Abstract
Plants, although sessile, can reorient growth axes in response to changing environmental conditions. Phototropism and gravitropism represent adaptive growth responses induced by changes in light direction and growth axis orientation relative to gravitational direction, respectively. The nearly 80-year-old Cholodny–Went theory [Went, F. W. & Thimann, K. V. (1937) Phytohormones (Macmillan, New York)] predicts that formation of a gradient of the plant morphogen auxin is central to the establishment of tropic curvature. Loss of tropic responses in seedling stems of Arabidopsis thaliana mutants lacking the auxin-regulated transcriptional activator NPH4/ARF7 has further suggested that a gradient of gene expression represents an essential output from the auxin gradient. Yet the molecular identities of such output components, which are likely to encode proteins directly involved in growth control, have remained elusive. Here we report the discovery of a suite of tropic stimulus-induced genes in Brassica oleracea that are responsive to an auxin gradient and exhibit morphologically graded expression concomitant with, or before, observable curvature responses. These results provide compelling molecular support for the Cholodny–Went theory and suggest that morphologically graded transcription represents an important mechanism for interpreting tropically stimulated gradients of auxin. Intriguingly, two of the tropic stimulus-induced genes, EXPA1 and EXPA8, encode enzymes involved in cell wall extension, a response prerequisite for differential growth leading to curvatures, and are up-regulated before curvature in the flank that will elongate. This observation suggests that morphologically graded transcription likely leads to the graded expression of proteins whose activities can directly regulate the establishment and modulation of tropic curvatures.
Keywords: gravitropism, NPH4/ARF7, phototropism
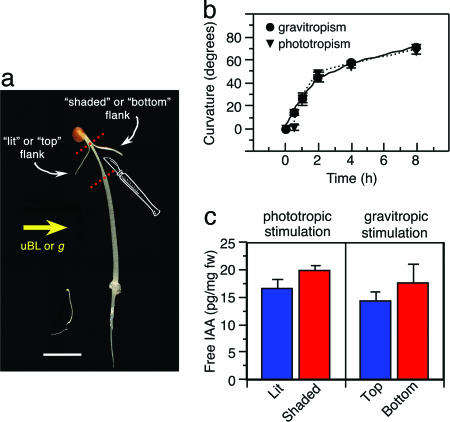
Plants exhibit a remarkable degree of morphological and developmental plasticity that allows them to adapt to ever-changing environmental conditions they experience as a result of their confined sessile nature. One way plants adapt to changes in perceived direction of physical stimuli (e.g., light, gravity, and touch) is through differential growth, or tropic curvature, of particular organs toward or away from those stimuli (1–3). Although different tropic stimuli are perceived by genetically diverged systems, it is generally accepted that the plant morphogen auxin functions as common tropic signaling molecule (3–6). In fact, nearly all current models of tropic responses are based in large part on the Cholodny–Went theory, which holds that tropic stimuli induce the lateral redistribution of auxin, resulting in unequal accumulation between opposing flanks of a responding organ such that differential growth is promoted (7). Identification of loss-of-function mutations in the auxin-regulated transcriptional activator NPH4/ARF7 that disrupt photo- and gravitropic responses in the Arabidopsis seedling stem (hypocotyl) (8–11) have led to the hypothesis that a gradient of gene expression represents a critical molecular response to the differential gradient of auxin established in response to tropic stimulation (1, 12, 13). In particular, this hypothesis predicts that mRNA abundance should increase for a select set of genes in the hypocotyl flank farthest from the incident tropic stimulation (“shaded” and “bottom” flanks for phototropic and gravitropic stimulations, respectively; see Fig. 1A for example). Moreover, the region of increased mRNA abundance should correspond morphologically to where auxin accumulates, the ability of NPH4/ARF7 to function as a transcriptional activator is expected to be highest, and cell elongation is stimulated.
Fig. 1.
B. oleracea represents a model for studies of auxin-regulated phototropism and gravitropism. (a) Comparison of B. oleracea (on the right) and Arabidopsis (on the left) seedlings at the developmental age when assays are done. B. oleracea tissue samples were taken from the region between the dotted lines. uBL, unidirectional blue light; g, gravitational vector. (Scale bar, 1 cm.) (b) Time course of phototropism (triangles, dotted line) and gravitropism (circles, solid line) in 3-d-old etiolated B. oleracea seedlings. Data represent mean responses with associated SE for three independent experiments. (c) Measurements of free IAA in opposing hypocotyl flanks of phototropically or gravitropically stimulated B. oleracea seedlings. Data represent the mean measurement with associated SD from three independent experiments. Results from t tests comparing mean auxin levels between flanks within a given treatment are as follows: phototropic stimulation, t = 1.63, 0.2 > P > 0.1; gravitropic stimulation, t = 2.35, 0.1 > P > 0.05.
Despite its wealth of genetic resources for the study of tropic responses, Arabidopsis has thus far proven ill suited as a system to test the hypothesis just outlined. Previous transcript profiling experiments using phototropically stimulated and mock-treated whole wild-type and nph4/arf7-null mutant Arabidopsis seedlings as mRNA sources failed to yield any phototropically responsive NPH4/ARF7-dependent targets (R. Harper and E.L., unpublished results; microarray data can be found at http://genome-www5.stanford.edu). In hindsight, these findings are not particularly surprising, because collection of whole seedlings might be expected to “homogenize” differences in mRNA abundances that exist between hypocotyl flanks. The obvious solution to this “homogenization” effect is to isolate opposing tissue flanks. However, the isolation of unfixed opposing hypocotyl flanks from Arabidopsis seedlings is essentially precluded by their small size (≈1 cm in length and 200 μm in diameter; see ref. 10). In contrast, several properties of Brassica oleracea, a close relative of Arabidopsis (14, 15), suggested that it might represent an ideal alternative organism of choice in which to address this hypothesis. First, the dramatically larger size of B. oleracea in comparison with Arabidopsis allows for the simple isolation of target tissue (opposing flanks from the most tropically responsive region of the hypocotyl without central vasculature) by hand sectioning (Fig. 1A). Next, like Arabidopsis (13), B. oleracea seedlings exhibit time-dependent and saturable phototropic and gravitropic responses (Fig. 1B), making it possible to ask both spatial and temporal questions about differential gene expression. Last, the substantial homology between Brassica and Arabidopsis nuclear genomes (≈85% identity in protein coding sequences; see refs. 14 and 15) suggested that B. oleracea mRNAs could be used to probe Arabidopsis microarrays with a high degree of confidence in signal authenticity, allowing for a gene-rich and unbiased examination of expression patterns. A recent study in which anther-specific B. oleracea mRNAs were used to probe Arabidopsis cDNA macroarrays (16) supports this contention.
Based on the aforementioned properties, we have used opposing hypocotyl flanks of tropically stimulated B. oleracea seedlings as a source of mRNAs to probe Affymetrix Arabidopsis ATH1 whole-genome microarrays to test the hypothesis that tropic stimulation results in increased expression of particular genes in the flank farthest from compared with that closest to the incident stimulation. In parallel with these microarray experiments, we measured the free auxin content in opposing hypocotyl flanks to verify that the lateral auxin gradient predicted by the Cholodny–Went theory (7) in fact exists. Results from these studies indicate that a relatively small set of genes are differentially expressed across the tropically responding hypocotyl of B. oleracea, and that they are expressed at the highest levels in the elongating portion of the hypocotyl where auxin levels are measurably highest. RT-PCR studies showed that the morphologically graded expression of these genes depends upon auxin, likely through the action of NPH4/ARF7. Increased expression of these genes in the elongating flank was also found to coincide with, or precede, observable curvature, suggesting that the encoded proteins may function as regulators of the growth response itself.
Results
Arabidopsis Microarrays Can Be Used to Address Gene Expression in Brassica. When cRNAs derived from B. oleracea were used to probe Affymetrix Arabidopsis whole-genome arrays, hybridization signals were observed for ≈4,500 of the ≈24,000 genes represented on the arrays, independent of the experimental growth conditions used (for additional information, contact E.L.). Although the number of genes expressed (called present) might seem low based on studies by using Arabidopsis cRNAs as probes (17, 18), it likely reflects the genome divergence between Brassica and Arabidopsis (14, 15) and the low sequence divergence tolerated by the Affymetrix perfect match/mismatch oligonucleotide platform. Statistical analyses of these data (Figs. 5 and 6, which are published as supporting information on the PNAS web site; for additional information, contact E.L.) indicate that the hybridization signals observed in this cross-species experiment are highly reproducible, significant, and within the expected range of variation for homospecies comparisons.
Tropic Stimulation Induces Accumulation of Specific mRNAs in the Region of the Hypocotyl Farthest from the Incident Stimulation. The central facet of the hypothesis driving this study is that a common set of tropic stimulus-induced (TSI) genes should be identifiable that exhibit higher expression in the elongating (“shaded” and “bottom;” Fig. 1 A) versus nonelongating flanks (“lit” and “top;” Fig. 1 A) of both phototropically and gravitropically stimulated B. oleracea seedlings. Eight TSI genes were identified that reproducibly exhibited such a pattern of differential expression after 2 h of tropic stimulation: At1g69530/EXPA1, At2g40610/EXPA8, At4g25240/SKS1, At4g27260/GH3.5, At4g34760/SAUR50, At5g15160/bHLH134, At5g47370/HAT2, and At5g54510/GH3.6/DFL1 (Table 1, which is published as supporting information on the PNAS web site; TSI gene and protein identities shown in this table reflect those of the Arabidopsis ortholog, per www.tigr.org/tdb/e2k1/ath1/ath1.shtml; ref. 19).
A Lateral Gradient of Auxin Is Formed in Brassica Hypocotyls in Response to Tropic Stimulation. The hypothesis that tropic stimulation would lead to the accumulation of specific transcripts in the elongating hypocotyl flank farthest from the incident stimulation is based on two key predictions: (i) that auxin would accumulate in the elongating flank, and (ii) that this would in turn lead to higher NPH4/ARF7-dependent transcriptional activation in that flank. With the identification of TSI genes in the aforementioned microarray experiments, it became important to address these predictions. Consistent with predictions of the Cholodny–Went theory (7) and measurements made in other species (5, 20), tropically stimulated B. oleracea seedlings accumulated ≈20% more free indole-3-acetic acid (IAA), the native active auxin, in the hypocotyl flank farthest from the incident stimulation where elongation will occur, as compared with the flank closest the stimulation (Fig. 1C). Thus it would appear that at least the first prediction holds.
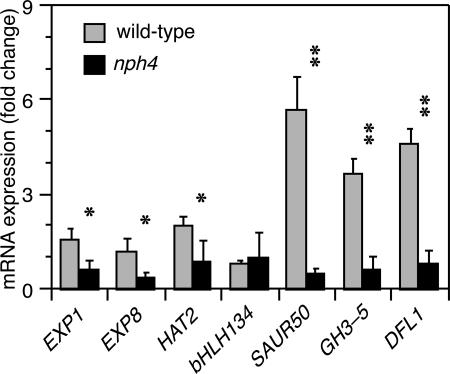
TSI Genes Likely Represent NPH4/ARF7 Transcriptional Targets. The second prediction that is key to our hypothesis is that NPH4/ARF7-dependent transcription would increase in the flank experiencing an increased auxin level. Although this prediction has not been tested directly in B. oleracea, it is intriguing that each of the eight TSI genes identified contains at least one consensus ARF-binding auxin-response element (AuxRE; TGTCnC or GnGACA) (21) within its promoter region (Table 2, which is published as supporting information on the PNAS web site), and that these genes are among a much larger group of genes that are induced by auxin treatment in Arabidopsis seedlings (17, 18, 22, 23). More germane to our expectation that TSI genes are likely targets for regulation by NPH4/ARF7 are the findings that the TSI genes exhibited little to no auxin-induced expression in an nph4/arf7-null mutant background (Fig. 2). NPH4/ARF7 dependence had been previously proposed for auxin-induced expression of EXPA8, GH3.6/DFL1, and HAT2, based on similar studies comparing wild-type and nph4/arf7 mutants (18, 24). The only gene that did not show apparent NPH4/ARF7 dependence in the present study was BHLH134. However, at the auxin concentration used in these experiments, BHLH134 was not auxin-induced in the wild-type background either (Fig. 2), making additional dose-dependency studies necessary to clearly address the possibility that BHLH134 expression also requires NPH4/ARF7.
Fig. 2.
Auxin-dependent accumulation of TSI transcripts in Arabidopsis requires NPH4/ARF7. Transcript accumulation was determined by quantitative real-time PCR from RNA isolated from 3-d-old etiolated wild-type and nph4-1 seedlings. Fold changes between mock and auxin-treated samples for each TSI gene were calculated by established methods (41). ACT2 was used as a normalizing control for all targets. Bars represent mean fold change with associated SD for three independent experiments. Asterisks refer to transcript accumulation in wild-type that is significantly greater than that in nph4 by a t test (*, P < 0.05; **, P < 0.001).
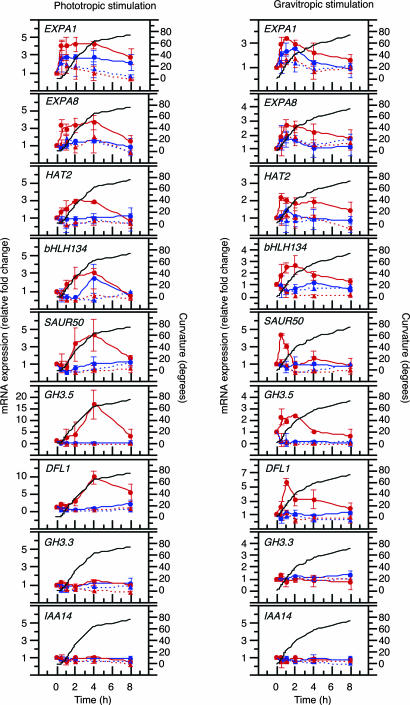
TSI Transcript Accumulation Depends upon Auxin and Occurs Concomitant with, or Before, the Curvature Responses. RT-PCR was used to verify microarray results, as well as to examine the auxin and temporal dependence of TSI transcript accumulation in B. oleracea seedlings (Fig. 3, as well as Fig. 7, which is published as supporting information on the PNAS web site). In tropically stimulated seedlings, each of the TSI genes exhibited a clear gradient in transcript abundance, with dramatically higher levels in the shaded or bottom flanks (solid red line) compared with the opposing lit or top flanks (solid blue line). It is interesting to note that, whereas some differences in the temporal patterns of expression were observed, each TSI gene was found to accumulate significantly in the shaded or bottom flanks concomitant with, or before, observable curvature (Fig. 3, compare solid red and blue with black lines).
Fig. 3.
Differential accumulation of TSI transcripts occurs in an auxin- and temporal-dependent fashion. Transcript accumulation was determined by semiquantitative RT-PCR in lit (blue lines) and shaded flanks (red lines) of phototropically stimulated seedlings (Left), as well as top (blue lines) and bottom flanks (red lines) of gravitropically stimulated seedlings (Right), in the absence (solid lines) or presence (dotted lines) of the auxin transport inhibitor NPA. The data represent the average relative RT-PCR expression values compared to unstimulated controls for each gene. Bars represent SD for experiments done in triplicate. Expression data are shown plotted against mean tropic curvature of siblings in the absence of NPA (black lines; although not shown, SDs of all curvature measurements were <11°).
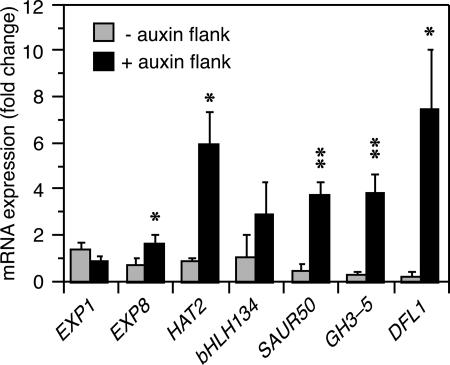
By contrast to the aforementioned experiments, when the polar auxin transport inhibitor 1-naphthylphthalamic acid (NPA) was applied to the apical region of seedlings immediately before tropic stimulation, neither tropic curvature (data not shown) nor differential TSI transcript accumulation was observed (Fig. 3, dotted red and blue lines). These results imply that the lateral gradient of auxin observed in tropically stimulated B. oleracea seedlings (Fig. 1C) is prerequisite to the differential accumulation of TSI transcripts and development of curvatures. If this conclusion is valid, it was reasoned that it should be possible to induce both differential TSI transcript accumulation and hypocotyl curvature in etiolated B. oleracea seedlings by unilateral application of auxin in the absence of tropic stimulation. It was found that these phenotypes can, in fact, be phenocopied in large part by unilateral application of IAA to the elongating region of B. oleracea hypocotyls (Fig. 4). Moreover, sibling seedlings exposed to the same auxin treatment exhibited a mean organ curvature of 35.6° (SE = 2.1°; n = 30) away from the site of auxin application.
Fig. 4.
Unilateral application of auxin induces differential transcript accumulation in B. oleracea seedlings. Transcript accumulation was determined by quantitative real-time PCR from RNA isolated from opposing flanks of 3-d-old etiolated seedlings. IAA–lanolin paste was applied to one side of hypocotyls, as described in Materials and Methods. The data represent the average fold change in transcript accumulation 4 h after IAA–lanolin application, as compared with seedlings treated with lanolin alone, for each gene. Bars represent mean fold change with associated SD for three independent experiments. Asterisks refer to transcript accumulation in the plus auxin flank that is significantly greater than that in the minus auxin flank by a t test (*, P < 0.05; **, P < 0.001).
Although it appears that auxin acts as a primary inductive agent of both differential transcript accumulation and hypocotyl curvatures, it is clear that the relatively subtle increase in free auxin concentration observed in the hypocotyl flank distal to an incident tropic stimulation (Fig. 1C) is not sufficient to stimulate expression of all auxin-responsive genes. For example, At2g23170/GH3.3 (21) and AT4G14550/SLR/IAA14 (25), although expressed in B. oleracea hypocotyls segments, did not exhibit differential expression in response to tropic stimulation (Fig. 3).
Discussion
The studies presented here were initiated to test the hypothesis that tropic stimulations result in accumulation of specific transcripts in a responding organ, in this case a seedling stem, at a position opposite to the incident tropic stimulation where cell elongation will occur. In addition, we wished to address two presumptions that led to development of this hypothesis; namely, that this differential transcript accumulation depends upon the formation of a lateral gradient of the plant morphogen auxin and the subsequent activation of the auxin-responsive transcriptional activator NPH4/ARF7. The studies presented herein show that transcripts from at least eight genes exhibit the hypothesized pattern of differential accumulation in B. oleracea, and that expression of these TSI genes depends upon formation of an auxin gradient. Moreover, auxin-induced expression of TSI genes in Arabidopsis genetically depends upon NPH4/ARF7. If we assume that the observed patterns of morphologically graded transcript abundance will be reflected by similar graded differences in protein abundance, the temporal coincidence of the TSI transcript accumulation and hypocotyl curvature responses begs an obvious next question: Do any of the identified TSI genes encode proteins that could function as regulators of the differential growth response leading to tropic curvatures?
The observation that two members of the α-expansin family (26–28), EXPA1 and EXPA8, exhibited differential transcript accumulation before noticeable tropic curvature (Fig. 3, compare solid red and blue with black lines) is certainly exciting in this context. Members of the α-expansin protein family mediate cell wall extension (26–28), a prerequisite for cell elongation processes like those occurring in the stem flank opposite to an incident tropic stimulus (5), suggesting that EXPA1 and EXPA8 may play a direct role in the establishment of tropic curvatures. Interestingly, expansin activity is stimulated by low pH (maximally between pH 3.5 and 4.5) (29), and auxin is known to rapidly (within 20 min of application) stimulate a plasma-membrane H+-ATPase, which results in acidification of the apoplastic space (30). Thus it would appear that an increase in auxin concentration in the hypocotyl flank distal to a tropic stimulation (Fig. 1C) is capable of stimulating both the local accumulation of EXPA1 and EXPA8 mRNAs (Fig. 3) and activity of the encoded proteins with sufficient speed to be directly involved in the differential growth response (Fig. 1B). Although not examined in detail here, we predict that the differential transcript accumulation of another TSI gene, SKS1, will also precede the curvature responses. SKS1 encodes a member of a family of glycosylphosphatidylinositol-anchored putative oxidases that includes SKU5 (31, 32), a protein previously shown to modulate differential growth of Arabidopsis roots likely via alterations in cell wall expansion (31).
Although not likely involved in the direct regulation of growth, it is worth noting that two of the TSI genes, GH3.5 and GH3.6/DFL1, encode IAA-amido synthetases (33) and thus could be involved in feedback mechanisms that dampen responsiveness over time by converting free active IAA to inactive amido-IAA (34). Feedback repression of morphogen-dependent signaling through down-regulation of both morphogen levels and signaling events represents a common regulatory mechanism to achieve efficient and highly controlled morphogenetic switches (35). A relatively simple regulatory feedback loop for the control of auxin-dependent tropic responses has been proposed previously in which the transcriptional activity of NPH4/ARF7 is controlled by the Aux/IAA repressor protein MSG2/IAA19 (13). Based on the results presented here, we propose modifying this hypothesized feedback loop to include additional regulation through control of free auxin levels via action of IAA-amido synthetases, as discussed below.
In hypocotyls of unstimulated wild-type seedlings, NPH4/ARF7 is thought to exist as a heterodimer with MSG2/IAA19, such that its transcriptional activity toward target genes is repressed (13, 25). Upon tropic stimulation, a gradient of free auxin is established across the hypocotyl (refs. 5 and 20; also see Fig. 1C). An increase in free auxin in the flank distal to the incident tropic stimulation is then believed to promote the degradation of MSG2/IAA19 (13, 25) via a ubiquitination and proteasome-dependent process (36), allowing for homodimerization of NPH4/ARF7 (13, 25) and subsequent transcriptional activation of target genes, including MSG2/IAA19 (13, 21) and GH3.6/DFL1 (18). Newly translated MSG2/IAA19 would then compete with NPH4/ARF7 monomers for dimerization with DNA-bound NPH4/ARF7 (21, 25) to rerepress the system, whereas synthesis of amido-IAA conjugates by GH3.6/DFL1 (33) would be predicted to reduce free IAA levels and thus further strengthen repression of NPH4/ARF7 activity by reducing auxin-dependent (37, 38) turnover of MSG2/IAA19. The coordinate regulation of GH3.5 and GH3.6/DFL1 transcript abundance in response to tropic stimulation (Fig. 3) suggests that both GH3 proteins (and possibly others) may be necessary for modulation of auxin levels. We are in a position to use combination loss- and gain-of-function genetic approaches in Arabidopsis to test the aforementioned hypotheses, as well as others, and to address the functional relevance of the various TSI gene products in establishment and regulation of tropic growth responses.
Materials and Methods
Plant Material. B. oleracea seeds (Park Seed, Greenwood, SC) were surface-sterilized with 30% (vol/vol) bleach, rinsed thoroughly with sterile distilled H2O, and planted in 1-ml displacement tips on 0.5 × MS medium. Tips were then transferred to tip boxes at 4°C for 2 d, after which they were placed in red light at 22°C for 1 h to induce uniform seed germination (10). Tips were then transferred to darkness at 22°C for 3 d to obtain etiolated seedlings. For Arabidopsis experiments, seeds of wild-type Columbia ecotype and the nph4/arf7-null mutant, nph4–1 (12), were handled as described (10).
Tropic Stimulations. Phototropic stimulations were done by exposing etiolated B. oleracea seedlings to unidirectional blue light (0.1 μmol m–2·s–1) (10), whereas gravitropic stimulations were done by rotating seedlings 90° along their growth axis, so they were perpendicular instead of parallel to the gravitational vector. At the indicated times, seedlings were collected for RNA isolation (see below) or measurement of curvatures as described for Arabidopsis (10).
Microarray Data Collection. A 15° ophthalmic scalpel (Feather, Osaka) was used to isolate tissue flanks (≈1 cm in length; see Fig. 1A) from etiolated B. oleracea seedlings that had received one of three treatments: (i) a mock stimulation, (ii) a 2-h phototropic stimulation, or (iii) a 2-h gravitropic stimulation. Only the outer third of the hypocotyl was collected for each flank section, and opposing flanks were collected into separate pools. Hence, there were five distinct tissue samples (two flanks for each of two tropic stimulations plus one set of control flanks), with each sample being generated and collected in triplicate for a total of 15 biological samples. Tissue flanks were immediately quick-frozen in liquid N2 and stored at –80°C until RNAs were isolated. Total RNA was extracted from frozen tissue by using the RNeasy kit (Qiagen, Valencia, CA), followed by cRNA synthesis (ENZO Diagnostics). cRNA probe generation, as well as microarray hybridization, washing, and scanning, was performed as described by manufacturer protocols (Affymetrix). Gene signal intensities for each Affymetrix Genechip Microarray were computed by using Affymetrix microarray suite, Version 5.0, software. The signal field for each gene was then log2-transformed, and the transformed signals were compared graphically in a scatter-plot matrix (see Figs. 5 and 6). There were no patterns present that were outside of expected bounds. The data were then processed in two stages by using a statistical mixed model to account for the systematic array effects (39). These normalized data represent that the analytic dataset was used for all subsequent analysis and statistical modeling.
Microarray Data Analysis. To identify genes exhibiting differential expression between opposing flanks of tropically stimulated seedlings, we initially used a rank comparison test with Affymetrix data mining tool, Version 3.0. Only genes that exhibited at least a 1.3-fold change in expression (in the same direction) between opposing flanks in six of the nine comparisons done that were also identified as changing relative to controls were considered for further analysis. This list of genes was then compared with lists of genes generated by two independent statistical methods. First, the analytic dataset described above was modeled by using a mixed model to test for treatment by gene interactions. An F test across all treatments and t tests for all pair-wise comparisons were then computed. Next, the analytic dataset was modeled by using nonparametric techniques in a fashion analogous to the parametric procedures. The Kruskal–Wallis test was used for the three treatment comparisons, and the Wilcoxon rank sum test was used for the pair-wise comparisons. A single list of TSI genes was finally compiled that represented the overlap between the three analyses, and this gene list was in turn culled for interesting trends.
Measurements of Endogenous Auxin. Approximately 20–30 flanks (total fresh weight of 10–20 mg) were pooled from each of the 12 2-h tropic stimulation samples. Tissue extraction, extract purification, and free IAA determination by GC-selected reaction monitoring MS were made of each tissue pool, as described (40). Calculation of isotopic dilution was based on the addition of 500 pg of [13C6]IAA/sample.
RT-PCR Analysis. Total RNA was isolated from hypocotyl flanks as described above. Forty nanograms of total RNA from each sample was used for first-strand cDNA synthesis with an olgo-(dT)24 primer and SuperScript II reverse transcriptase (Invitrogen). Five percent of the first-strand reaction was then subjected to PCR amplification (95° for 30 s, 55° for 30 s, 72° for 1 min) for various numbers of cycles. We determined that 28 cycles were optimal to produce a clear product within the linear nonsaturating range of amplification for each of the genes, and thus this cycle protocol was used in all subsequent experiments. Primers for gene-specific amplification of TSI genes, as well as controls, were generated from Arabidopsis sequences and are available from the corresponding author upon request. Relative band intensity comparisons and quantifications were made by using fovea pro 3.0 (Reindeer Graphics, Asheville, NC). Expression levels were normalized in comparison with ACT2 transcripts. We were unable to achieve reproducible results with a number of different primer combinations for At4g25240/SKS1, and thus those data are not shown.
Quantitative Real-Time PCR Analysis. Quantitative real-time PCR was performed by using Platinum SYBR green qPCR Supermix UDG with ROX reference dye following the manufacturer's guidelines (Invitrogen) by using cDNA templates derived from total RNA from either B. oleracea or Arabidopsis (isolated as described above for B. oleracea) with Moloney murine leukemia virus reverse transcriptase (Promega). PCR was performed by using DNA Engine Opticon 2 Continuous Fluoresence Detector (MJ Research, Cambridge, MA). Data analyses to determine cycle thresholds (CT) were performed by using opticon monitor analysis software (MJ Research). Melting curves were generated for each TSI-specific and control primer. All primers were identical to those used for semiquantitative RT-PCR, except those for HAT2 (all primer sequences are available from E.L. upon request). Fold-change values for each TSI gene were calculated by using the Pfaffl (ΔCT/ΔCT) equation (41). ACT2 was used as a normalizing control for all targets.
Auxin and Auxin Transport Inhibitor Treatments. For B. oleracea studies, IAA or NPA was mixed with melted lanolin (as an inert carrier) to a final concentration of 10 μM. After cooling, lanolin paste was applied as follows: For unilateral auxin treatments, IAA–lanolin paste was applied to a 1-cm region along one side (just below the apical hook; Fig. 1A) of 10 etiolated seedling hypocotyls by using a toothpick. For auxin transport inhibitor experiments, NPA–lanolin paste was applied to the shoot apex of 10 etiolated seedlings with a toothpick, so the entire apex of the seedlings below the cotyledons was coated. After NPA–lanolin pasting, seedlings received either unilateral light or gravity stimulation for the indicated times, then tissue was collected and handled as described for RT-PCR analysis. Flanks from seedlings treated with lanolin alone were collected at the same time points and used as controls. For Arabidopsis studies, 3-d-old etiolated seedlings were treated with 10 μM IAA (in solvent, 0.04% ethanol) or solvent alone by simply pipetting 5 ml of solution onto each plate. After 1 h, seedlings were harvested into liquid N2, and total RNAs were isolated as described above for B. oleracea.
Supplementary Material
Acknowledgments
We thank members of the Liscum laboratory and Drs. Stephen Alexander, Walter Gassmann, and Steve Kay for critical reading of the manuscript in its various incarnations. G.S. is host principle investigator for K.L.'s contribution. We also thank the University of Missouri–Columbia DNA Core for cRNA preparation and microarray hybridizations. This research was supported by Grants MCB-0077312 and IBN-0415970 from the National Science Foundation (to E.L.). C.A.E. was supported by a University of Missouri Life Sciences Predoctoral Fellowship. A.G.T. was supported by the MU-Monsanto Undergraduate Research Program.
Author contributions: C.A.E. and E.L. designed research; C.A.E., A.G.T., G.S., and K.L. performed research; C.A.E., L.B.H., and E.L. analyzed data; and C.A.E. and E.L. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The microarray data have been deposited in the Gene Expression Omnibus database (GEO accession no. GSE3847).
Abbreviations: AuxRE, auxin response element; IAA, indole-3-acetic acid; NPA, 1-naphthylphthalamic acid; TSI, tropic stimulus-induced; CT, cycle threshold.
References
- 1.Liscum, E. (2002) in The Arabidopsis Book, eds. Somerville, C. R. & Meyerowitz, E. M. (Am. Soc. Plant Biologists, Rockville, MD).
- 2.Morita, M. T. & Tasaka, M. (2004) Curr. Opin. Plant Biol. 7, 712–718. [DOI] [PubMed] [Google Scholar]
- 3.Esmon, C. A., Pedmale, U. V. & Liscum, E. (2005) Int. J. Dev. Biol. 49, 665–674. [DOI] [PubMed] [Google Scholar]
- 4.Trewavas, T., Briggs, W. R., Bruinsma, J., Evans, M. L., Firn, R., Hertel, R., Iino, M., Jones, A. M., Leopold, A. C., Pilet, P. E., et al. (1992) Plant Cell Environ. 15, 759–794.11541800 [Google Scholar]
- 5.Iino, M. (2001) in ESP Comprehensive Series in Photosciences (Elsevier, Amsterdam), Vol. 1.
- 6.Friml, J., Wisniewska, J., Benkova, E., Mendgen, K. & Palme, K. (2002) Nature 415, 806–809. [DOI] [PubMed] [Google Scholar]
- 7.Went, F. W. & Thimann, K. V. (1937) Phytohormones (Macmillan, New York).
- 8.Liscum, E. & Briggs, W. R. (1996) Plant Physiol. 112, 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watahiki, M. K. & Yamamoto, K. T. (1997) Plant Physiol. 115, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stowe-Evans, E. L., Harper, R. M., Motchoulski, A. V. & Liscum, E. (1998) Plant Physiol. 118, 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watahiki, M. K., Tatematsu, K., Fujihira, K., Yamamoto, M. & Yamamoto, K. T. (1999) Planta 207, 362–369. [DOI] [PubMed] [Google Scholar]
- 12.Harper, R. M., Stowe-Evans, E. L., Luesse, D. R., Muto, H., Tatematsu, K., Watahiki, M. K., Yamamoto, K. & Liscum, E. (2000) Plant Cell 12, 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatematsu, K., Kumagi, S., Muto, H., Sato, A., Watahiki, M. K., Harper, R. M., Liscum, E. & Yamamoto, K. T. (2004) Plant Cell 16, 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayele, M., Haas, B. J., Kumar, N., Wu, H., Xiao, Y. L., Van Aken, S., Utterback, T. R., Wartman, J. R., White, O. R. & Town, C. D. (2005) Genome Res. 15, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang, T. J., Kim, J. S., Lim, K. B., Kwon, S. J., Kim, J. A., Jin, M., Park, J. Y., Lim, M. H., Kim, H. I., Kim, S. H., et al. (2005) Comp. Funct. Genom. 6, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amagai, M., Ariizumi, T., Endo, M., Hatakeyama, K., Kuwataa, C., Shibata, D., Toriyama, K. & Watanabe, M. (2003) Sex Plant Reprod. 15, 213–220. [Google Scholar]
- 17.Nemhauser, J. L., Mockler, T. C. & Chory, J. (2004) PLoS Biol. 2, E258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okushima, Y., Overvoorde, P. J., Arima, K., Alonso J. M., Chan, A., Chang, C., Ecker, J. R., Hughes, B., Lui, A., Nguyen, D., et al. (2005) Plant Cell 17, 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas, B. J., Wortman, J. R., Ronning, C. M., Hannick, L. I., Smith, R. K., Maiti R., Chan, A. P., Yu, C., Farzad, M., et al. (2005) BMC Biol. 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs, I., Phillipar, K., Ljung, K., Sandberg, G. & Hedrich, R. (2003) Proc. Natl. Acad. Sci. USA 100, 11795–11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagen, G. & Guilfoyle, T. J. (2002) Plant Mol. Biol. 49, 373–385. [PubMed] [Google Scholar]
- 22.Pufky, J., Qui, Y., Rao, M. V., Hurban, P. & Jones, A. M. (2003) Funct. Integr. Genom. 3, 135–143. [DOI] [PubMed] [Google Scholar]
- 23.Zhao, Y., Dai, X., Blackwell, H. E., Schreiber, S. L. & Chory, J. (2003) Science 301, 1107–1110. [DOI] [PubMed] [Google Scholar]
- 24.Wilmoth, J. C., Wang, S., Tiwari, S. B., Joshi, A. D., Hagen, G., Guilfoyle, T. J., Alonso, J. M., Ecker, J. R. & Reed, J. W. (2005) Plant J. 43, 118–130. [DOI] [PubMed] [Google Scholar]
- 25.Liscum, E. & Reed, J. (2002) Plant Mol. Biol. 49, 387–400. [PubMed] [Google Scholar]
- 26.Cosgrove, D. J. (2000) Nature 407, 321–326. [DOI] [PubMed] [Google Scholar]
- 27.Li, Y., Jones, L. & McQueen-Mason, S. (2003) Curr. Opin. Plant Biol. 6, 603–610. [DOI] [PubMed] [Google Scholar]
- 28.Kende, H., Bradford, K. J., Brummell, D. A., Cho, H.-T., Cosgrove, D. J., Fleming, A. J., Gehring, C., Lee, Y., McQueen-Mason, S., Rose, J. K. C., et al. (2004) Plant Mol. Biol. 55, 311–314. [DOI] [PubMed] [Google Scholar]
- 29.McQueen-Mason, S., Durachko, D. M. & Cosgrove, D. J. (1992) Plant Cell 4, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hager, A. (2003) J. Plant Res. 116, 483–505. [DOI] [PubMed] [Google Scholar]
- 31.Sedbrook, J. C., Carroll, K. L., Hung, K. F., Masson, P. H. & Somerville, C. R. (2002) Plant Cell 14, 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borner, G. H. H., Lilley, K. S., Stevens, T. J. & Dupree, P. (2003) Plant Physiol. 132, 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staswick, P. E., Serban, B., Rowe, M., Tiryaki, I., Maldonado, M. T., Maldonado, M. C. & Suza, W. (2005) Plant Cell 17, 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljung, K., Hull, A. K., Kowalczyk, M., Marchant, A., Celenza, J., Cohen, J. D. & Sandberg, G. (2002) Plant Mol. Biol. 50, 309–332. [DOI] [PubMed] [Google Scholar]
- 35.Freeman, M. & Gurdon, J. B. (2002) Annu. Rev. Cell Dev. Biol. 18, 515–539. [DOI] [PubMed] [Google Scholar]
- 36.Smalle, J. & Vierstra, R. D. (2004) Annu. Rev. Plant Biol. 55, 555–590. [DOI] [PubMed] [Google Scholar]
- 37.Dharmasiri, N., Dharmasiri, S. & Estelle, M. (2005) Nature 435, 441–445. [DOI] [PubMed] [Google Scholar]
- 38.Kepinski, S. & Leyser, O. (2005) Nature 435, 446–451. [DOI] [PubMed] [Google Scholar]
- 39.Wolfinger, R. D., Gibson, G., Wolfinger, E. D., Bennett, L., Hamadeh, H., Bushel, P., Afshari, C. & Paules, R. S. (2001) J. Comput. Biol. 8, 625–637. [DOI] [PubMed] [Google Scholar]
- 40.Edlund, A., Eklöf, S., Sundberg, B., Moritz, T. & Sandberg, G. (1995) Plant Physiol. 108, 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaffl, M.W. (2001) Nucleic Acids Res. 29, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.