Abstract
The lifespan and survival of dendritic cells (DC) in vivo are potentially critical to the expansion of T cell immune responses. We have previously reported that DC loaded with specific antigen are rapidly eliminated by cytotoxic T lymphocytes (CTL) in vivo, but the site, mechanism, and consequences of DC elimination were not defined. In this article we show that DC elimination in vivo occurs in a perforin-dependent manner and does not require IFN-γ or the presence of CD4+CD25+ regulatory T cells. Most importantly, failure to eliminate DC had profound consequences on the CTL immune response. Perforin-deficient mice showed a progressive increase in the numbers of antigen-specific CD8+ T cells after repeated immunizations with DC. In contrast, in control mice the number of antigen-specific CD8+ T cells did not notably increase with repeated immunizations. Lastly, we also show that CTL-mediated elimination of DC occurs in peripheral tissues but not in the lymph node. Our data suggest that CTL act as “gatekeepers” that control access of antigen-loaded DC into the lymph node, thereby preventing continued expansion of antigen-specific T cells.
Keywords: cytotoxic T cells, immunoregulation, killing
Dendritic cells (DC) are powerful antigen-presenting cells that are critical for the initiation of CD4+ and CD8+ T cell responses. DC reside in peripheral tissues where they take up antigens from the external environment, then migrate to the lymph nodes where they interact with antigen-specific T cells and induce their activation to proliferation and effector function (1, 2). The lifespan of DC once in the lymph node is thought to be relatively brief, but experimental estimates have not yielded a consistent figure (3–5).
The mechanism and regulation of DC survival and death (6, 7) are likely to be important in maintaining the homeostatic balance of the immune system. A few reports have linked extended survival of DC to enhanced or dysregulated T cell immune responses and lymphoproliferative disease (8–10). In contrast, reduced survival of DC has been associated with impaired immune responses (7). It is presently unclear whether T cells also influence the lifespan of DC in an antigen-specific fashion. In lymph nodes of mice adoptively transferred with CD4+ T cell receptor (TCR) transgenic T cells, DC presenting specific antigen disappear more rapidly than DC not presenting antigen (11), suggesting that T cells may have a role in regulating DC numbers and survival. Experiments using infection with Listeria or malaria also suggest that the number of antigen-presenting cells becomes limiting during the early phases of CD8+ immune responses and prevents the continued expansion of antigen-specific T cells (12, 13). Together, these experiments suggest the attractive hypothesis that T cells may be able to regulate their own responses in a feedback fashion, by affecting the survival of antigen-presenting DC.
We have previously reported that DC loaded with antigen and injected s.c. into immune mice are rapidly eliminated by CD8+ T cells (14). However, the consequences of this antigen-specific DC elimination remain unclear. DC elimination may simply be a means to dispose of DC that are no longer required for T cell activation and may not affect the final size of the immune response. Alternatively, DC elimination may serve a critical regulatory function by which activated cytotoxic T lymphocytes (CTL) prevent further T cell activation by clearing antigen-loaded antigen-presenting cells.
In this article we examine the site and mechanism of DC elimination and its consequences to the immune response. We show that in immune mice antigen-loaded DC are eliminated before they reach the draining lymph node and are thus prevented from interacting with lymph node-resident memory and/or naïve T cells. We also show that in perforin-deficient (PKO) mice DC are not eliminated by activated CD8+ T cells. DC survival in PKO mice correlates with an improved ability of the DC to induce expansion of antigen-specific CD8+ T cells during secondary and subsequent immune responses. We conclude that perforin-dependent elimination of DC acts as a regulatory feedback mechanism to prevent the access of antigen-loaded DC to the lymph node and the further activation of antigen-specific T cells. These findings may explain the observation that PKO mice develop exaggerated immune responses, principally after viral infections (15, 16) but also after immunization with antigen-loaded DC (17).
Materials and Methods
Mice. C57BL/6 and C57BL/6-background PKO and IFN-γ–/– mice were from The Jackson Laboratory. CD45-congenic B6.SJL-Ptprca mice were from the Animal Resources Centre, Perth, Australia. Line 318 (L318) mice carry a transgenic TCR specific for fragment 33-41 of the lymphocytic choriomeningitis virus glycoprotein (gp33) in association with H-2Db. L318 mice that are PKO (PKO-L318) were generated by conventional breeding. All experimental protocols were approved by the Wellington School of Medicine and Victoria University Animal Ethics Committee and performed according to institutional guidelines.
In Vitro Culture Media and Reagents. All cultures were in complete medium composed of Iscove's modified Dulbecco medium, 2 mM glutamax, 1% penicillin-streptomycin, 5 × 10–5 M 2-mercapto-ethanol, and 5% FBS (all Invitrogen). The synthetic peptide gp33 (KAVYNFATM) was from Chiron Mimotopes, Clayton, Australia. LPS from Escherichia coli was from Sigma–Aldrich.
Immunization with DC. To generate DC, bone marrow cells from C57BL/6 or CD45-congenic mice were cultured in complete medium containing 20 ng/ml recombinant murine IL-4 and 10 ng/ml recombinant murine granulocyte–macrophage colony-stimulating factor for 7 days as described (18). On day 7 cultures typically contained 70–90% CD11c+ cells. In some experiments DC were activated by transferring to a fresh plate or adding 100 ng/ml LPS during the last 20 h of culture. On day 7 DC were harvested and loaded with 10 μM gp33 peptide for 2 h at 37°C. Mice were injected with 2 × 105 gp33-loaded DC or identically treated DC not loaded with peptide. CD4+CD25+ regulatory T cells (Treg) were depleted by i.p. injection of 100 μg purified anti-CD25 antibody (PC61) on the day before DC immunization as described (19). Treatment conditions were determined in pilot experiments and considerably diminished the percentage of CD25+ cells in recipient blood on days 1–12 after antibody injection.
DC Labeling and in Vivo DC Elimination Assays. DC were harvested from culture, washed, and labeled with carboxy-fluorescein diacetate succinimidyl ester (CFSE, Molecular Probes) or the orange fluorescent dye chloromethyl-benzoyl-aminotetramethyl-rhodamine (CMTMR, Molecular Probes) with the protocols described (20). The target DC populations used throughout this study were not treated with LPS, and treatment with 0.1–1 μg/ml LPS did not change their susceptibility to elimination in vivo (K. Andrew and F.R., unpublished work).
In vivo elimination of antigen-loaded DC was assessed as described (20). Mice were injected s.c. in the forelimb or intradermally into the ear with 1–2 × 106 cells containing equal numbers of CFSE-labeled DC loaded with 1 μM peptide (DC+) and CMTMR-labeled control DC (DC–). At the indicated times, draining lymph nodes or ears were removed, digested in collagenase (Invitrogen) and DNase I (Sigma), and analyzed for the presence of fluorescent cells by flow cytometry. The percent surviving antigen-loaded DC in each sample was calculated as (DC+/DC–) × 100. This value was normalized to the ratio in the relevant control sample when available.
Adoptive Transfer of Naïve or Activated T Cells. Single cell suspensions were prepared from pooled lymph nodes and spleens of L318 or PKO-L318 mice, and the equivalent of ≈4 × 106 Vα2+Vβ8+ transgenic T cells was injected i.v. into the lateral tail vein of C57BL/6 or PKO recipient mice. CD8+ T cells from L318 mice were activated in vitro on anti-CD3 and anti-CD28 for 5 days, and then cultured in 100 units/ml of recombinant human IL-2 and 10 ng/ml of recombinant human IL-6 for 2 more days as described (21). In some experiments, CD8+ T cells were cultured with LPS-treated DC and 10 μM gp33 for 4 days, and then in 100 units/ml of recombinant human IL-2 for 3 more days. On day 7 activated T cells were harvested and washed in Iscove's modified Dulbecco medium, and 107 cells were injected i.v. into the lateral tail vein of recipient mice.
FACS Analysis. Anti-FcγRII (2.4G2), anti-MHC II (3JP), and anti-CD11c (N418) mAbs were affinity-purified from hybridoma culture supernatants and conjugated to biotin, FITC, or allophycocyanin. Anti-Vα2-PE, anti-Vβ8.1, 8.2-biotin, anti-CD25-FITC, anti-CD45.2APC, and anti-CD45.2PE were from Pharmingen. All samples were analyzed on a FACSort (Becton Dickinson) by using cellquest (Becton Dickinson) or flowjo (Tree Star, Ashland, OR) software. Dead cells were excluded from analysis with propidium iodide staining (Pharmingen).
Results
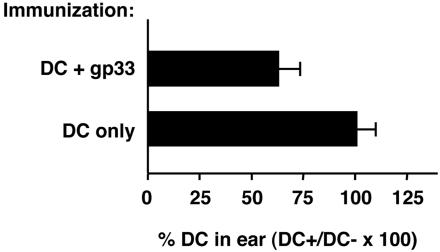
Effector CTL Eliminate Antigen-Loaded DC Before They Reach the Lymph Node, Whereas DC in Lymph Nodes Are Not Eliminated. We have previously reported that antigen-loaded DC are eliminated by specific CD8+ T cells in vivo and fail to accumulate in the draining lymph node (20). In vitro experiments (data not shown) also showed that CTL can kill DC in an antigen-specific fashion, providing further support for a direct killing mechanism in vivo. We wanted to establish whether DC are eliminated immediately at the site of injection, in the lymph node, or at both sites. To establish whether DC are eliminated in tissues, C57BL/6 mice were immunized with gp33-loaded DC and injected 7 days later with CD45-congenic target DC given intradermally into the ear. The target DC population was a mixture of CFSE-labeled DC loaded with the specific antigen gp33 (DC+) and CMTMR-labeled DC not loaded with antigen (DC–). Two days later, mice were killed, and cell suspensions were prepared from the injected ears. As shown in Fig. 1, by 48 h after injection the number of gp33-loaded DC was only ≈60% of the number of DC not loaded with antigen, indicating that DC are specifically eliminated at the injection site in tissues.
Fig. 1.
Antigen-loaded DC are eliminated in tissues of DC-immunized mice and are sensitive to CTL killing in vitro. Groups of CD45-congenic mice were immunized s.c. in the flank with LPS-treated congenic DC as indicated. Seven days later mice were injected intradermally in the ear with equal numbers of C57BL/6 DC labeled with CFSE or CMTMR and loaded with gp33 or left untreated, respectively. After 48 h mice were killed, and ear cell suspensions were prepared, stained with CD45.2-specific antibodies, and examined for the presence of fluorescent donor DC by FACS. Bars indicate the average proportion of gp33-loaded DC (DC+) remaining in tissue, as a percent of the coinjected control DC (DC–), +SD for four samples.
To establish whether DC could be eliminated in lymph nodes, antigen-specific L318 CTL populations were generated in culture and injected into mice. Preliminary experiments showed that CTL transferred in vivo could be recovered from several different tissues, including lymph nodes, between days 1 and 3 after injection (data not shown). To evaluate the survival of DC in the presence of specific CTL, mice were also injected s.c. with mixtures of labeled DC that were given at different times with respect to the CTL transfer. In one group of mice DC were injected 1 day after the adoptive transfer of CTL. In this situation DC may interact with CTL immediately after injection, before migrating to the draining lymph node. In the second group of mice, DC were injected 1 day before CTL transfer. In this case DC are able to reach the lymph node before they interact with CTL. As shown in Fig. 2A, gp33-loaded DC given after CTL transfer selectively failed to accumulate in the lymph node, suggesting that they were eliminated. In contrast, gp33-loaded DC given 1 day before CTL transfer could be recovered from the draining lymph node at 24 and 48 h after CTL transfer, times that are expected to be sufficient for CTL to recirculate through the lymph node. Similar results were also obtained in mice immunized with DC and then given a second, carefully timed injection of target DC. Target DC given before the CTL response had time to develop survived in the lymph node for several days, whereas DC given after the CTL response had developed failed to accumulate in the lymph node (Fig. 6, which is published as supporting information on the PNAS web site). Together, these results indicate that DC in lymph nodes are not eliminated even in the presence of a host CTL response.
Fig. 2.
Antigen-loaded DC survive in lymph nodes and induce proliferation of specific CTL adoptively transferred i.v. (A) Groups of C57BL/6 mice were injected s.c. in both forelimbs with mixtures of fluorescent target DC as described in the legend to Fig. 1. The same mice were also injected i.v. with 10 million in vitro-activated CTL or with no CTL as indicated on the left. CTL were injected either 24 h before or 24 h after the DC, to allow DC time to reach the lymph node. Mice were killed 24 h or 48 h after the last injection as indicated, and the presence of labeled DC in lymph nodes was determined. Bars indicate the average proportion of gp33-loaded DC (DC+) remaining in lymph nodes, expressed as a percentage of control DC (DC–), + SD, for groups of five mice. (B) Groups of CD45-congenic mice were injected with CD45-congenic, gp33-loaded DC in the forelimb (Right) or left untreated (Left). One day later both groups were injected i.v. with 10 million CFSE-labeled, in vitro-activated CTL. T cell proliferation in the draining lymph node was examined 72 h after CTL transfer. Results show total, live-gated lymph node cells from individual mice that were representative of groups of three.
To determine whether DC in lymph nodes survived because they failed to express sufficient levels of antigen or interact with transferred CTL we asked whether they were able to induce proliferation of activated T cells in lymph nodes. CD45-congenic mice were injected s.c. with gp33-loaded CD45-congenic DC, and the next day they were injected i.v. with CFSE-labeled specific CTL. T cell proliferation in lymph nodes was examined 3 days later. As shown in Fig. 2B, donor T cells proliferated vigorously in mice injected with gp33-loaded DC. Because in vivo the gp33 peptide is transferred from donor to host antigen presenting cells very poorly, if at all (22), this result suggests that injected DC were available to interact with activated T cells in the lymph node.
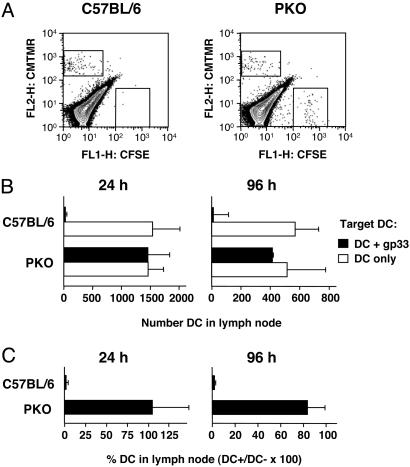
DC Elimination in Vivo Requires Perforin But Not IFN-γ. To establish the mechanism of DC elimination in vivo, we used PKO and IFN-γ–/– mice as perforin and IFN-γ are critical molecules in the function of CTL. Groups of C57BL/6 and PKO mice were immunized with gp33-loaded DC, and 7 days later they were injected s.c. with equal numbers of labeled DC that were loaded with gp33 or left untreated. Results from this experiment are shown in Fig. 3A as representative FACS profiles, in Fig. 3B as numbers of DC recovered from lymph node, and in Fig. 3C as gp33-loaded DC as percent of control DC not loaded with antigen. DC not loaded with antigen were recovered from the draining lymph nodes of immune C57BL/6 mice, whereas the gp33-loaded DC population failed to accumulate. In contrast, both DC populations were recovered from the lymph nodes of PKO mice. Of note, similar numbers of DC not loaded with antigen were recovered in C57BL/6 and PKO mice, indicating that DC migration and survival were not otherwise affected in PKO hosts. No preferential decline in the number of gp33-loaded DC was observed in PKO hosts even by 96 h after DC injection, indicating that no other cytotoxicity mechanisms were compensating for the lack of perforin function. Similar results were also obtained with immune L318 TCR transgenic mice (data not shown), in which the number of antigen-specific CTL is presumably not limiting. We therefore conclude that perforin is critical for the elimination of antigen-loaded DC in vivo.
Fig. 3.
Antigen-loaded DC are not eliminated in PKO mice. Groups of C57BL/6 and PKO mice were immunized by s.c. injection of gp33-loaded DC in the flank. One week later all mice were injected in both forelimbs with mixtures of fluorescent target DC as described in the legend to Fig. 1 and killed 24 or 96 h later as indicated. (A) Representative FACS profiles from C57BL/6 (Left) and PKO (Right) mice killed at 96 h. (B) Total number of DC recovered from the lymph nodes of C57BL/6 or PKO mice. (C) Average proportion of gp33-loaded DC (DC+) remaining in lymph nodes, expressed as percentage of control DC (DC–), + SD for groups of three mice.
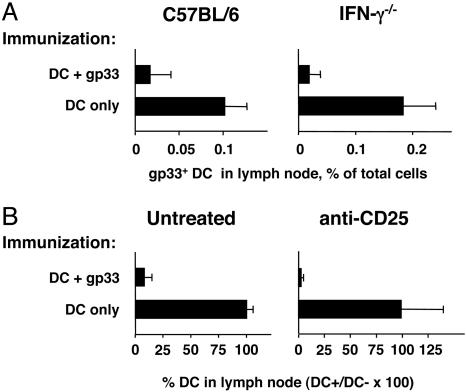
A similar experiment was carried out with IFN-γ–/– and C57BL/6 mice. Mice were immunized with DC only or DC loaded with gp33, and 7 days later they were injected in the forelimb with CFSE-labeled DC loaded with gp33. The accumulation of these DC in the draining lymph node was compared. As shown in Fig. 4A, antigen-loaded DC accumulated in the lymph nodes of mice immunized with DC only, but not in the lymph nodes of C57BL/6 or IFN-γ–/– mice immunized with gp33-loaded DC, indicating that IFN-γ was not critical for DC elimination.
Fig. 4.
IFN-γ and CD4+CD25+ Treg are not required for the elimination of DC in vivo. (A) Groups of C57BL/6 (Left) and IFN-γ–/– (Right) mice were immunized with DC only or DC loaded with gp33 peptide, by s.c. injection into the flank. Seven days later all mice received CFSE-labeled DC loaded with gp33 peptide by s.c. injection into each forelimb. Seventy-two hours later the draining lymph nodes were harvested and the percent DC was determined by FACS staining. Bars show the percentages of CFSE+ DC in lymph nodes; average + SD for groups of three mice are shown. (B) Groups of C57BL/6 mice were injected with 100 μg of the anti-CD25 antibody PC61 (Right) or left untreated (Left) and immunized with the indicated DC the next day. Target fluorescent DC were injected on day 7. Bars indicate the average proportion of gp33-loaded DC (DC+) remaining in lymph node, expressed as a percent of control DC (DC–), + SD, for groups of four mice.
Recent studies have investigated the role of perforin or granzyme B in the function of Treg populations. Under certain circumstances, induced human Treg and activated naturally occurring Treg have the potential to kill target cells in a perforin-dependent manner (23). Murine CD4+CD25+ T cells, however, do not show the same perforin dependence (24). To address the role of naturally occurring CD4+CD25+ T cells in our system, mice were treated with the anti-CD25 antibody PC61 under conditions that effectively deplete CD25+ cells from blood (Fig. 7, which is published as supporting information on the PNAS web site), and that have been shown effective at enhancing antitumor immunity in a DC vaccination model (19). Treated and control mice were immunized with gp33-loaded DC 1 day after anti-CD25 administration, and elimination of target DC was assessed 1 week after immunization. As shown in Fig. 4B, depletion of Treg cells did not restore DC survival, indicating that CD4+CD25+ Treg cells were not required for DC elimination.
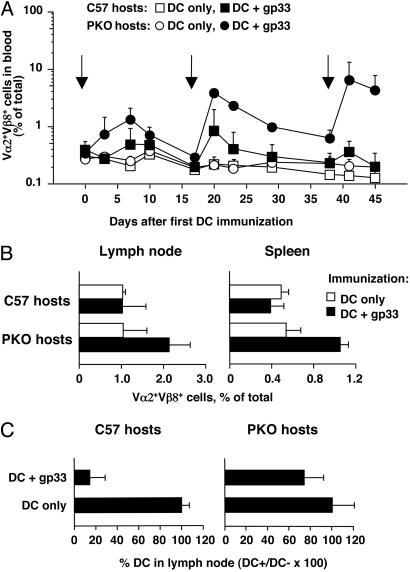
Multiple Immunizations with DC Lead to Greater Increases in the Frequency of Antigen-Specific CD8+ T Cells in PKO Mice Compared with C57BL/6 Mice. As shown in the previous section, antigen-loaded DC accumulate in the lymph nodes of immune PKO mice, where they remain for several days. In contrast, very few antigen-loaded DC are found in the lymph nodes of immune C57BL/6 mice. We reasoned that DC that accumulate in the lymph nodes of PKO mice should be able to induce further immune activation by inducing the proliferation of resident T cells. If this was the case, multiple immunizations with DC should elicit larger numbers of antigen-specific T cells in PKO mice compared with perforin-sufficient C57BL/6 mice. To test this hypothesis, we adoptively transferred TCR transgenic T cells that were perforin-sufficient or PKO into C57BL/6 or PKO mice and immunized both groups with DC only or gp33-loaded DC administered s.c. every 2–3 weeks. Immune responses were monitored by determining the percentage of Vα2+Vβ8+ cells in the blood every 3–5 days. As shown in Fig. 5A, primary immunization of C57BL/6 or PKO mice with gp33-loaded DC led to an increase in the proportion of Vα2+Vβ8+ cells in peripheral blood, which peaked at day 7 after immunization and then declined. In some experiments, including the one in Fig. 5, the increase in Vα2+Vβ8+ cells was more marked in PKO than in C57BL/6 mice, but this was not always the case. Additional DC immunizations given at the same site again caused an increase in the proportion of Vα2+Vβ8+ T cells in blood, which in C57BL/6 hosts was restored to the levels observed after one immunization, and then declined again. In contrast, in PKO mice the percentage of Vα2+Vβ8+ cells in blood increased to above the level observed after primary immunization and stabilized at a level that was higher than the level in C57BL/6 mice. A similar increase was observed after the third DC immunization. Representative profiles of blood cells on day 41, corresponding to the peak response after the third DC immunization, are shown in Fig. 8, which is published as supporting information on the PNAS web site.
Fig. 5.
Multiple DC immunizations lead to increased CD8+ T cell expansion in PKO mice compared with C57BL/6. Groups of C57BL/6 mice or PKO mice were injected with TCR transgenic T cells from L318 or PKO-L318 mice, respectively, and repeatedly immunized s.c. with gp33-loaded DC or DC only at the times indicated by arrows. Immunizing DC were activated by plastic adherence (A and C) or treatment with 100 ng/ml LPS (B). (A) The percentage of Vα2+Vβ8+ T cells in peripheral blood was determined by FACS analysis at different times after immunization. Data points represent the average + SD for groups of three mice. (B) The percentage of Vα2+Vβ8+ T cells in lymph nodes and spleen was determined in C57BL/6 (Left) and PKO (Right) mice 14 days after the fourth DC immunization. Bars represent the average + SD for groups of five (DC + gp33) or three (DC only) mice. (C) Survival of gp33-loaded DC in multiply immunized mice. Seven days after the third DC immunization, mice were injected in both forelimbs with mixtures of fluorescent target DC as described in the legend to Fig. 1. The proportion of gp33-loaded DC (DC+) remaining in lymph node, expressed as a percent of the coinjected control DC (DC–), was determined 40 h after injection. Bars represent average + SD for groups of three mice. Data in A and C refer to the same experiment. Data in B are from a similar but independent experiment that gave comparable results.
A higher percentage of antigen-specific T cells was also recovered from the lymph nodes and spleens of multiply immunized PKO hosts. As shown in Fig. 5B, the proportion of Vα2+Vβ8+ T cells was increased in lymph nodes and spleens of PKO mice given a fourth DC immunization 2 weeks earlier, but was similarly low in all other groups. This finding indicated that by 2 weeks after immunization the proportion of antigen-specific T cells had reverted to a resting level, which was higher in PKO than in C57BL/6 hosts.
It was possible that multiple DC immunizations may allow the generation of cytotoxic activity even in PKO mice. We tested this possibility by injecting multiply immunized mice with labeled DC, and again testing the DC's ability to accumulate in the draining lymph nodes. As shown in Fig. 5C, only a few gp33-loaded DC could be recovered from the lymph nodes of multiply immunized C57BL/6 mice. In contrast, both gp33-loaded and not antigen-loaded DC were recovered from the lymph nodes of immune PKO mice.
Discussion
In this article we show that the accumulation of antigen-loaded DC in the lymph nodes of immune mice is limited by a CTL-dependent, perforin-dependent mechanism. By preventing the accumulation of antigen-loaded DC in the lymph node, effector CTL presumably also limit the DC's ability to induce further T cell proliferation and differentiation to effector function during secondary immune responses. DC elimination fails to operate in PKO mice, where CTL are unable to control DC access to the lymph node. This failure is associated with the accumulation of higher proportions of antigen-specific T cells in multiply immunized PKO mice.
Previous reports have documented how PKO mice generate exaggerated immune responses after infections with lymphocytic choriomeningitis virus (15, 25) or Listeria (17) and during graft-versus-host disease (26). Similar findings are also reported in humans deficient in perforin function (27), who are susceptible to developing lymphoproliferative disease with accumulation of activated T cells as a consequence of viral infections. In mice, the larger immune responses of PKO mice do not require infection, but are also observed after immunization with antigen-loaded DC (17), indicating that they are not simply caused by the inability of the PKO host to control the spread of infection. The basis for this increased immune response has not been clarified, and failure to eliminate antigen-presenting DC or impaired fratricidal elimination of T cells both have been proposed as possible mechanisms.
In this article we present evidence in favor of the hypothesis that failure to eliminate DC contributes to the increased immune responses observed in PKO mice. We show that in normal immune mice antigen-loaded DC are rapidly eliminated in tissues or during transit to the draining lymph nodes. Because T cell division appears to occur only in lymph nodes (28, 29), these DC would be unable to induce proliferation of antigen-specific T cells. We also show that in PKO mice DC remain untouched by CTL activity and accumulate in the draining lymph nodes where they can present antigen to, and presumably induce proliferation and effector activity of, resident naïve and/or central memory T cells. In support of our hypothesis, we show that the expansion of CD8+ T cells is greater in PKO mice that have been immunized with DC than in C57BL/6 mice and that the difference between the two strains becomes more marked with subsequent immunizations. When in the context of viral infection and consequent inflammatory environment, such increased T cell expansion and continued antigen presentation could result in the mortality and lymphoproliferative phenotype already reported for PKO mice (16, 27).
The interaction of CTL with target cells has been shown to allow transfer of surface molecules from the target cell to the T cell (30) and sensitization of T cells to attack from other CTL (31). T cell–T cell killing may also be occurring in our system and affect the accumulation of normal, but not PKO, T cells. However, T cell fratricide seems a less attractive explanation for our findings. Higher concentrations of antigen on the antigen-presenting cells compared with T cells would make DC especially susceptible to killing. In addition, although there are described physiological mechanisms to maximize the interaction of T cells and DC (32), the opportunity for T cell–T cell contact in vivo may remain limited.
Perforin deficiency may also affect the function of other cell populations besides CTL. Human activated natural Treg cells have been reported to kill DC and T cells through a perforin-dependent mechanism (23). In contrast, murine CD4+CD25+ Treg from PKO mice were found to have normal suppressive function in vitro (24). Treg did not appear to contribute to DC elimination in our system, as DC elimination was not prevented by in vivo depletion of CD25+ cells. In addition, hosts adoptively transferred with in vitro-activated CD8+ T cells acquired the ability to immediately eliminate DC. The same activated CD8+ T cells were also able to kill DC in vitro (data not shown), providing further support for a direct effect of CD8+ T cells on DC. We conclude that a potentially defective Treg function in PKO mice is unlikely to explain our observations.
In physiological situations, several factors may affect the susceptibility of endogenous DC to CTL-mediated elimination. Cross-presentation of exogenous antigen is regulated during DC maturation, with immature DC expressing only low levels of MHC I–antigen complexes at the cell surface (33). Specific CD8+ T cells have been reported to induce antigen loss from the surface of DC (34). Low expression of MHC I–antigen complexes because of either of the above causes may decrease the susceptibility of DC to CTL, but it appears unlikely to prevent it altogether given the capacity of CTL to eliminate targets that express as few as one to three MHC I–antigen complexes at their surface (35).
Previous reports have indicated that DC become resistant to CTL killing by up-regulating expression of the granzyme inhibitor serpin 6 upon activation (36) or by polarized exocytosis of secretory lysosomes upon interaction with CD8+ T cells (37). Furthermore, perforin has been reported to be critical for DC elimination by alloreactive CTL (38), but not for DC elimination by CTL elicited by viral infection (39). A number of differences exist between our experimental system and those described, which may explain some of the discrepancies in the results. Reconciling such differences, to establish the physiological significance of DC elimination in the regulation of immune responses, will require in vivo analysis of the susceptibility of endogenous DC to CTL-mediated killing during immune responses to infectious agents or tumors.
We show in this article that DC injected into immune mice are susceptible to immediate CTL elimination at the site of injection, but survive for at least 24–48 h once they reach the lymph node. This is despite activated T cells interacting with injected DC in the lymph node, as indicated by the induction of T cell proliferation. This differential susceptibility of DC to CTL action may be caused by mature DC in lymph node becoming resistant to CTL (36) or the described exclusion of effector CTL from the lymph node environment (40, 41). We favor the latter possibility; we have previously shown that DC injected into naïve TCR transgenic mice can be recovered from the lymph nodes in high numbers at day 1, but disappear by day 3 (14). This finding indicates that, in the presence of high numbers of antigen-specific T cells, DC in lymph nodes are eliminated by the T cells they activate. A similar observation was also made in “memory” C57BL/6 mice, where memory T cells becoming activated to effector function eliminate DC in the lymph node (14). Together, these results support the view that DC remain sensitive to CTL elimination even when in the lymph node. However, the low numbers of effector CTL normally present in lymph nodes allow DC to survive relatively protected from CTL attack.
Together, our results suggest an attractive scenario of feedback regulation of the immune response. In the presence of a vigorous effector T cell response, CTL effectively patrol peripheral tissues where they eliminate DC that have taken up antigen, thus preventing them from reaching the draining lymph node and interacting with central memory and/or naïve T cells. Once the effector response in peripheral tissues declines to levels that are insufficient to contain antigen spread, DC again become able to migrate to lymph nodes where they can restimulate T cell responses. Thus, T cells would act as “gatekeepers” to regulate T cell expansion by controlling the access of stimulatory DC to the lymph node. The observation that CD8+ T cells require only one division to acquire cytotoxic function (42) suggests that the proposed feedback mechanism may rapidly become operational during the first few days of an immune response. In situations such as infections, where microbial replication in tissues is likely to provide for continued antigen presentation, the entry of antigen-loaded DC into the lymph node would rapidly decline once a CTL response is established, thereby limiting further expansion of antigen-specific T cells. This possibility is consistent with the observation that clonal burst size and the ability to present antigen to T cells appear not to correlate with antigen load (43), but are determined in the first few days of infection (13), and can be manipulated by increasing the number of antigen-presenting cells (12).
Supplementary Material
Acknowledgments
We thank the staffs of the Biomedical Research Unit at the Wellington School of Medicine (Wellington, New Zealand) and the Malaghan Institute Experimental Research Facility for animal husbandry and care and the staff of the Malaghan Institute for useful suggestions and discussion. This work was supported by research grants from the Health Research Council of New Zealand and New Zealand Cancer Society (to F.R.) and an equipment grant from the New Zealand Lottery Health Board.
Author contributions: R.S.M., I.F.H., and F.R. designed research; J.Y., S.P.H., R.S.M., and I.F.H. performed research; J.Y., S.P.H., R.S.M., and I.F.H. analyzed data; and F.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CFSE, carboxy-fluorescein diacetate succinimidyl ester; CMTMR, chloromethyl-benzoyl-aminotetramethyl-rhodamine; CTL, cytotoxic T lymphocytes; DC, dendritic cells; gp33, fragment 33–41 of the lymphocytic choriomeningitis virus glycoprotein; L318, line 318; PKO, perforin-deficient; TCR, T cell receptor; Treg, regulatory T cells.
References
- 1.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 2.Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. (2002) Annu. Rev. Immunol. 20, 621–667. [DOI] [PubMed] [Google Scholar]
- 3.Ruedl, C., Koebel, P., Bachmann, M., Hess, M. & Karjalainen, K. (2000) J. Immunol. 165, 4910–4916. [DOI] [PubMed] [Google Scholar]
- 4.Kamath, A. T., Henri, S., Battye, F., Tough, D. F. & Shortman, K. (2002) Blood 100, 1734–1741. [PubMed] [Google Scholar]
- 5.Garg, S., Oran, A., Wajchman, J., Sasaki, S., Maris, C. H., Kapp, J. A. & Jacob, J. (2003) Nat. Immunol. 4, 907–912. [DOI] [PubMed] [Google Scholar]
- 6.Hou, W. S. & Van Parijs, L. (2004) Nat. Immunol. 5, 583–589. [DOI] [PubMed] [Google Scholar]
- 7.Hon, H., Rucker, E. B., 3rd, Hennighausen, L. & Jacob, J. (2004) J. Immunol. 173, 4425–4432. [DOI] [PubMed] [Google Scholar]
- 8.Wang, J., Zheng, L., Lobito, A., Chan, F. K., Dale, J., Sneller, M., Yao, X., Puck, J. M., Straus, S. E. & Lenardo, M. J. (1999) Cell 98, 47–58. [DOI] [PubMed] [Google Scholar]
- 9.Nopora, A. & Brocker, T. (2002) J. Immunol. 169, 3006–3014. [DOI] [PubMed] [Google Scholar]
- 10.Ashcroft, A. J., Cruickshank, S. M., Croucher, P. I., Perry, M. J., Rollinson, S., Lippitt, J. M., Child, J. A., Dunstan, C., Felsburg, P. J., Morgan, G. J. & Carding, S. R. (2003) Immunity 19, 849–861. [DOI] [PubMed] [Google Scholar]
- 11.Ingulli, E., Mondino, A., Khoruts, A. & Jenkins, M. K. (1997) J. Exp. Med. 185, 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafalla, J. C., Morrot, A., Sano, G., Milon, G., Lafaille, J. J. & Zavala, F. (2003) J. Immunol. 171, 964–970. [DOI] [PubMed] [Google Scholar]
- 13.Wong, P. & Pamer, E. G. (2003) Immunity 18, 499–511. [DOI] [PubMed] [Google Scholar]
- 14.Hermans, I. F., Ritchie, D. S., Yang, J. P., Roberts, J. M. & Ronchese, F. (2000) J. Immunol. 164, 3095–3101. [DOI] [PubMed] [Google Scholar]
- 15.Matloubian, M., Suresh, M., Glass, A., Galvan, M., Chow, K., Whitmire, J. K., Walsh, C. M., Clark, W. R. & Ahmed, R. (1999) J. Virol. 73, 2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badovinac, V. P., Hamilton, S. E. & Harty, J. T. (2003) Immunity 18, 463–474. [DOI] [PubMed] [Google Scholar]
- 17.Badovinac, V. P., Tvinnereim, A. R. & Harty, J. T. (2000) Science 290, 1354–1357. [DOI] [PubMed] [Google Scholar]
- 18.Garrigan, K., Moroni-Rawson, P., McMurray, C., Hermans, I., Abernethy, N., Watson, J. & Ronchese, F. (1996) Blood 88, 3508–3512. [PubMed] [Google Scholar]
- 19.Prasad, S. J., Farrand, K. J., Matthews, S. A., Chang, J. H., McHugh, R. S. & Ronchese, F. (2005) J. Immunol. 174, 90–98. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie, D. S., Hermans, I. F., Lumsden, J. M., Scanga, C. B., Roberts, J. M., Yang, J. P., Kemp, R. A. & Ronchese, F. (2000) J. Immunol. Methods 246, 109–117. [DOI] [PubMed] [Google Scholar]
- 21.Kemp, R. A. & Ronchese, F. (2001) J. Immunol. 167, 6497–6502. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie, D. S., Yang, J., Hermans, I. F. & Ronchese, F. (2004) Scand. J. Immunol. 60, 543–551. [DOI] [PubMed] [Google Scholar]
- 23.Grossman, W. J., Verbsky, J. W., Barchet, W., Colonna, M., Atkinson, J. P. & Ley, T. J. (2004) Immunity 21, 589–601. [DOI] [PubMed] [Google Scholar]
- 24.Gondek, D. C., Lu, L. F., Quezada, S. A., Sakaguchi, S. & Noelle, R. J. (2005) J. Immunol. 174, 1783–1786. [DOI] [PubMed] [Google Scholar]
- 25.Jordan, M. B., Hildeman, D., Kappler, J. & Marrack, P. (2004) Blood 104, 735–743. [DOI] [PubMed] [Google Scholar]
- 26.Maeda, Y., Levy, R. B., Reddy, P., Liu, C., Clouthier, S. G., Teshima, T. & Ferrara, J. L. (2005) Blood 105, 2023–2027. [DOI] [PubMed] [Google Scholar]
- 27.Stepp, S. E., Dufourcq-Lagelouse, R., Le Deist, F., Bhawan, S., Certain, S., Mathew, A. P., Henter, J. I., Bennett, M., Fischer, A., de Saint Basile, G. & Kumar, V. (1999) Science 286, 1957–1959. [DOI] [PubMed] [Google Scholar]
- 28.Harris, N., Watt, V., Ronchese, F. & Le Gros, G. (2002) J. Exp. Med. 195, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhardt, R. L., Bullard, D. C., Weaver, C. T. & Jenkins, M. K. (2003) J. Exp. Med. 197, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, J. F., Yang, Y., Sepulveda, H., Shi, W., Hwang, I., Peterson, P. A., Jackson, M. R., Sprent, J. & Cai, Z. (1999) Science 286, 952–954. [DOI] [PubMed] [Google Scholar]
- 31.Hwang, I., Huang, J. F., Kishimoto, H., Brunmark, A., Peterson, P. A., Jackson, M. R., Surh, C. D., Cai, Z. & Sprent, J. (2000) J. Exp. Med. 191, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bajenoff, M., Granjeaud, S. & Guerder, S. (2003) J. Exp. Med. 198, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delamarre, L., Holcombe, H. & Mellman, I. (2003) J. Exp. Med. 198, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedl, R. M., Schaefer, B. C., Kappler, J. W. & Marrack, P. (2002) Nat. Immunol. 3, 27–32. [DOI] [PubMed] [Google Scholar]
- 35.Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. (1996) Immunity 4, 565–571. [DOI] [PubMed] [Google Scholar]
- 36.Medema, J. P., Schuurhuis, D. H., Rea, D., van Tongeren, J., de Jong, J., Bres, S. A., Laban, S., Toes, R. E., Toebes, M., Schumacher, T. N., et al. (2001) J. Exp. Med. 194, 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardella, S., Andrei, C., Lotti, L. V., Poggi, A., Torrisi, M. R., Zocchi, M. R. & Rubartelli, A. (2001) Blood 98, 2152–2159. [DOI] [PubMed] [Google Scholar]
- 38.Loyer, V., Fontaine, P., Pion, S., Hetu, F., Roy, D. C. & Perreault, C. (1999) J. Immunol. 163, 6462–6467. [PubMed] [Google Scholar]
- 39.Ludewig, B., Bonilla, W. V., Dumrese, T., Odermatt, B., Zinkernagel, R. M. & Hengartner, H. (2001) Eur. J. Immunol. 31, 1772–1779. [DOI] [PubMed] [Google Scholar]
- 40.Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. (1999) Nature 401, 708–712. [DOI] [PubMed] [Google Scholar]
- 41.Masopust, D., Vezys, V., Marzo, A. & Lefrancois, L. (2001) Science 291, 2413–2417. [DOI] [PubMed] [Google Scholar]
- 42.Oehen, S. & Brduscha-Riem, K. (1998) J. Immunol. 161, 5338–5346. [PubMed] [Google Scholar]
- 43.Badovinac, V. P., Porter, B. B. & Harty, J. T. (2002) Nat. Immunol. 3, 619–626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.