichman Y, Bar-El Z, and Shor H. et al. Cognitive, interpersonal, and behavioral predictors of patient's and spouse's depression. J Affect Disord. 2003 74:247–256. [DOI] [PubMed] [Google Scholar]
- Halford WK, Bouma R, and Kelly A. et al. Individual psychopathy and marital distress: analyzing the association and implications for therapy. Behav Modif. 1999 23:179–216. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, and Kolodner K. et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997 277:1362–1368. [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, and Williams JB. et al. Physical symptoms in primary care: predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994 3:774–779. [DOI] [PubMed] [Google Scholar]
- Gareri P, Falconi U, and De Fazio P. et al. Conventional and new antidepressant drugs in the elderly. Prog Neurobiol. 2000 61:353–396. [DOI] [PubMed] [Google Scholar]
- Zisook S, Dunn LB.. Dementia and bereavement: adverse life experiences complicating depression in later life. Prim Care Companion J Clin Psychiatry. 2000;2(suppl 5):25–31. [Google Scholar]
- Pignone MP, Gaynes BN, and Rushton JL. et al. Screening for depression in adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002 136:765–766. [DOI] [PubMed] [Google Scholar]
- Pfizer Incorporated. Patient Health Questionnaire (PHQ-9). Available at: http://www.pfizer.com/pfizer/phq-9/index.jsp. Accessed Nov 21, 2005. [Google Scholar]
- Lowe B, Unutzer J, and Callahan CM. et al. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004 42:1194–1201. [DOI] [PubMed] [Google Scholar]
- Marangell LB.. Switching antidepressants for treatment-resistant major depression. J Clin Psychiatry. 2001;62(suppl 18):12–17. [PubMed] [Google Scholar]
- Burt VK.. Plotting the course to remission: the search for better outcomes in the treatment of depression. J Clin Psychiatry. 2004;65(suppl 5):12–17. [PubMed] [Google Scholar]
Relationship Between Physical Symptoms and Depression Treatment Response or Resistance in Patients With Comorbid Medical Conditions
David A. Fishbain, M.D., F.A.P.A., began the lecture by stating that a patient's response to or remission from depression may depend on the resolution of somatic symptoms. Somatic symptoms may be related to pain rather than depression, but antidepressants have analgesic properties and can address pain and associated somatic symptoms. It may be necessary to target each somatic symptom with pharmacologic treatment in order to achieve remission of depression.
Somatic Symptoms in Depression and Pain
The most common somatic symptoms of depression are decreased energy, decreased sleep, headaches, psychomotor changes, gastrointestinal problems, appetite changes, and vague aches and pains. A study1 found that the more somatic symptoms a patient displays, the higher the likelihood that the patient has a mood disorder. A similar relationship also exists between pain and depression. In a study2 of 483 outpatients at a neurology clinic, approximately 161 patients were diagnosed with depression. Of the 161 patients, 75% had depression accompanied with pain. The odds ratio for having pain increased in those with depression (2.4) and the odds ratio for having depression increased in those with pain (2.4), confirming an association between the two.
The presence of pain is usually indicative of other somatic symptoms in patients with major depression. In a large population study of the characteristics of pain and depression (N = 18980),3 4% had MDD. Of the patients with MDD, 43% experienced chronic pain and were more likely to suffer from severe fatigue, insomnia, severe psychomotor agitation, weight gain, and difficulty concentrating than those without MDD.
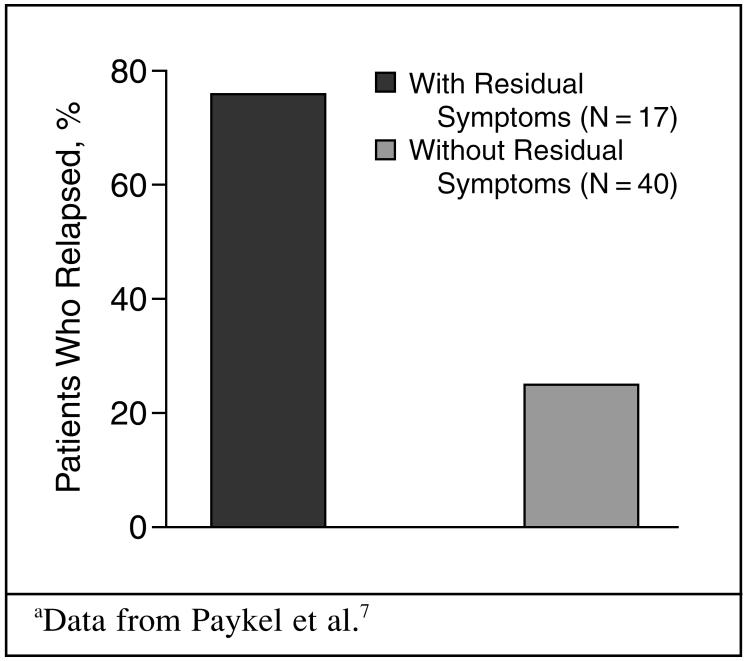
Dr. Fishbain continued by explaining that somatic symptoms, especially pain, are directly related to antidepressant nonresponse, remission failure, and relapse. For example, studies show that the greater the number of somatic symptoms4,5 or pain6 reported at baseline, the greater the nonresponse. Supporting this concept, one study7 found that 94% of nonremitters experienced a greater number of baseline somatic symptoms. Also, residual physical symptoms are associated with the inability to achieve remission.8 Paykel et al.7 reported that a patient's continued psychiatric and physical symptoms after depression treatment can seriously increase the risk of relapse (Figure 3).
Figure 3.
Relapse in Patients With Residual Symptoms of Depression Compared With Those Withouta
Antidepressant Effects on Pain
Antidepressants can have antinociceptive qualities, Dr. Fishbain remarked, despite the fact that this treatment has been previously viewed as nonanalgesic. For instance, one meta-analysis9 of patients with psychogenic somatoform pain disorder indicated that antidepressants greatly decreased pain intensity, regardless of the effect on depression. Other meta-analyses10,11 on fibromyalgia showed that antidepressants decreased pain and fatigue, increased sleep, and contributed to the patient's overall well-being.
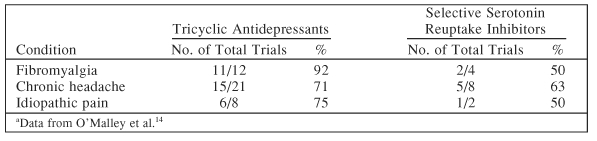
To further analyze antidepressant analgesic effects, Dr. Fishbain and colleagues12 grouped antidepressants into three main categories (noradrenergic, serotonergic [SSRIs], and dualaction—a combination of the 2) and analyzed the effects of each type of antidepressant class on depression and pain. Animal neuropathic pain subjects, when treated with the different antidepressants, showed a 100% improvement in pain with dualaction agents as opposed to 88.9% effectiveness with noradrenergic and only 14.3% for serotonergic. In human patients, dualaction antidepressants were shown to be consistent in their analgesic effect on chronic low back pain, osteoarthritis, rheumatoid arthritis, and foot ulcers.13 A meta-analysis of 94 trials14 indicated that tricyclic antidepressants produced greater results than SSRIs in fibromyalgia, headache pain, and idiopathic pain (Table 5).
Table 5.
Percentage of Antidepressant Trials Reporting Efficacy by Type of Antidepressanta
A recently approved dualaction antidepressant, duloxetine, appears to be an effective treatment for pain as a somatic symptom of MDD. When treated with duloxetine, patients with MDD and pain demonstrated an improvement in back pain, shoulder pain, pain while awake, and interference with daily activities as opposed to placebo.15 This improvement in overall pain severity is associated with a higher estimated probability of remission.16 A path analysis of duloxetine in patients with painful diabetic neuropathy showed that 88.6% of the total pain improvement was direct while only 11.4% of the total pain improvement was indirect through change in depressive symptoms.17 Duloxetine-treated patients with fibromyalgia have also shown pain improvement independent of MDD severity at treatment onset.18
Dr. Fishbain described other dualaction antidepressants and their effects on pain in fibromyalgia. Milnacipran-treated patients demonstrated pain improvement that was independent of mood.19 Similarly, a path analysis20 of venlafaxine determined that 83.3% of the pain decrease resulted from pain change, 15.3% of the pain improvement was linked to depression change, and 1.5% of the pain change was linked to anxiety change. Not only do these studies emphasize the antinociceptive qualities of dual-action antidepressants, but they also recognize a separation of analgesic from antidepressive effects.
To emphasize that depression and pain are not dependent on one another, Dr. Fishbain described a study21 of 573 patients with chronic depression who received the SSRIs fluoxetine, paroxetine, or sertraline. All 3 antidepressants improved depression but had minimal effect on physical symptoms, especially pain. Similarly, in a trial22 of 13 patients with pain and depression treated with electroconvulsive therapy (ECT), patients showed improvement in depression but no change in physical functioning, pain, stiffness, morning tiredness, number of tender reports, or self-reported pain.
Treating Somatic Symptoms
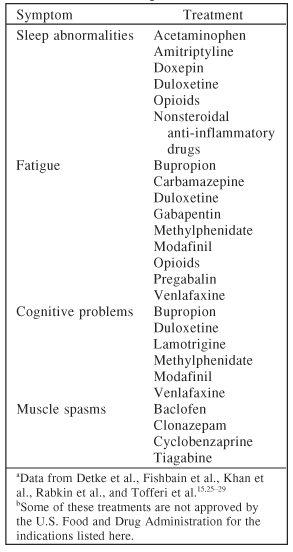
Some common somatic symptoms in depression, such as sleep disturbances, fatigue, cognitive (memory or concentration) problems, muscle spasms, autonomic disturbances, restless leg syndrome, headaches, non-organic physical findings, and sexual dysfunction may be strictly pain-related and, as a result, will improve if pain improves (Table 6).23,24 In fact, Dr. Fishbain stressed each somatic symptom may need to be addressed individually in order to improve patients' response to depression. Although there is not yet concrete proof for this treatment strategy, pain clinicians have been using this method for years. Table 715,25–29 outlines some specific medications that may be helpful in treating somatic symptoms in patients with depression.
Table 6.
Somatic Symptoms in Depression That May Be Pain-Related and Improve if Pain Improvesa
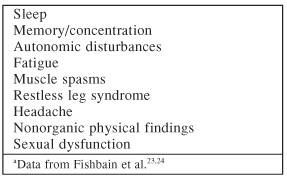
Table 7.
Medication Options for Somatic Symptoms in Patients With Chronic Pain and Depressiona,b
Conclusion
Patients with somatic problems are more likely to have severe depression, nonremitting depression, and a high relapse risk, resulting in complex symptomatology and treatment difficulty. These patients may also have other psychiatric comorbidities and poor treatment outcome, and they may require polypharmacy in order to be treated effectively. Dr. Fishbain recommended isolating individual symptoms, targeting pain aggressively, and using antidepressants with analgesic properties as an appropriate course of treatment for patients with depression who also experience chronic pain and other somatic symptoms.
REFERENCES
- Kroenke K, Spitzer RL, and Williams JB. et al. Physical symptoms in primary care: predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994 3:774–779. [DOI] [PubMed] [Google Scholar]
- Williams LS, Jones WJ, and Shen J. et al. Prevalence and impact of depression and pain in neurology outpatients. J Neurol Neurosurg Psychiatry. 2003 74:1587–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM.. Specific characteristics of the pain/depression association in the general population. J Clin Psychiatry. 2004;65(suppl 12):5–9. [PubMed] [Google Scholar]
- Papakostas GI, Petersen TJ, and Iosifescu DV. et al. Somatic symptoms as predictors of time to onset of response to fluoxetine in major depressive disorder. J Clin Psychiatry. 2004 65:543–546. [DOI] [PubMed] [Google Scholar]
- Denninger JW, Mahal Y, and Merens W. et al. The relationship between somatic symptoms and depression. In: New Research Abstracts of the 155th Annual Meeting of the American Psychiatric Association. 21May2002 Philadelphia, Pa: Abstract NR251: 68–69. [Google Scholar]
- Karp JF, Weiner D, and Seligman K. et al. Body pain and treatment response in late-life depression. Am J Geriatr Psychiatry. 2005 13:188–194. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, and Cooper Z. et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995 25:1171–1180. [DOI] [PubMed] [Google Scholar]
- Keller MB, Berndt ER.. Depression treatment: a lifelong commitment? Psychopharmacol Bull. 2002;36(suppl 2):133–141. [PubMed] [Google Scholar]
- Fishbain DA, Cutler RB, and Rosomoff HL. et al. Do antidepressants have an analgesic effect in psychogenic pain and somatoform disorder? a meta-analysis. Psychosom Med. 1998 60:503–509. [DOI] [PubMed] [Google Scholar]
- O'Malley PG, Balden E, and Tomkins G. et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000 15:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LM, Keck PE Jr, Welge JA.. Antidepressant treatment of fibromyalgia: a meta-analysis and review. Psychosomatics. 2000;41:104–113. doi: 10.1176/appi.psy.41.2.104. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler RB, and Rosomoff HL. et al. Evidence-based data from animal and human experimental studies on pain relief with antidepressants: a structured review. Pain Med. 2000 1:310–316. [DOI] [PubMed] [Google Scholar]
- Fishbain DA.. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32:305–316. doi: 10.3109/07853890008995932. [DOI] [PubMed] [Google Scholar]
- O'Malley PG, Jackson JL, and Santoro J. et al. Antidepressant therapy for unexplained symptoms and symptom syndromes. J Fam Pract. 1999 48:980–990. [PubMed] [Google Scholar]
- Detke MJ, Lu Y, and Goldstein DJ. et al. Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. J Clin Psychiatry. 2002 63:308–315. [DOI] [PubMed] [Google Scholar]
- Fava M, Mallinckrodt C, and Detke M. et al. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher rates of remission? J Clin Psychiatry. 2004 65:521–530. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, and Detke MJ. et al. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005 116:109–118. [DOI] [PubMed] [Google Scholar]
- Arnold LM, Lu Y, and Crofford LJ. et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004 50:2974–2984. [DOI] [PubMed] [Google Scholar]
- Vitton O, Gendreau M, and Gendreau J. et al. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum Psychopharmacol. 2004 19(suppl 1):S27–S35. [DOI] [PubMed] [Google Scholar]
- Zijlstra TR, Barendregt PJ, and van de Laar MA. et al. Venlafaxine in fibromyalgia: results of a randomized, placebo-controlled, double-trial. Presented at the 66th annual scientific meeting of the American College of Rheumatology; Oct 25–29; New Orleans, La. Presentation 179. [Google Scholar]
- Bair MJ, Robinson RL, and Eckert GJ. et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004 66:17–22. [DOI] [PubMed] [Google Scholar]
- Huuhka MJ, Haanpaa ML, Lainonen EV.. Electroconvulsive therapy in patients with depression and fibromyalgia. Eur J Pain. 2004;8:371–376. doi: 10.1016/j.ejpain.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, and Cutler RB. et al. Is pain fatiguing? a structured evidence-based review. Pain Med. 2003 4:51–62. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, and Cutler RB. et al. A structured evidence-based review on the meaning of nonorganic physical signs: Waddell signs. Pain Med. 2003 4:141–181. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler RB, and Rosomoff HL. et al. Clonazepam open clinical treatment trial for myofascial syndrome associated chronic pain. Pain Med. 2000 1:332–339. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler RB, and Lewis J. et al. Modafinil for the treatment of pain-associated fatigue: review and case report. J Pain Palliat Care Pharmacother. 2004 18:39–47. [PubMed] [Google Scholar]
- Khan A, Ginsberg LD, and Asnis GM. et al. Effect of lamotrigine on cognitive complaints in patients with bipolar I disorder. J Clin Psychiatry. 2004 65:1483–1490. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, and Rabkin R. et al. Modafinil treatment for fatigue in HIV+ patients: a pilot study. J Clin Psychiatry. 2004 65:1688–1695. [DOI] [PubMed] [Google Scholar]
- Tofferi JK, Jackson JL, and O'Malley PG. et al. Treatment of fibromyalgia with cyclobenzaprine: a meta-analysis. Arthritis Rheum. 2004 51:9–13. [DOI] [PubMed] [Google Scholar]
Review of the Efficacy and Safety of Available Antidepressants in the Treatment of Depression in Patients With Comorbid Medical Illness
James M. Martinez, M.D., reviewed published randomized, controlled trials of antidepressant treatment of depression in patients with medical co-morbidities, specifically those with cancer, HIV/AIDS, and cardiovascular disease. He also briefly reviewed some general treatment principles when considering antidepressant treatment for patients with depression and comorbid hepatic or renal impairment.
Cancer
A literature review revealed a total of 7 published controlled clinical trials1–7 that evaluated the efficacy of various antidepressants in patients with comorbid depression and cancer. The results of these studies were generally positive. Imipramine improved depression in one small pilot study in hospitalized cancer patients.1 Two trials2,3 of mianserin treatment demonstrated significant improvement in depressive symptoms in patients with cancer and depression. Response rates from one placebo-controlled trial4 of fluoxetine were not promising. However, a different fluoxetine study5 reported reduced levels of depression and an improvement in the overall quality of life with fluoxetine treatment in patients with advanced cancer. In a small trial of fluoxetine versus desipramine,6 both treatments showed benefits in improving depression. In a multicenter trial in which 179 women with breast cancer were randomized to treatment with paroxetine or amitriptyline, both treatment groups displayed marked and similar improvements in depressive symptoms, although amitriptyline was associated with a greater frequency of anticholinergic side effects.7
Limitations of these trials include small sample sizes, short follow-up periods, and the exclusion of certain populations. Dr. Martinez emphasized that providing specific antidepressant medication recommendations for patients with cancer and depression is difficult due to the paucity of large, randomized, controlled trials. Additionally, it is difficult to generalize available data because of the heterogeneity of different cancer types, the exclusion of certain patient populations from existing studies, and the high study discontinuation rates.8
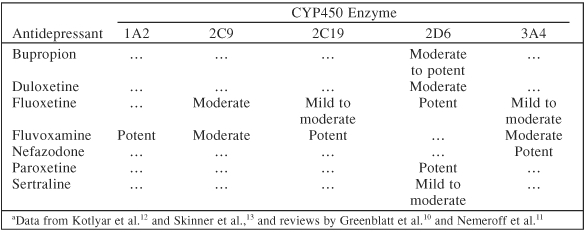
In patients with cancer and depression, several factors need to be taken into consideration for optimal treatment outcome when considering treatment with antidepressants. For example, clinicians should avoid antidepressants which have side effects that may exacerbate symptoms present due to the cancer or its treatments, such as gastrointestinal upset, somnolence, and fatigue. Also, one must consider concomitant organ system impairment, such as hepatic impairment, renal impairment, and cardiovascular disease, when choosing an antidepressant to avoid those agents which may be con-traindicated and potentially harmful. An additional concern is the potential for drug interactions, especially within the cytochrome P450 (CYP) enzyme system.9 Many chemotherapeutic agents are metabolized by P450 enzymes, and antidepressants with significant cytochrome P450 enzyme inhibition may inhibit their metabolism.9 Table 810–13 outlines some potential cytochrome P450 enzyme inhibition by selected antidepressants. Extensive reviews of P450 enzyme inhibition by antidepressants have been published elsewhere.10,11
Table 8.
Potential Inhibitory Effects of Selected Antidepressants on Cytochrome P450 (CYP450) Enzymesa
HIV/AIDS
Six published controlled trials14–19 have examined the treatment efficacy of antidepressants in patients with depression and HIV/AIDS. In trials of imipramine14 and fluoxetine,15 both medications showed a high response rate in study completers, regardless of the severity of immunodeficiency, and neither drug affected CD4 counts during the trials. A trial of imipramine versus paroxetine16 demonstrated comparable efficacy between these agents, although a large number of participants discontinued imipramine treatment due to side effects. Two studies17,19 that examined the efficacy of fluoxetine in conjunction with group therapy versus group therapy alone resulted in different outcomes. The first study17 reported an improvement in depression in both groups while the second19 found that fluoxetine plus group therapy was associated with a greater improvement in depression compared to group therapy alone. Finally, in a small comparator trial of desipramine and methylphenidate, both desipramine and methyl-phenidate demonstrated a similar reduction in depressive symptoms.18
Dr. Martinez asserted that the available evidence supports the efficacy of antidepressants in the treatment of depression in patients with HIV/AIDS. However, as with cancer, certain issues must be taken into account when prescribing medication (i.e., side effects, safety concerns, and drug interactions). For example, many protease inhibitors and non-nucleoside reverse transcriptase inhibitors are metabolized by enzymes within the cyto-chrome P450 system; therefore, clinicians should review the CYP450 enzyme inhibition potential of antide-pressants to avoid choosing an agent that may lead to increased blood levels of concomitant antiretroviral medications. Selective serotonin reuptake inhibitors (SSRIs) are generally considered appropriate treatment, although serotonin syndrome has been reported when fluoxetine is used with certain antiretroviral agents,20 and medications within the SSRI class differ with respect to their potential for CYP450 enzyme inhibition. Treatments for HIV/AIDS may also affect the metabolism of other drugs, including some antidepressants, through the inhibition or induction of CYP450 enzymes.21–24 For example, ritonavir has multiple complex potential inhibitory and induction effects on several CYP450 enzymes.21–24 Additionally, the inhibitory effects of ritonavir, efavirenz, and nelfinavir on CYP2B6 may lead to potential drug interactions with bupropion.23
Cardiovascular Disease
Dr. Martinez reviewed 3 randomized, controlled trials25–27 that examined the efficacy and safety of antide-pressant treatment of depression in patients with ischemic heart disease. A small, placebo-controlled study of fluoxetine25 in patients with depression 3 to 12 months after a first myocardial infarction indicated an improvement in depression, with a trend favoring fluoxetine over placebo, and found no echocardiogram evidence of decreased cardiac function associated with fluoxetine treatment. The efficacy and safety of sertraline in the treatment of depression in patients with an acute myocardial infarction or unstable angina were examined in a large, randomized controlled trial.26 In this trial, sertraline was found to be an effective treatment for patients with recurrent depression and did not adversely affect left ventricular ejection fraction or other cardiac safety measures.26 Finally, a trial27 of paroxetine versus nortriptyline in patients with depression and ischemic heart disease found that both treatments were effective in improving depression, but more nortriptyline-treated patients discontinued treatment due to medication side effects.
Dr. Martinez noted that the available evidence supports the relative safety of SSRIs, particularly sertraline, in patients with depression and comor-bid ischemic heart disease.25–27 However, given the paucity of controlled trials, there is no clear consensus on specific antidepressants that should be used in this setting. SSRIs are generally preferred because they have been studied more than other new-generation antidepressants and have a safer cardiovascular profile than tricyclic antidepressants (TCAs). SSRIs may normalize platelet activation28,29 and do not appear to have quinidine-like effects like the TCAs do.30 SSRIs produce minimal heart rate changes and do not significantly affect heart rate variability, cardiac conduction, or QT variability.29 The use of TCAs should be avoided in patients with ischemic heart disease. TCAs have numerous undesirable side effects, including antihistaminic, anticholinergic, and antiadrenergic effects. TCAs also increase the risk for orthostatic hypotension.31 Additionally, TCAs have type 1A antiarrhythmic effects and other type 1A antiarrhythmics have been associated with increased mortality in patients with ischemic heart disease.32 Table 933 shows a number of cardiovascular medications that are metabolized by the cytochrome P450 enzyme system and may interact with antidepressants.
Table 9.
Selected Cytochrome P450 (CYP450) Substrates in Patients With Cardiovascular Diseasea

Hepatic and Renal Impairment
Hepatic impairment may be associated with a number of changes that can affect antidepressant metabolism, disposition, and safety. These changes may include alterations in binding protein availability and affinity, impairments in hepatic metabolism, the presence of ascites, and the emergence of hepatic encephalopathy.34 Since most antidepressants are highly protein bound, a decrease in the availability or affinity of binding proteins may lead to increased blood levels of unbound antidepressants. Additionally, antide-pressants may compete with other highly protein-bound medications for protein binding sites, which may affect the free fractions of those agents as well. Thus, clinicians should consider the therapeutic index of medications (both psychotropic and non-psychotropic) when prescribing them to patients with hepatic impairment. In patients with impaired hepatic metabolism, the breakdown of many anti-depressants may also be affected. In general, when treating patients with depression and hepatic impairment, Dr. Martinez recommended using lower doses, less frequent dosage schedules, and slower titrations. Additionally, clinicians should avoid medications with a known risk for hepatotoxicity, such as nefazodone, and medications that have not been well-studied in hepatically impaired patients, such as duloxetine. Also, Dr. Martinez recommended checking the current prescribing information and consulting a hepatologist, especially in cases of severe hepatic impairment.
Renal impairment may also be associated with a number of changes that can affect antidepressant use, including changes in drug absorption, metabolism, distribution, and elimination.34 When using antidepressants to treat renally impaired patients with depression, Dr. Martinez suggested using lower starting dosages, slow titrations, and lower target doses. Additionally, clinicians should strongly consider consulting with a nephrologist, particularly when patients are on dialysis.
Conclusion
Depression frequently coexists with major medical illnesses. Due to a paucity of large, randomized trials, limited controlled data and open trials should be evaluated for the efficacy and safety of the administration of antidepressants in patients with comorbid medical conditions. Side effects and drug interactions should be examined carefully before prescribing antidepressant medications to patients with comorbid medical illnesses.
Drug names: amiodarone (Cordarone, Pacerone, and others), azathioprine (Azasan, Imuran, and others), baclofen (Lioresal, Kemstro, and others), bupropion (Wellbutrin and others), carbamazepine (Equetro, Tegretol, and others), clonazepam (Klonopin and others), clonidine (Catapres-TTS-1,2 and others), cyclobenzaprine (Flexeril), cycloserine (Seromycin), desipramine (Norpramine and others), digoxin (Lanoxin), doxepin (Sinequan and others), duloxetine (Cymbalta), efavirenz (Sustiva), flecainide (Tambacor and others), fluoxetine (Prozac and others), fluvastatin (Lescol), gabapentin (Neurontin), imipramine (Tofranil, Surmontil, and others), indomethacin (Indocin), lamotrigine (Lamictal), lidocaine (Xylocaine and others), methylphenidate (Ritalin, Concerta, and others), mirtazapine (Remeron and others), modafinil (Provigil), nelfinavir (Viracept), nortriptyline (Aventyl, Pamelor, and others), paroxetine (Paxil), pregabalin (Lyrica), propranolol (Inderal and others), reserpine (Serpalan), ritonavir (Norvir), r-warfarin (Coumadin and others), sertraline (Zoloft), s-warfarin (Coumadin and others), tiagabine (Gabitril), venlafaxine (Effexor).
Disclosure of off-label usage: The chair has determined that, to the best of his knowledge, bupropion is not approved by the U.S. Food and Drug Administration specifically for the treatment of fatigue and cognitive problems in depression; carbamazepine, gabapentin, and pregabalin are not approved for the treatment of fatigue in depression; clonazepam and tiagabine are not approved for the treatment of muscle spasms in depression; doxepin, venlafaxine, and amitriptyline are not approved specifically for sleep abnormalities in depression; duloxetine is not approved specifically for sleep abnormalities, fatigue, and cognitive problems in depression, back pain, shoulder pain, or pain associated with fibromyalgia; lamotrigine, methylphenidate, and modafinil are not approved for the treatment of cognitive problems in depression; encainide is not approved for the treatment of cardiovascular disease; mianserin is not approved for the treatment of depression; milnacipran is not approved for the treatment of fibromyalgia; and phenacetin is not approved for the treatment of nonsteroidal anti-inflammatory use. If you have questions, contact the medical affairs department of the manufacturer for the most recent prescribing information.
Pretest and Objectives
Instructions and Posttest
Registration Form
Footnotes
This ACADEMIC HIGHLIGHTS section of The Primary Care Companion to The Journal of Clinical Psychiatry presents the highlights of the series of planning teleconferences “Identifying and Managing Depression in the Medical Patient,” which was held in August and September 2005. This report was prepared by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Eli Lilly and Company.
The teleconference was chaired by Maurizio Fava, M.D., Depression Clinical and Research Program, Massachusetts General Hospital and Harvard University, Boston. The faculty were Larry Culpepper, M.D., M.P.H., Department of Family Medicine, Boston University, Boston, Mass.; David A. Fishbain, M.D., F.A.P.A., Departments of Psychiatry and Neurological Surgery and Anesthesiology, Miller School of Medicine, University of Miami, and the Rosomoff Comprehensive Pain and Rehabilitation Center, Miami, Fla.; and James M. Martinez, M.D., Mood Disorders Center and Menninger Department of Psychiatry, Baylor College of Medicine, Houston, Tex.
Faculty disclosure: In the spirit of full disclosure and in compliance with all ACCME Essential Areas and Policies, the faculty for this CME article were asked to complete a statement regarding all relevant financial relationships between themselves or their spouse/partner and any commercial interest (i.e., a proprietary entity producing health care goods or services) occurring within the 12 months prior to joining this activity. The CME Institute has resolved any conflicts of interest that were identified. The disclosures are as follows: Dr. Fava has received research support from Abbott, Lichtwer Pharma GmbH, and Lorex; has received honoraria from Bayer AG, Biovail, BrainCells, Cypress, Compellis, Dov, Fabre-Kramer, Grunenthal GmbH, Janssen, Knoll, Lundbeck, Sepracor, and Somerset; and has received both research support and honoraria from Aspect Medical Systems, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, Johnson & Johnson, Novartis, Organon, Pharmavite, Pfizer, Roche, Sanofi-Synthelabo, Solvay, and Wyeth. Dr. Culpepper is a consultant and a member of the advisory boards for Eli Lilly, Forest, Pfizer, and Wyeth. Dr. Fishbain is a consultant and a member of the speakers or advisory boards for Eli Lilly and Endo and has received honoraria from Eli Lilly, Endo, and Purdue. Dr. Martinez is a consultant for Bristol-Myers Squibb, Cyberonics, Eli Lilly, and GlaxoSmithKline; has received grant/research support from Bristol-Myers Squibb, Cyberonics, Eli Lilly, GlaxoSmithKline, and Neuromics; has received honoraria from and is a member of the speakers or advisory boards for AstraZeneca, Bristol-Myers Squibb, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Pfizer, and Wyeth; and his spouse/partner has received grant/research support from Abbott, Organon, Forest, and Pfizer.
The opinions expressed herein are those of the faculty and do not necessarily reflect the views of the CME provider and publisher or the commercial supporter.
REFERENCES
- Purohit DR, Navlakha PL, and Modi RS. et al. The role antidepressants in hospitalised cancer patients: a pilot study. J Assoc Physicians India. 1978 26:245–248. [PubMed] [Google Scholar]
- Costa D, Mogos I, Toma T.. Efficacy and safety of mianserin in the treatment of depression of women with cancer. Acta Psychiatr Scand Suppl. 1985;320:85–92. doi: 10.1111/j.1600-0447.1985.tb08081.x. [DOI] [PubMed] [Google Scholar]
- Van Heeringen K, Zivkov M.. Pharmacological treatment of depression in cancer patients: a placebo-controlled study of mi-anserin. Br J Psychiatry. 1996;169:440–443. doi: 10.1192/bjp.169.4.440. [DOI] [PubMed] [Google Scholar]
- Razavi D, Allilaire JF, and Smith M. et al. The effect of fluoxetine on anxiety and depression symptoms in cancer patients. Acta Psychiatr Scand. 1996 94:205–210. [DOI] [PubMed] [Google Scholar]
- Fisch MJ, Loehrer PJ, and Kristeller J. et al. Fluoxetine versus placebo in advanced cancer outpatients: a double-blind trial of the Hoosier Oncology Group. J Clin Oncol. 2003 21:1937–1943. [DOI] [PubMed] [Google Scholar]
- Holland JC, Romano SJ, and Heiligenstein JH. et al. A controlled trial of fluoxetine and desipramine in depressed women with advanced cancer. Psychooncology. 1998 7:291–300. [DOI] [PubMed] [Google Scholar]
- Pezzella G, Moslinger-Gehmayr R, Contu A.. Treatment of depression in patients with breast cancer: a comparison between paroxetine and amitriptyline. Breast Cancer Res Treat. 2001;70:1–10. doi: 10.1023/a:1012518831494. [DOI] [PubMed] [Google Scholar]
- Fisch M.. Treatment of depression in cancer. J Natl Cancer Inst Monogr. 2004;32:105–111. doi: 10.1093/jncimonographs/lgh011. [DOI] [PubMed] [Google Scholar]
- Wynn GH, Cole MA. Oncology. In: Cozza KL, Armstrong SC, Oesterheld JR, eds. Concise Guide to Drug Interaction Principles for Medical Practices: Cytochrome P450s, UGTs, P-Glycoproteins. 2nd ed. Washington, DC: APPI. 2003 [Google Scholar]
- Greenblatt DJ, von Moltke LL, and Harmatz JS. et al. Drug interactions with newer an-tidepressants: role of human cytochromes P450. J Clin Psychiatry. 1998 59(suppl 15):19–27. [PubMed] [Google Scholar]
- Nemeroff CB, DeVane CL, Pollock BG.. Newer antidepressants and the cytochrome P450 system. Am J Psychiatry. 1996;153:311–320. doi: 10.1176/ajp.153.3.311. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Brauer LH, and Tracy TS. et al. Inhibition of CYP2D6 activity by bupro-pion. J Clin Psychopharmacol. 2005 25:226–229. [DOI] [PubMed] [Google Scholar]
- Skinner MH, Kuan HY, and Pan A. et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther. 2003 73:170–177. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Rabkin R, and Harrison W. et al. Effect of imipramine on mood and enumerative measures of immune status in depressed patients with HIV illness. Am J Psychiatry. 1994 151:516–523. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Wagner GJ, Rabkin R.. Fluoxe-tine treatment for depression in patients with HIV and AIDS: a randomized, placebo-controlled trial. Am J Psychiatry. 1999;156:101–107. doi: 10.1176/ajp.156.1.101. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Uldall KK, and Bergam K. et al. Randomized, placebo-controlled trial of paroxetine versus imipramine in depressed HIV-positive outpatients. Am J Psychiatry. 1998 155:367–372. [DOI] [PubMed] [Google Scholar]
- Targ EF, Karasic DH, and Diefenbach PN. et al. Structured group therapy and fluoxetine to treat depression in HIV-positive persons. Psychosomatics. 1994 35:132–137. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Levy JK, and Samley HR. et al. Effects of methylphenidate in HIV-related depression: a comparative trial with desip-ramine. Int J Psychiatry Med. 1995 25:53–67. [DOI] [PubMed] [Google Scholar]
- Zisook S, Peterkin J, and Goggin KJ. et al. Treatment of major depression in HIV-seropositive men: HIV Neurobehavioral Research Center Group. J Clin Psychiatry. 1998 59:217–224. [DOI] [PubMed] [Google Scholar]
- DeSilva KE, Le Flore DB, and Marston BJ. et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001 15:1281–1285. [DOI] [PubMed] [Google Scholar]
- Tseng AL, Foisy MM.. Significant interactions with new antiretrovirals and psycho-tropic drugs. Ann Pharmacother. 1999;33:461–473. doi: 10.1345/aph.18240. [DOI] [PubMed] [Google Scholar]
- Angelino AF, Treisman GJ.. Management of psychiatric disorders in patients infected with human immunodeficiency virus. Clin Infect Dis. 2001;33:847–856. doi: 10.1086/322679. [DOI] [PubMed] [Google Scholar]
- Hesse LM, von Moltke LL, and Shader RI. et al. Ritonavir, efavirenz, and nelfinavir inhibit CYP2B6 activity in vitro: potential drug interactions with bupropion. Drug Metab Dispos. 2001 29:100–102. [PubMed] [Google Scholar]
- Smith B. Antiretrovirals. In: Cozza KL, Armstrong SC, Oesterheld JR, eds. Concise Guide to Drug Interaction Principles for Medical Practices: Cytochrome P450s, UGTs, P-Glycoproteins. 2nd ed. Washing-ton, DC: APPI. 2003 233–241. [Google Scholar]
- Strik JJ, Honig A, and Lousberg R. et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med. 2000 62:783–789. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O'Connor CM, and Califf RM. et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002 288:701–709. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Kennedy JS, and Pollock BC. et al. Treatment of major depression with nor-triptyline and paroxetine in patients with ischemic heart disease. Am J Psychiatry. 1999 156:1024–1028. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Glassman AH, and Malinin AI. et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003 108:939–944. [DOI] [PubMed] [Google Scholar]
- Roose SP.. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262–268. doi: 10.1016/s0006-3223(03)00320-2. [DOI] [PubMed] [Google Scholar]
- Roose SP, Laghrissi-Thode F, and Kennedy JS. et al. Comparison of paroxetine and nor-triptyline in depressed patients with ische-mic heart disease. JAMA. 1998 279:287–291. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT Jr, and Giardina EV. et al. Clinical characteristics of imipramine-induced orthostatic hypotension. Lancet. 1979 1:468–472. [DOI] [PubMed] [Google Scholar]
- Effect of the antiarrythmic agent mori-cizine on survival after myocardial, infarction. The Cardiac Arrythmia Suppression Trial II Investigators. N Engl J Med. 1992;327:227–233. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- Wynn GH. Internal Medicine. In: Cozza KL, Armstrong SC, Oesterheld JR, eds. Concise Guide to Drug Interaction Principles for Medical Practices: Cytochrome P450s, UGTs, P-Glycoproteins. 2nd ed. Washington, DC: APPI. 2003 192–199. [Google Scholar]
- Beliles K, Stoudemire A.. Psychopharmaco-logic treatment of depression in the medically ill. Psychosomatics. 1998;39:S2–S19. doi: 10.1016/S0033-3182(98)71339-8. [DOI] [PubMed] [Google Scholar]