Abstract
Although fucose-α(1-2)-galactose [Fucα(1-2)Gal] carbohydrates have been implicated in cognitive processes such as long-term memory, the molecular mechanisms by which these sugars influence neuronal communication are not well understood. Here, we present molecular insights into the functions of Fucα(1-2)Gal sugars, demonstrating that they play a role in the regulation of synaptic proteins and neuronal morphology. We show that synapsins Ia and Ib, synapse-specific proteins involved in neurotransmitter release and synaptogenesis, are the major Fucα(1-2)Gal glycoproteins in mature cultured neurons and the adult rat hippocampus. Fucosylation has profound effects on the expression and turnover of synapsin in cells and protects synapsin from degradation by the calcium-activated protease calpain. Our studies suggest that defucosylation of synapsin has critical consequences for neuronal growth and morphology, leading to stunted neurite outgrowth and delayed synapse formation. We also demonstrate that Fucα(1-2)Gal carbohydrates are not limited to synapsin but are found on additional glycoproteins involved in modulating neuronal architecture. Together, our studies identify important roles for Fucα(1-2)Gal sugars in the regulation of neuronal proteins and morphological changes that may underlie synaptic plasticity.
Keywords: fucose, glycosylation, neurite outgrowth, glycoprotein, calpain
Fucose-α(1-2)-galactose [Fucα(1-2)Gal], which exists as a terminal carbohydrate modification to N- and O-linked glycoproteins, has been implicated in cognitive processes such as learning and memory. For instance, preventing formation of Fucα(1-2)Gal linkages by incorporation of 2-deoxy-d-galactose (2-dGal) into glycan chains has been shown to cause reversible amnesia in animals (1–3). 2-dGal also interferes with the maintenance of long-term potentiation (LTP), a form of synaptic plasticity that is closely associated with learning and memory (4). Moreover, injection of a monoclonal antibody specific for Fucα(1-2)Gal has been found to impair memory formation in animals, presumably by blocking the Fucα(1-2)Gal epitope (5, 6). These studies suggest important roles for Fucα(1-2)Gal carbohydrates and their associated proteins in modulating neuronal communication in the brain.
Interestingly, evidence suggests that protein fucosylation is regulated in response to synaptic activity. Both task-specific learning and LTP have been shown to induce the fucosylation of proteins at the synapse (7, 8), and addition of exogenous fucose or 2′fucosyllactose was found to enhance LTP in hippocampal slices (9). The activity of fucosyltransferases, enzymes involved in the transfer of fucose to glycoproteins, has also been demonstrated to increase substantially during synaptogenesis (10) and upon passive avoidance training in animals (11). Together, these studies suggest that protein fucosylation may be a highly regulated process in the brain and may contribute to neuronal development and synaptic plasticity. Despite these intriguing observations, little is known about the molecular mechanisms by which Fucα(1-2)Gal sugars influence neuronal communication. Surprisingly, no Fucα(1-2)Gal glycoproteins have been characterized from the brain, and the precise roles of the sugars in regulating the structure and function of neuronal proteins are presently unclear.
Here, we show that Fucα(1-2)Gal carbohydrates are expressed on several glycoproteins during neuronal development and demonstrate that synapsins Ia and Ib are the predominant Fucα(1-2)Gal glycoproteins in the adult rat brain. We also present molecular insights into the function of the Fucα(1-2)Gal epitope in regulating neuronal proteins, revealing that fucosylation increases the half-life of synapsin in cells and modulates neurite outgrowth. Our studies suggest important roles for Fucα(1-2)Gal sugars in the regulation of synaptic proteins and morphological changes that may underlie synaptic plasticity.
Materials and Methods
Neuronal Cultures and Immunocytochemistry. Hippocampal and cortical neurons were cultured and immunostained as described (12). Synapsin I knockout mice (13) were generously provided by H. T. Kao and P. Greengard (The Rockefeller University, New York). Antibody A46-B/B10 (5) was a generous gift from U. Karsten (Max-Delbrück Centre for Molecular Medicine, Berlin-Buch, Germany) and was incubated in 3% BSA (2.5 μg/ml) overnight at 4°C. The anti-tubulin (1:500; Sigma), anti-synapsin (1:5,000; Molecular Probes), and anti-spinophilin [1:10,000 (14)] antibodies were added in 3% BSA for 2 h at 37°C. Goat anti-mouse IgM-AlexaFlour 488 or goat anti-rabbit IgG AlexaFluor 568 (1:250; Molecular Probes) was added for 1 h at 37°C in 3% BSA.
Synaptic Vesicle Purification from Adult Rat Brain. Synaptic vesicles were purified by using a sucrose density gradient, as described (15).
Treatment of Cells with Deoxy-Galactose Analogues. Rat neuronal cultures were treated after 7 days, as described (12). Neuronal cultures from C57BL/6 embryonic day (E)16 mice were treated after 7 days in vitro (DIV) with 2-dGal (0, 5, 10, or 15 mM) in PBS for 5 days. Neurons from C57BL/6 and synapsin I knockout postnatal day 0 mice were cultured for 2 days and then treated for 3 days with 15 mM 2-dGal. HeLa cells were seeded at 6 × 105 cells per 60-mm dish in DMEM supplemented with 10% FCS and incubated at 37°C/5% CO2 for 24 h. After pretreatment with the deoxy-galactose analogues (0.5–10 mM) for 1 h, the cells were transfected at ≈60% confluence with the plasmid pCMV-FLAG-Synapsin Ia (see Supporting Text, which is published as supporting information on the PNAS web site) and pSV-β-galactosidase (Promega) by using Lipofectamine 2000 (Invitrogen). After 22 h, the cells were harvested, resuspended in PBS, and either lysed in 1% boiling SDS (70% of the cells) or analyzed for transfection efficiency by using a β-galactosidase assay (30% of the cells). For synapsin degradation experiments, cells were treated with 2-dGal or PBS as above, followed by treatment 4 h posttransfection with bafilomycin A1 (100 nM, Acros, Geel, Belgium), MG132 (5 μM, Sigma), ammonium chloride (25 mM, Fisher), calpain inhibitor peptide (33 μM, Sigma), or calpeptin (2 μM, Alexis Biochemicals, Lausen, Switzerland). Cells were lysed as above after 15 h of treatment, resolved by SDS/PAGE, and analyzed by immunoblotting with chemiluminescence detection (Pierce).
Results
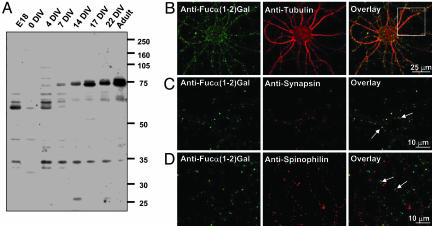
Expression of Fucα(1-2)Gal on Glycoproteins in the Hippocampus. We investigated whether Fucα(1-2)Gal glycoproteins are present in the hippocampus, a brain structure important for spatial learning and memory (16). Cell lysates from adult rat hippocampus, E18 hippocampus, and cultured embryonic hippocampal neurons were analyzed by Western blotting by using an antibody (A46-B/B10) selective for Fucα(1-2)Gal (5). Antibody A46-B/B10 has been shown to induce amnesia in animals (6), suggesting that it recognizes one or more physiologically relevant epitopes. We found that the Fucα(1-2)Gal epitope is present on several distinct proteins during neuronal development (Fig. 1A). In E18 hippocampal tissue, three major glycoproteins of ≈35, 60, and 65 kDa are prominently observed, whose expression is significantly reduced in the adult hippocampus. In contrast, glycoproteins of ≈73 and 75 kDa are found in mature cultured neurons and in adult brain tissue. Interestingly, expression of Fucα(1-2)Gal is observed on multiple proteins in developing neurons cultured for 4 and 7 DIV, periods when axons, dendrites, and functional synapses are being formed. Thus, expression and/or fucosylation of Fucα(1-2)Gal glycoproteins appear to change extensively during the course of neuronal development.
Fig. 1.
Fucα(1-2)Gal is expressed on several glycoproteins in the hippocampus and is enriched in presynaptic nerve terminals. (A) Comparison of the glycoproteins present in E18 rat hippocampus, embryonic hippocampal neurons cultured for the indicated times, and adult rat hippocampus. Cellular lysates were resolved by SDS/PAGE and probed by Western blotting with antibody A46-B/B10. (B) Coimmunostaining of hippocampal neurons cultured for 14 DIV with antibody A46-B/B10 (green) and an antibody selective for the neuronal marker tubulin (red, B), the presynaptic marker synapsin (red, C), or the postsynaptic marker spinophilin (red, D). C and D are enlargements of the area indicated by the rectangle in B.
Fucα(1-2)Gal Is Enriched at Synapses. We next investigated the subcellular localization of Fucα(1-2)Gal sugars in neurons. Hippocampal neurons were cultured for 14 DIV to allow for synapse formation and were subsequently fixed, permeabilized, and coimmunostained with antibody A46-B/B10 and an antibody against the neuronal marker tubulin. The Fucα(1-2)Gal epitope exhibited a punctate pattern consistent with enriched localization to neuronal synapses (Fig. 1B). To examine whether the sugar was present at pre- or postsynaptic terminals, neurons were coimmunostained for Fucα(1-2)Gal and the presynaptic marker synapsin I or the postsynaptic marker spinophilin. Fucα(1-2)Gal labeling was observed in a subpopulation of the synapses (58 ± 2%; n = 350), overlapping with synapsin-positive puncta (Fig. 1C) and generally apposing spinophilin-positive puncta (Fig. 1D). Membrane delipidation by using methanol/chloroform did not alter the immunostaining pattern, which confirms the staining of glycoproteins rather than glycolipids (Fig. 6, which is published as supporting information on the PNAS web site). These findings indicate that Fucα(1-2)Gal sugars are enriched on glycoproteins in presynaptic nerve terminals.
Synapsins Ia and Ib Are the Major Fucα(1-2)Gal Glycoproteins in the Hippocampus. We next sought to identify neuronal glycoproteins modified by the Fucα(1-2)Gal epitope. Attempts to purify Fucα(1-2)Gal glycoproteins from brain extracts by using antibody A46-B/B10 were unsuccessful due to the relatively weak binding affinity of the antibody for the carbohydrate epitope. Fucose-specific lectins such as Lotus tetragonolobus lectin (LTL) and Ulex europaeus agglutinin I (UEA-I) also displayed weak affinity or broad specificity, resulting in limited enrichment of Fucα(1-2)Gal glycoproteins. To circumvent these challenges, we identified potential glycoproteins using a combination of subcellular fractionation, gel electrophoresis, and MALDI-TOF MS. Adult rat hippocampal lysates were enriched in synaptic proteins by using standard subcellular fractionation procedures. The crude synaptosomal fractions were resolved by 1D or 2D gel electrophoresis and analyzed by Western blotting with antibody A46-B/B10 or stained with Coomassie brilliant blue. As observed previously, two major glycoproteins of ≈73 and 75 kDa were recognized by antibody A46-B/B10. Proteins of interest were identified by immunoblotting and excised from the corresponding Coomassie-stained gel, digested with trypsin, and identified by MALDI-TOF MS. MS analysis revealed three potential Fucα(1-2)Gal-containing glycoproteins: synapsin Ia, synapsin Ib, and NSF. Eleven measured peptides matched the masses calculated from the National Center for Biotechnology Information (NCBI) nonredundant database for both synapsins Ia and Ib with >50 ppm accuracy, and the unmodified matching peptides covered 11.2% of the amino acid sequence (Table 1, which is published as supporting information on the PNAS web site). For NSF, 24 matching peptides were detected within 50 ppm, which provided 29.7% overall sequence coverage (Table 2, which is published as supporting information on the PNAS web site).
To establish whether synapsins Ia/Ib and NSF were indeed recognized by antibody A46-B/B10, each protein was immunoprecipitated and examined by Western blotting with antibody A46-B/B10. Upon immunoprecipitation, synapsins Ia and Ib were specifically detected by the antibody, whereas NSF was not recognized (Fig. 2A). Deletion mutagenesis of synapsin Ia revealed that the synapsin fucosylation sites are localized to the D domain, a region found only in the synapsin I isoforms (Fig. 7, which is published as supporting information on the PNAS web site). In addition, loss of the fucosylated bands corresponding to synapsins Ia and Ib was observed by Western blot analysis of adult hippocampal lysates from synapsin I-deficient mice, confirming that synapsins Ia and Ib are the predominant Fucα(1-2)Gal glycoproteins (Fig. 8, which is published as supporting information on the PNAS web site). Treatment of purified synapsin I with PNGaseF and EndoH, enzymes that cleave N-linked oligosaccharides from proteins, did not abolish the interaction with antibody A46-B/B10, which suggests that the Fucα(1-2)Gal moiety is present on an O-linked glycan (data not shown). Together, these studies suggest that synapsins Ia and Ib are glycosylated in the D domain with an O-linked fucosyl oligosaccharide.
Fig. 2.
Synapsins Ia and Ib are Fucα(1-2)Gal glycoproteins. (A) Immunoprecipitated synapsin I but not NSF is detected by antibody A46-B/B10. Input, lysate used for immunoprecipitation; Control, immunoprecipitation in the absence of antibody; Synapsin or NSF IP, immunoprecipitated synapsin or NSF. Left Upper and Right Upper were immunoblotted with an anti-synapsin (Left) or anti-NSF (Right) antibody, and Left Lower and Right Lower were probed with antibody A46-B/B10. Synapsin Ia appeared in darker exposures of the blot (data not shown). (B) Structures of the mono- and disaccharide competitors used in this study. (C) Competition ELISA shows that binding of antibody A46-B/B10 to bovine synapsin I is selectively inhibited by monosaccharides containing l-fucose and the disaccharide l-Fucα(1-2)Gal-β-OEt. (D) Treatment of bovine synapsin I with α-(1-2)-fucosidase significantly reduces the fucosylation of synapsin, as demonstrated by Western blotting with antibody A46-B/B10 (Left). In contrast, α-(1-3,4)-fucosidase had no effect on synapsin fucosylation (Right). Gray bars indicate synapsin protein levels, and black bars indicate synapsin fucosylation levels after treatment with the fucosidase for the indicated times.
Characterization of the Carbohydrate Structure on Synapsin. Having identified the synapsins, we turned our attention to establishing the presence of the Fucα(1-2)Gal epitope. To gain insight into the structural determinants recognized by antibody A46-B/B10, we performed competition ELISA experiments using a series of fucose analogues (Fig. 2 B and C). Synapsins Ia and Ib from bovine brain were immobilized on a microtiter plate, and binding of antibody A46-B/B10 to synapsin was assessed in the presence of varying concentrations of the competitors. The monosaccharides ethyl α/β-l-fucopyranoside (l-Fuc-α-OEt and l-Fuc-β-OEt) and ethyl α/β-d-fucopyranoside (d-Fuc-α-OEt and d-Fuc-β-OEt), were synthesized from l- or d-fucose by using a modified Fischer glycosylation reaction (17). Disaccharide l-Fucα(1-2)Gal-β-OEt was synthesized from the known O-(2,3,4-tri-O-benzyl-α-l-fucopyranosyl)trichloroacetimidate (Fig. 9, which is published as supporting information on the PNAS web site). l-Fucose, l-Fuc-α-OEt, l-Fuc-β-OEt, and l-Fucα(1-2)Gal-β-OEt were effective at inhibiting antibody binding to synapsins Ia and Ib. Interestingly, the IC50 values of l-fucose, l-Fuc-α-OEt, and l-Fuc-β-OEt were comparable to that of the disaccharide, consistent with the specificity observed for some fucose-binding lectins such as Anguilla anguilla agglutinin (18). In contrast, the corresponding d-fucose sugars and naturally occurring sugars such as d-glucose (d-Glc), d-galactose (d-Gal), and N-acetyl-d-glucosamine (d-GlcNAc) did not compete with synapsin for antibody binding (Fig. 2C). These results suggest that antibody A46-B/B10 selectively recognizes l-fucose sugars on synapsin.
As independent confirmation, we examined the ability of l-fucose-specific lectins to bind to synapsin. Lotus tetragonolobus lectin (LTL) and Ulex europaeus agglutinin I (UEA-I) lectins have been reported to interact strongly with terminal Fucα(1-2)Gal carbohydrates, whereas Anguilla anguilla agglutinin (AAA) prefers Fucα(1-3)Gal carbohydrates and interacts only weakly with Fucα(1-2)Gal (19). Consistent with the presence of a Fucα(1-2)Gal moiety on synapsin, both LTL and UEA-I readily detected synapsins Ia and Ib (Fig. 10, which is published as supporting information on the PNAS web site). However, AAA also recognized synapsin, indicating that lectins cannot be used to determine the nature of the fucose-galactose linkage on synapsin.
Because fucosidases have been shown to hydrolyze specific glycosidic linkages, we treated synapsins Ia and Ib with an α-(1-2)-fucosidase or an α-(1-3,4)-fucosidase from Xanthomonas manihotis. Rapid deglycosylation of synapsin was observed upon treatment with the α-(1-2)-fucosidase (Fig. 2D). In contrast, the α-(1-3,4)-fucosidase, which hydrolyzes both Fucα(1-3) and Fucα(1-4) linkages, had no effect on the fucosylation levels of synapsin, even after 6 h of treatment (Fig. 2D and data not shown). Together, these results provide strong evidence that synapsins Ia and Ib are covalently modified by the critical Fucα(1-2)Gal epitope.
Synapsin I Is Fucosylated in Various Subcellular Compartments. We next investigated the extent of fucosylation on neuronal synapsin I. Subcellular fractions of rat forebrain lysates were analyzed for levels of fucosylated or total synapsin (Fig. 3). Fucosylated synapsin was present in all subcellular fractions containing synapsin. Moreover, the relative level of fucosylated synapsin to total synapsin was equivalent in the fractions examined. Quantitative analysis revealed that the membrane-associated to soluble ratio (LP2:LS2) of fucosylated synapsin was similar to that of synapsin (39:1 and 38:1 for fucosylated synapsin and synapsin, respectively; Fig. 11, which is published as supporting information on the PNAS web site). These results suggest that neuronal synapsin is extensively fucosylated in various subcellular compartments, and that fucosylation does not alter the subcellular localization of synapsin.
Fig. 3.
Distribution of synapsins Ia/Ib and fucosylated synapsin in subcellular fractions from adult rat forebrain. Protein extracts from the indicated subcellular fractions were immunoblotted with an anti-synapsin I antibody or A46-B/B10. H, homogenate; S1, low speed supernatant; S2, postnuclear supernatant; S2′, supernatant of P2; P2′, crude synaptosomes; LS1, supernatant of LP1; LP1, crude synaptic plasma membranes; LS2, supernatant of LP2 (synaptosol); LP2, crude synaptic vesicles; SG, synaptic vesicles after sucrose gradient. Each lane contains 100 μg of protein, with the exception of the SG fraction, which has 10 μg.
To gain insight into the stoichiometry of fucosylation, we compared synapsin I purified from bovine brain against a fucosylated BSA standard (Fig. 12, which is published as supporting information on the PNAS web site). 2′-Fucosyllactose [Fucα(1-2)Galβ(1-4)GlcNAc] was conjugated to BSA by using reductive amination chemistry, and an epitope density of ≈3.0 ± 0.8 mol of fucose per BSA molecule was determined by using the Habeeb assay (20). Comparison of the relative binding of antibody A46-B/B10 to synapsin I versus this standard revealed a stoichiometry of ≈1.5–3.2 Fucα(1-2)Gal epitopes per synapsin molecule. Together with the subcellular fractionation studies, these results indicate that a significant fraction of synapsin I is likely fucosylated in neurons.
Inhibiting Synapsin Fucosylation Significantly Decreases Its Cellular Half-Life. To investigate the impact of fucosylation on the functional properties of synapsin, we inhibited the fucosylation of synapsin in cells using 2-dGal. 2-dGal has been shown to prevent the fucosylation of glycoproteins (1). Upon cellular uptake, 2-dGal is converted via the Leloir pathway to the corresponding activated uridine diphosphate (UDP) analogue (1, 21). UDP-2-deoxy-galactose competes with UDP-galactose for incorporation into glycan chains and thereby terminates the chain by preventing formation of the Fucα(1-2)Gal linkage (1).
We first established that incubation of HeLa cells with 2-dGal leads to the biosynthesis of UDP-2-deoxy-galactose. Significant formation of UDP-2-deoxy-galactose was observed by LC-MS analysis of cell extracts, demonstrating that 2-dGal is an efficient unnatural substrate for the Leloir pathway enzymes (Fig. 13, which is published as supporting information on the PNAS web site). We next investigated the effects of 2-dGal on synapsin I expressed in HeLa cells. Cell lysates containing equivalent amounts of transfected protein were resolved by SDS/PAGE, and the fucosylation and protein levels of synapsin were measured by immunoblotting. Consistent with the presence of a Fucα(1-2)Gal epitope on synapsin, 2-dGal had a dramatic effect on the fucosylation level of synapsin (Fig. 4A). Unexpectedly, the 2-dGal treatment also led to a significant decrease in the level of synapsin protein. The effects appear to be specific to 2-dGal, because treatment with other deoxy-galactose sugars, including 6-deoxy-d-galactose (6-dGal), had no effect on either the fucosylation or protein levels of synapsin. Thus, 2-dGal was found to affect synapsin levels and fucosylation specifically through the C2 position of galactose.
Fig. 4.
Inhibition of synapsin fucosylation using 2-dGal decreases the expression and half-life of synapsin I. (A) Effects of 2- and 6-dGal on synapsin fucosylation (Left) and synapsin expression (Right) levels. Synapsin I-transfected HeLa cells were treated with the indicated amounts of the deoxy sugar for 24 h. Fucosylation and synapsin expression levels were measured by immunoblotting with antibody A46-B/B10 and an anti-synapsin antibody, respectively. (B) Pulse–chase experiments demonstrate that defucosylation significantly decreases the cellular half-life of synapsin from 18 to 5.5 h. Synapsin I-transfected HeLa cells were treated with 2-dGal or a vehicle control (PBS), pulse-labeled with 35S-l-cysteine and 35S-l-methionine, as described in Supporting Text, and chased for the indicated times. 35S-labeled synapsin levels were measured by autoradiography. (C) Inhibition of the calcium-activated protease calpain using a calpain inhibitor peptide or calpeptin protects synapsin from degradation. The lysosomal inhibitors bafilomycin A1 and ammonium chloride and the proteasome inhibitor MG132 had no effect on synapsin degradation. Synapsin I-transfected HeLa cells were treated with the indicated inhibitors or vehicle control (DMSO, PBS, or MeOH) in the presence or absence of 2-dGal for 15 h. After lysis, the cell extracts were analyzed for synapsin expression levels by immunoblotting.
Based on these results, we postulated that fucosylation might be critical for the half-life and turnover of synapsin in cells. We conducted pulse–chase experiments of synapsin Ia expressed in HeLa cells in the presence or absence of 2-dGal. Cells were pulse-labeled with 35S-l-cysteine and 35S-l-methionine and then incubated for various times in the absence of radioisotopes. After the indicated chase times, synapsin Ia was immunoprecipitated from the cell lysates. A relatively long half-life of 18 h was observed for synapsin Ia (Fig. 4B), consistent with previous studies of endogenous synapsin I in cultured hippocampal neurons (t1/2 ≈ 20 h) (22). In contrast, treatment of the cells with 2-dGal led to a dramatic reduction in synapsin half-life to 5.5 h. These results indicate that defucosylation of synapsin induces its degradation in cells.
Synapsin Degradation Is Mediated by the Calcium-Dependent Protease Calpain. To investigate the molecular mechanisms responsible for synapsin turnover, cells expressing synapsin were treated with various inhibitors of protein degradation in the presence or absence of 2-dGal. Specifically, we used the lysosomal inhibitors bafilomycin A1 and ammonium chloride, the proteasome inhibitor MG132, and two inhibitors of the calcium-dependent protease calpain. With the exception of MG132, the inhibitors had minimal effects on synapsin expression levels in the absence of 2-dGal (Fig. 4C). As before, 2-dGal treatment of the cells significantly reduced levels of synapsin expression. Notably, inhibition of the protease calpain using a calpain inhibitor peptide or calpeptin rescued the effects of 2-dGal, significantly attenuating the loss of synapsin, whereas the lysosomal and proteasomal inhibitors could not rescue synapsin from degradation. These data suggest that fucosylation protects synapsin from rapid degradation mediated at least in part by the Ca2+-dependent protease calpain.
Fucosylation Modulates the Expression of Synapsin in Neurons and Neurite Outgrowth. To examine the effects of 2-dGal on synapsin fucosylation in neurons, neurons were cultured for 7 DIV to allow for adequate expression of synapsin and subsequently treated with either 2- or 6-dGal. 2-dGal dramatically reduced the expression of synapsin I in cultured neurons (Fig. 5A). Importantly, the effects of 2-dGal appeared to be selective, because treatment with another deoxy sugar, 6-dGal, did not alter the expression of synapsin. Moreover, the effects of 2-dGal were specific to synapsin as the expression of other synaptic proteins, including NSF, synaptotagmin, syntaxin, PSD-95, the AMPA receptor subunit GluR1, and spinophilin, was unchanged by the 2-dGal treatment.
Fig. 5.
2- but not 6-dGal reduces synapsin I expression levels in cultured neurons and induces neurite retraction. (A) E18 cortical neurons cultured for 7 DIV were treated with 2- or 6-dGal (15 mM) for 3 days. Protein lysates were analyzed by Western blotting by using antibodies selective for the indicated proteins. A significant reduction in the expression of synapsin Ib was observed, whereas other synapse-associated proteins were unaffected by the 2-dGal treatment. (B) 2-dGal induces neurite retraction and collapse of synapses in cultured neurons. Neurons were cultured for 7 DIV and treated with either 2- or 6-dGal (15 mM) for 3 days. After fixation, neurites were immunostained by using anti-tau antibodies. The effects of 2-dGal were partially reversed by treatment with galactose. (C) Synapsin-deficient neurons display reduced neurite retraction relative to wild-type neurons upon treatment with 2-dGal. (Upper) Neurons from synapsin I-deficient (Syn KO) or wild-type (WT) mice were cultured for 2 days, treated in the presence or absence of 2-dGal (15 mM) for 3 days, and examined by confocal fluorescence microscopy. (Lower) Neurons treated with 2-dGal were analyzed for neurite length by using nih image 1.62 software, and the mean neurite length was compared by the ANOVA test. Error bars represent the SEM from 50 total neurons in three separate experiments (*, P < 0.003).
Because the synapsins play important roles in neuronal development and synaptogenesis (13, 23), we investigated whether 2-dGal might influence neuronal growth and morphology. Hippocampal neurons were cultured for 7 DIV as above to establish synapses and subsequently incubated for 3–5 days with 2-dGal at various concentrations. Treatment with 2-dGal induced a dramatic retraction of neurites and collapse of synapses, whereas 6-dGal had no effect (Fig. 5B and Fig. 14, which is published as supporting information on the PNAS web site). The effects of 2-dGal could be partially rescued by subsequent incubation of the neurons with d-Gal, which is expected to reestablish the Fucα(1-2)Gal linkage (Fig. 5B; 2.06 ± 0.14-fold rescue; n = 50; P < 0.0001). These results suggest that disruption of the Fucα(1-2)Gal linkage on neuronal glycoproteins has a profound impact on neurite outgrowth and neuronal morphology.
One potential mechanism by which 2-dGal might influence neuronal morphology is by regulating the function and/or expression of synapsin in presynaptic terminals. Notably, the phenotypic effects of 2-dGal on neurite outgrowth at 5 mM concentration are similar to deletion of the synapsin I gene, which results in retarded neurite outgrowth and delayed synapse formation (13) (Fig. 14). However, because other neuronal proteins bear the Fucα(1-2)Gal modification (Fig. 1A), these proteins might also contribute to the morphological effects observed upon defucosylation.
To examine the relative contribution of synapsin I to the effects of 2-dGal, neurons were cultured from synapsin I-deficient or wild-type mice for 2 days, treated in the presence or absence of 2-dGal for 3 days, and examined by confocal fluorescence microscopy. Fig. 5C a and b are representative images of untreated wild-type and synapsin I-deficient neurons after 5 days in culture, respectively. Neurons from wild-type mice treated with 2-dGal had shorter neurites relative to their untreated wild-type counterparts (Fig. 5C, compare c and a). The effects of defucosylation at a concentration of 15 mM 2-dGal were more pronounced than elimination of the synapsin I gene (Fig. 5C, compare c and b). Finally, treatment with 2-dGal induced a more dramatic neurite retraction in wild-type relative to synapsin-deficient neurons (Fig. 5C, compare c and d). Although the length and extensive overlap among processes for untreated wild-type neurons precluded a quantitative analysis of neurite length, 2-dGal treatment led to neurite retraction and enabled quantification. We found that synapsin-deficient neurons displayed longer neurites than wild-type neurons upon treatment with 2-dGal (Fig. 5C, bar graph).
Discussion
Increasing evidence has linked synaptic activity with changes in the levels of protein fucosylation in the brain. For instance, both task-specific learning and LTP have been shown to enhance protein fucosylation at the synapse (7, 8). Moreover, the activity of fucosyltransferases increases substantially during synaptogenesis (10) and upon passive avoidance training in animals (11). Together, these studies suggest that protein fucosylation may relate to the dynamic regulation of synaptic proteins. Studies have implicated a particular carbohydrate, Fucα(1-2)Gal, in cognitive processes such as learning and memory (1–4). Although Fucα(1-2)Gal has been postulated to covalently modify synaptic glycoproteins, the identity of such proteins has remained elusive. In this study, we identify synapsins Ia and Ib as the major Fucα(1-2)Gal glycoproteins in maturing neuronal cultures and the adult rat hippocampus. Our results provide molecular insights into the functions of Fucα(1-2)Gal and demonstrate that these carbohydrates play a critical role in the regulation of synaptic proteins.
The synapsins are a family of highly conserved neuron-specific proteins that are associated with synaptic vesicles (24). Studies indicate that these proteins regulate multiple aspects of neuronal function. The synapsins have been shown to modulate neurotransmitter release by regulating the supply of releasable vesicles during periods of high activity (24, 25). In addition, synapsin I has recently been found to control synaptic vesicle dynamics in developing neurons via a cAMP-dependent pathway (26). Accordingly, synapsin-deficient mice show reduced numbers of synaptic vesicles within nerve terminals and exhibit significant alterations in neurotransmitter release and synaptic depression (24, 27). The synapsins have also been implicated in diverse aspects of neuronal development, including axon outgrowth, nerve terminal development, synapse formation, and synapse maintenance (13, 28, 29).
Our studies indicate that fucosylation of synapsin critically impacts its expression and turnover in presynaptic nerve terminals. The potential to modulate the expression level of synapsin is expected to have important consequences for neuronal function. For instance, the addition of exogenous synapsin I to embryonic Xenopus spinal neurons has been shown to accelerate structural and functional maturation of neuromuscular synapses, including the early compartmentalization of synaptic vesicles into nerve terminals and a mature form of quantal secretion (30, 31). Conversely, reduction in synapsin expression levels also has profound effects: synapsin-deficient mice exhibit significant delays in axonal extension, neuronal differentiation, and synapse formation.
We found that defucosylation of synapsin promoted its degradation by calcium-activated calpain proteases, which are a family of nonlysosomal neutral cysteine proteases. These observations corroborate and extend recent findings suggesting a role for calpain in the regulation of both synaptic transmission and neuronal morphology (32, 33). Interestingly, calpain has been suggested to be critical for refilling depleted vesicle stores in sensory motor synapses of Aplysia via a mechanism involving the cleavage of potential substrates such as synapsin (34). In addition, calpain was found to degrade synaptosomal-associated protein of 25 kDa (SNAP-25), a protein essential for neurotransmitter release, in a calcium-dependent manner (35). The proteolytic activity of calpain was also shown to induce cytoskeletal rearrangements, leading to both facilitation and inhibition of neurite outgrowth (33, 36, 37). Although further studies will be necessary to understand more fully the mechanisms of calpain-mediated synapsin degradation, our results suggest an expanded role for this protease family in presynaptic nerve terminals and reveal that fucosylation has profound effects on the half-life of synapsin, possibly preventing synapsin from calcium-activated degradation.
In this study, we also investigated the role of synapsin fucosylation on neuronal growth and morphology. 2-dGal, a small molecule inhibitor of Fucα(1-2)Gal linkages, served as a valuable tool to defucosylate synapsin and dissect the role of the carbohydrate in modulating synapsin function. Treatment of neurons with 2-dGal led to stunted neurite outgrowth and delayed synapse formation. Moreover, significant differences were observed between wild-type and synapsin-deficient neurons upon treatment with 2-dGal. We believe that the extent of neurite retraction in synapsin-deficient mice is less pronounced because the primary target of 2-dGal, synapsin I, is missing. Indeed, the bar graph shown in Fig. 5C likely represents a lower estimate of the contribution of synapsin because neurites from synapsin-deficient neurons are shorter than those from wild-type neurons before treatment with 2-dGal. Based on these results, we propose that defucosylation may disrupt synapsin function, leading to its degradation and neurite retraction. Although further studies will be needed to resolve whether synapsin fucosylation stimulates or inhibits neurite outgrowth, these results strongly support the notion that synapsin fucosylation plays a role in modulating neuronal growth and morphology.
Our data also implicate other Fucα(1-2)Gal glycoproteins in the regulation of neuronal morphology. We demonstrate that Fucα(1-2)Gal carbohydrates are not limited to synapsin but are found on several additional proteins in developing neurons. Expression of the sugar and/or these glycoproteins changes dramatically during the course of neuronal development. We found that disruption of synapsin fucosylation contributed, but was not fully sufficient, to account for the striking neurite retraction induced by 2-dGal. For instance, 2-dGal had stronger effects on neurite outgrowth at high concentrations relative to deletion of the synapsin I gene, suggesting that 2-dGal may disrupt the fucosylation of other Fucα(1-2)Gal glycoproteins that influence neuronal morphology. Moreover, 2-dGal was still capable of inducing partial neurite retraction in synapsin-deficient neurons and young cultured neurons where synapsin expression is low (12). Thus, Fucα(1-2)Gal sugars appear to modulate the functions of multiple proteins involved in neuronal morphology and exert their effects via several distinct molecular mechanisms.
Finally, our findings may shed light on behavioral and electrophysiological studies implicating Fucα(1-2)Gal in long-term memory storage. Alterations in neuronal morphology, such as dynamic changes in dendritic spine number and shape, occur during memory consolidation and LTP (38, 39). Future studies will investigate whether Fucα(1-2)Gal sugars and their associated glycoproteins contribute to structural remodeling events that underlie synaptic plasticity.
Supplementary Material
Acknowledgments
We thank Drs. H. T. Kao, T. E. Wilson, and S. B. Ficarro for assistance and helpful discussions. This research was supported by National Institutes of Health Grants R01 NS045061 (to L.C.H.-W. and C.I.G.) and R01 MH070898 (to B.P.), National Institutes of Health Training Grants T32 GM08501 (to C.I.G.) and T32 GM07616 (to H.E.M.), and the Alfred P. Sloan Foundation.
Author contributions: H.E.M., C.I.G., and L.C.H.-W. designed research; H.E.M., C.I.G., S.A.K., W.-I.L., and E.M.D. performed research; B.P. contributed new reagents/analytic tools; H.E.M., C.I.G., S.A.K., E.M.D., and L.C.H.-W. analyzed data; and H.E.M., C.I.G., and L.C.H.-W. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Fucα(1-2)Gal, fucose-α(1-2)-galactose; LTP, long-term potentiation; DIV, days in vitro; En, embryonic day n; NSF, N-ethylmaleimide-sensitive factor; 2-dGal, 2-deoxy-d-galactose; 6-dGal, 6-deoxy-d-galactose.
References
- 1.Bullock, S., Potter, J. & Rose, S. P. R. (1990) J. Neurochem. 54, 135–142. [DOI] [PubMed] [Google Scholar]
- 2.Rose, S. P. R. & Jork, R. (1987) Behav. Neural Biol. 48, 246–258. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzini, C. G. A., Baldi, E., Bucherelli, C., Sacchetti, B. & Tassoni, G. (1997) Neurobiol. Learn. Mem. 68, 317–324. [DOI] [PubMed] [Google Scholar]
- 4.Krug, M., Jork, R., Reymann, K., Wagner, M. & Matthies, H. (1991) Brain Res. 540, 237–242. [DOI] [PubMed] [Google Scholar]
- 5.Karsten, U., Pilgrim, G., Hanisch, F. G., Uhlenbruck, G., Kasper, M., Stosiek, P., Papsdorf, G. & Pasternak, G. (1988) Br. J. Cancer 58, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jork, R., Smalla, K. H., Karsten, U., Grecksch, G., Ruthrich, H. L. & Matthies, H. (1991) Neurosci. Res. Commun. 8, 21–27. [Google Scholar]
- 7.McCabe, N. R. & Rose, S. P. R. (1985) Neurochem. Res. 10, 1083–1095. [DOI] [PubMed] [Google Scholar]
- 8.Pohle, W., Acosta, L., Ruthrich, H., Krug, M. & Matthies, H. (1987) Brain Res. 410, 245–256. [DOI] [PubMed] [Google Scholar]
- 9.Matthies, H., Staak, S. & Krug, M. (1996) Brain Res. 725, 276–280. [DOI] [PubMed] [Google Scholar]
- 10.Matsui, Y., Lombard, D., Massarelli, R., Mandel, P. & Dreyfus, H. (1986) J. Neurochem. 46, 144–150. [DOI] [PubMed] [Google Scholar]
- 11.Popov, N., Schmidt, S., Schulzeck, S., Jork, R., Lossner, B. & Matthies, H. (1983) Pharmacol. Biochem. Behav. 19, 43–47. [DOI] [PubMed] [Google Scholar]
- 12.Kalovidouris, S. A., Gama, C. I., Lee, L. W. & Hsieh-Wilson, L. C. (2005) J. Am. Chem. Soc. 127, 1340–1341. [DOI] [PubMed] [Google Scholar]
- 13.Chin, L. S., Li, L., Ferreira, A., Kosik, K. S. & Greengard, P. (1995) Proc. Natl. Acad. Sci. USA 92, 9230–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen, P. B., Ouimet, C. C. & Greengard, P. (1997) Proc. Natl. Acad. Sci. USA 94, 9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huttner, W. B., Schiebler, W., Greengard, P. & De Camilli, P. (1983) J. Cell Biol. 96, 1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankland, P. W. & Bontempi, B. (2005) Nat. Rev. Neurosci. 6, 119–130. [DOI] [PubMed] [Google Scholar]
- 17.Vermeer, H. J., van Dijk, C. M., Kamerling, J. P. & Vliegenthart, J. F. G. (2001) Eur. J. Org. Chem. 2001, 193–203. [Google Scholar]
- 18.Haselhorst, T., Weimar, T. & Peters, T. (2001) J. Am. Chem. Soc. 123, 10705–10714. [DOI] [PubMed] [Google Scholar]
- 19.Alonso, E., Saez, F. J., Madrid, J. F. & Hernandez, F. (2003) J. Histochem. Cytochem. 51, 239–243. [DOI] [PubMed] [Google Scholar]
- 20.Habeeb, A. F. (1966) Anal. Biochem. 14, 328–336. [DOI] [PubMed] [Google Scholar]
- 21.Holden, H. M., Rayment, I. & Thoden, J. B. (2003) J. Biol. Chem. 278, 43885–43888. [DOI] [PubMed] [Google Scholar]
- 22.Daly, C. & Ziff, E. B. (1997) J. Neurosci. 17, 2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira, A., Li, L., Chin, L. S., Greengard, P. & Kosik, K. S. (1996) Mol. Cell. Neurosci. 8, 286–299. [DOI] [PubMed] [Google Scholar]
- 24.Hilfiker, S., Pieribone, V. A., Czernik, A. J., Kao, H. T., Augustine, G. J. & Greengard, P. (1999) Philos. Trans. R. Soc. London B 354, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chi, P., Greengard, P. & Ryan, T. A. (2002) Nat. Neurosci. 4, 1187–1193. [DOI] [PubMed] [Google Scholar]
- 26.Bonanomi, D., Menegon, A., Miccio, A., Ferrari, G., Corradi, A., Kao, H. T., Benfenati, F. & Valtorta, F. (2005) J. Neurosci. 25, 7299–7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gitler, D., Xu, Y. M., Kao, H. T., Lin, D. Y., Lim, S. M., Feng, J., Greengard, P. & Augustine, G. J. (2004) J. Neurosci. 24, 3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira, A. & Rapoport, M. (2002) Cell. Mol. Life Sci. 59, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, L., Chin, L. S., Shupliakov, O., Brodin, L., Sihra, T. S., Hvalby, O., Jensen, V., Zheng, D., McNamara, J. O., Greengard, P., et al. (1995) Proc. Natl. Acad. Sci. USA 92, 9235–9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu, B., Greengard, P. & Poo, M. M. (1992) Neuron 8, 521–529. [DOI] [PubMed] [Google Scholar]
- 31.Valtorta, F., Iezzi, N., Benfenati, F., Lu, B., Poo, M. M. & Greengard, P. (1995) Eur. J. Neurosci. 7, 261–270. [DOI] [PubMed] [Google Scholar]
- 32.Denny, J. B., Polan-Curtain, J., Ghuman, A., Wayner, M. J. & Armstrong, D. L. (1990) Brain Res. 534, 317–320. [DOI] [PubMed] [Google Scholar]
- 33.Shea, T. B., Cressman, C. M., Spencer, M. J., Beermann, M. L. & Nixon, R. A. (1995) J. Neurochem. 65, 517–527. [DOI] [PubMed] [Google Scholar]
- 34.Khoutorsky, A. & Spira, M. E. (2005) Learn. Mem. 12, 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ando, K., Kudo, Y. & Takahashi, M. (2005) J. Neurochem. 94, 651–658. [DOI] [PubMed] [Google Scholar]
- 36.Robles, E., Huttenlocher, A. & Gomez, T. M. (2003) Neuron 38, 597–609. [DOI] [PubMed] [Google Scholar]
- 37.Wilson, M. T., Kisaalita, W. S. & Keith, C. H. (2000) J. Neurobiol. 43, 159–172. [DOI] [PubMed] [Google Scholar]
- 38.Luscher, C., Nicoll, R. A., Malenka, R. C. & Muller, D. (2000) Nat. Neurosci. 3, 545–550. [DOI] [PubMed] [Google Scholar]
- 39.Trachtenberg, J. T., Chen, B. E., Knott, G. W., Feng, G. P., Sanes, J. R., Welker, E. & Svoboda, K. (2002) Nature 420, 788–794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.