Abstract
Btk plays crucial roles in the differentiation and activation of B and myeloid cells. Despite drastic reductions of other Ig isotypes, paradoxically high IgE responses have been known in btk mutant mice. Here we show that btk–/– dendritic cells exhibit a more mature phenotype and a stronger in vitro and in vivo T cell-stimulatory ability than wild-type cells. Increased IgE responses were induced by adoptive transfer of btk–/– dendritic cells into mice. Consistent with the stronger T cell-stimulatory ability of btk–/– dendritic cells, btk–/– mice exhibited enhanced inflammation in Th2-driven asthma and Th1-driven contact sensitivity experiments. These negative regulatory functions of Btk in dendritic cells appear to be mediated mainly through autocrine secretion of IL-10 and subsequent activation of Stat3.
Keywords: allergy, IgE, immune regulation, Btk
Immune responses are initiated by the interaction of naïve T cells with dendritic cells (DCs) in secondary lymphoid organs. DCs capture antigen in peripheral tissues, process them to peptides, and load the peptides onto MHC molecules. DCs then migrate to draining lymph nodes and present peptide–MHC complexes to T cells, leading to the activation and proliferation of T cells. Between antigen capture and antigen presentation to T cells, DCs undergo a maturation program that governs their capacity to generate the MHC–peptide complexes, migrate toward lymph nodes, and stimulate T cells (1). DCs direct the differentiation of CD4+ T cells into either IFN-γ-producing T helper cell type (Th) 1 cells or interleukin (IL)-4-producing Th2 cells (2, 3).
Btk, a member of the Tec protein-tyrosine kinase family, plays crucial roles in differentiation, activation, survival, and apoptosis of B cells (4). Mutations in the btk gene cause severe defects in adaptive immunity, leading to X-linked agammaglobulinemia (XLA) in humans and X-linked immunodeficiency (xid) in mice. Btk mutations in XLA patients lead to a block in the pro-B to pre-B transition during B cell ontogeny, resulting in a deficit of mature B cells and serum immunoglobulins. Xid and btk–/– mice have milder phenotypes. B cells from btk mutant mice respond abnormally to crosslinking of several cell surface receptors, including BCR and some cytokine receptors. Btk's roles have also been shown in Fc receptor-mediated mast cell and myeloid cell activation and collagen receptor-mediated platelet functions. Unlike these immune/hematopoietic cells, T cells and natural killer cells lack Btk expression (5).
Paradoxically, negative regulatory functions of Btk have been suspected in certain immune responses, including IgE production. Early studies found that CBA/N(xid) mice manifest increased IgE responses compared with normal mice (6, 7). Supranormal IgE responses in xid mice were also caused by infection with parasites such as Nippostrongylus brasiliensis (8) and Schistosoma mansoni (9), indicating that xid mice tend to be skewed toward Th2-dominant immunity. On the other hand, xid mice with a BALB/c background were resistant to infection with parasites, such as Trypanosoma cruzi (10) and Leishmania major (11), by responding with augmented IFN-γ responses (12). Potentially related to the Th1 skewing in these parasite-infected xid mice, XLA patients are reported to frequently develop Th1-related diseases, such as rheumatoid arthritis or type 1 diabetes mellitus (13, 14).
In this study, we present evidence that Btk plays a negative regulatory role in the maturation and T cell-stimulatory function of DCs. Consistent with the negative regulatory roles in these antigen-presenting cells (APCs), enhanced inflammation was observed in Th1- and Th2-dominant immune responses in Btk-deficient mice. Mechanistically, these roles for Btk in DCs appears to be mediated, at least, in part, by autocrine secretion of IL-10 and subsequent activation of Stat3, the transcription factor critical for immune tolerance. Therefore, our results demonstrate a previously unappreciated role for Btk in DCs.
Results
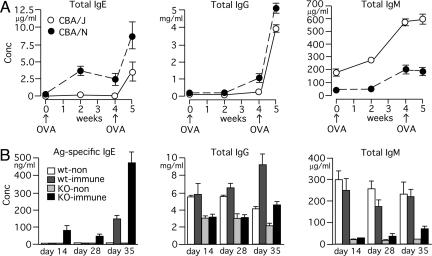
Increased IgE Responses and Exaggerated Airway Inflammation in Btk-Deficient Mice. We investigated IgE responses in xid and btk–/– mice by using different immunization conditions. First, we measured total serum IgE concentrations after immunizing CBA/J (WT) and CBA/N(xid) mice by i.p. injection of ovalbumin (OVA) in alum (Fig. 1A). Both primary and secondary IgE responses were significantly higher in xid mice. By contrast, IgM levels were severalfold lower in xid mice at all of the time points tested. Second, when mice were immunized with DNP-Asc in alum, btk–/– mice had higher serum levels of antigen-specific (antidinitrophenyl) and total IgE measured compared with WT mice (Fig. 1B and data not shown). Third, we induced airway inflammation by a standard OVA immunization/OVA aerosol inhalation method (15): Mice were i.p. immunized with OVA in alum twice (days 0 and 12) and exposed to 1% OVA or saline aerosol three times (days 22, 26, and 30) before serum collection on day 31. Both total and OVA-specific IgE levels were higher in saline- and OVA-challenged btk–/– mice compared with identically treated WT mice (Fig. 7A, which is published as supporting information on the PNAS web site). IgG1 levels were also higher in btk–/– mice. Therefore, these data indicate that increased IgE responses in xid and btk–/– mice can be ascribed to the lack of functional Btk protein but not the presence of the mutant Btk protein (R28C mutation) in xid mice.
Fig. 1.
High-serum IgE responses in btk mutant mice. (A) CBA/J(WT) and CBA/N(xid) mice were immunized with i.p. injection of 1 μg of OVA mixed with 1 mg of alum at week 0 and week 4. Sera were collected at the indicated times, and immunoglobulins were measured. Each value represents the mean ± SEM of 3–5 mice. Similar results are reproduced in another experiment. (B) WT and btk–/– mice were left unimmunized (n = 3 each) or immunized (n = 6 each) by i.p. injection of 10 μg of DNP-Asc mixed with 1 mg of alum on day 0 and day 28. Sera were collected on days 14, 28, and 35. Each value represents the mean ± SEM. Similar results are reproduced in another experiment.
In the airway inflammation experiments, airway hyperresponsiveness, measured on day 31 before serum collection and bronchoalveolar lavage (BAL) procedures, was slightly higher in btk–/– mice (data not shown). Remarkably, clear-cut differences were observed in inflammatory cells in BAL fluids: Total cell numbers and numbers of eosinophils and lymphocytes in the BAL fluids from OVA-challenged btk–/– mice were greater than those from OVA-challenged WT mice (Fig. 7B). Consistent with the increases in inflammatory cells in the BAL fluids from btk–/– mice, BAL fluids from these mice contained increased levels of IL-4, IL-5, and IL-13 (Fig. 7C). Interestingly, btk–/– BAL fluids also had higher levels of IFN-γ, in line with the increased IgG2a levels in these mice (Fig. 7A). Consistent with the increased inflammatory cells in btk–/– BAL fluids, infiltration of inflammatory cells in the lung was also more pronounced in the mutant mice (data not shown). These data indicate that the lack of Btk expression results in the increased airway inflammation.
Increased Contact Sensitivity in Btk-Deficient Mice. Given the increased Th1 cytokine in BAL fluids (Fig. 7C) and the increased IgG2a (which is IFN-γ-dependent) level in the btk–/– mice (Fig. 7A), we investigated the effects of Btk deficiency on a Th1-dominant immune response, the acute phase of contact sensitivity. Edematous inflammation was induced by epicutaneous challenge of the ear with dinitrofluorobenzene (DNFB) after T cell priming by epicutaneous administration of DNFB on the abdominal skin. We observed significantly greater ear thicknesses in DNFB-sensitized/DNFB-challenged btk–/– mice than in identically treated WT mice (Fig. 8A, which is published as supporting information on the PNAS web site). We also observed 2-fold higher relative IFN-γ/β-actin mRNA ratios in the ear and cervical lymph nodes in DNFB-sensitized/DNFB-challenged btk–/– mice than in similarly treated WT mice (Fig. 8B), confirming an enhanced Th1 skewing in btk–/– mice. Together with the increased Th2-driven IgE responses and airway inflammation, these results indicate that Btk deficiency leads to an enhancement in both Th1- and Th2-dominant immune responses.
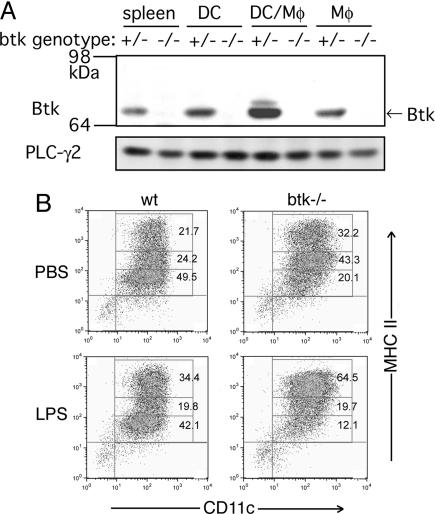
Btk Expression and Function in Dendritic Cells. Early studies suggested that the increased IgE response in xid mice is due to Btk's function extrinsic to B cells (16). Given that T cells do not express Btk (5), this observation implies that a cell type(s) other than B or T cells should contribute to the abnormal regulation of Th development and/or function in btk mutant mice. Consistent with this notion, splenic CD4+ T cells from btk–/– and WT mice proliferated and produced IL-2 in vitro similarly in response to stimulation with anti-CD3 or anti-CD3/anti-CD28 (data not shown). We next tested the possibility that APCs in btk–/– mice might have a stronger ability to stimulate T cells. However, no significant differences were observed in T cell-stimulatory ability of unfractionated splenocytes between WT and btk–/– mice (data not shown). Btk is expressed in APCs such as B cells and macrophages. However, there has been no data on whether Btk is expressed in mouse DCs, the central APCs for determining Th development and function. To address this issue, we cultured bone marrow cells in granulocyte/ macrophage colony-stimulating factor (GM-CSF) to generate CD11c+ dendritic cells (BMDCs). Immunoblotting of highly purified DCs (>95% CD11c+ cells, see Fig. 2B) clearly demonstrated that a good amount of Btk is expressed in BMDCs (Fig. 2A) and splenic CD11c+ cells (data not shown).
Fig. 2.
Btk expression and surface expression of MHC II in WT and btk–/– BMDCs. (A) Bone marrow cells were incubated in recombinant mouse GM-CSF-containing medium for 7 days. More than 95%-pure CD11c+ BMDCs (DC) were used. Adherent cells (Mφ) after 7 days in culture and a mixed population of adherent and nonadherent cells (DC/Mφ) after 3 days in culture were also analyzed together with another control, unfractionated spleen cells (spleen). Cell lysates were analyzed by immunoblotting. The 64- to 98-kDa portion of the blot was probed with anti-Btk antibody, and the higher Mr portion with anti-PLC-γ2 antibody. (B) BMDCs were incubated with PBS or 10 ng/ml LPS for 18 h. The cells were stained with FITC-conjugated anti-CD11c and PE-conjugated anti-I-A/I-E (M5/114.15.2) mAbs before flow cytometric analysis. Percentages of cell populations in each box are shown. The MHC phenotypes shown are representative of at least 48 independent BMDC cultures.
Given the critical role for Btk in the differentiation and/or activation of B and myeloid cells (4), we next investigated whether the differentiation of DCs is under the control of Btk. To this end, BMDCs were incubated with Escherichia coli lipopolysaccharide (LPS) for 18 h before flow cytometric analysis of surface expression of MHC class II, B7, and B7-2 molecules, the hallmarks of DC maturation and function (17). Control (PBS) treatment yielded a slightly more abundant population of MHC IIhigh mature DCs from btk–/– mice than from WT mice (Fig. 2B). As expected, LPS induced an expansion of the MHC IIhigh population in the cells of both genotypes. Importantly, LPS induced this population more vigorously in btk–/– BMDCs than in WT BMDCs (Fig. 2B). LPS also induced the up-regulation of B7-2 (CD86) and B7 (CD80) costimulatory molecules more vigorously in btk–/– BMDCs (Fig. 9, which is published as supporting information on the PNAS web site). Therefore, these results suggest that Btk plays a negative regulatory role in LPS-induced DC maturation.
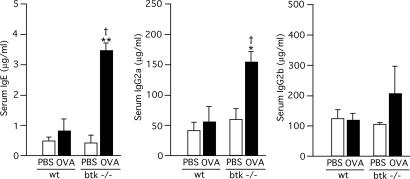
Increased IgE Responses in Mice Adoptively Transferred with Btk-Deficient DCs. Given the more mature phenotype in LPS-stimulated btk–/– DCs, we hypothesized that Btk-deficient DCs contribute to the increased IgE responses in btk mutant mice. To test this possibility directly, WT and btk–/– BMDCs were incubated with OVA or PBS, and the BMDCs were adoptively transferred to B6 mice. Serum Ig levels were measured 6 days later. More than 4-fold higher levels of IgE were observed in mice that had received OVA-loaded btk–/– BMDCs than in mice that had received OVA-loaded WT BMDCs or PBS-incubated btk–/– BMDCs (Fig. 3). IgG2a levels were also increased in mice that had received OVA-loaded btk–/– BMDCs than in the other cohorts, whereas IgG2b levels were not significantly different among the experimental groups. Control transfer experiments by using fluorescently labeled BMDCs indicated that i.v. injected btk–/– BMDCs reached the spleen slightly less efficiently (albeit statistically insignificant) than WT BMDCs (data not shown), indicating that the increased IgE and IgG2a responses in mice that had received btk–/– BMDCs are not due to increased migratory activity of these cells to the spleen and other lymphoid organs. These results suggest that Btk-deficient DCs have higher potency to stimulate Th2-dependent IgE responses and Th1-dependent IgG2a responses than WT DCs.
Fig. 3.
Increased production of IgE and IgG2a in mice adoptively transferred with Btk-deficient dendritic cells. WT and btk–/– BMDCs were incubated with 100 μg/ml OVA or PBS for 3 h, and the BMDCs (3 × 105 cells) were adoptively transferred to B6 mice. Total IgE, IgG2a, and IgG2b levels in sera were measured 7 days later. Data represents the mean ± SEM and analyzed by two-way ANOVA. *, P < 0.05, and **, P < 0.005 (vs. the PBS control of the same genotype); †, P < 0.05 (vs. the WT control). Similar results are reproduced in three independent experiments.
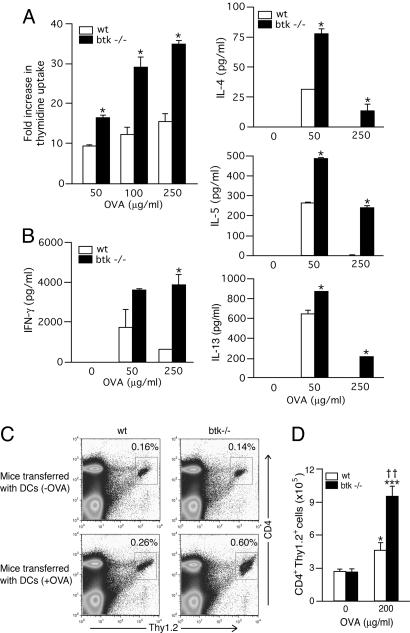
Increased T Cell-Stimulatory Function of Btk-Deficient DCs in Vitro and in Vivo. Given the increased ability of btk–/– DCs to induce IgE and IgG2a responses, we directly tested whether Btk deficiency affects T cell-stimulatory function of DCs. For this purpose, we used MHC class II (I-Ab)-restricted OVA-specific T cells from OTII TCR transgenic mice (18). Naïve OTII T cells were stimulated with WT or btk–/– splenic CD11c+ DCs (I-Ab) in the presence of OVA for 3–4 days, and T cell proliferation and cytokine production were analyzed. T cell proliferation induced by 50–250 μg/ml of OVA was ≈2-fold stronger in the presence of btk–/– DCs than in the presence of WT DCs (Fig. 4A). T cell proliferation was antigen-dependent and not a result of potential minor genetic differences between OTII mice and btk–/– mice, because no significant thymidine uptake was seen in the absence of OVA (data not shown). Production of both Th1 (IFN-γ) and Th2 (IL-4, IL-5, and IL-13) cytokines was antigen-dependent and more abundant in cultures with btk–/– DCs than in those with WT DCs (Fig. 4B). Therefore, these results indicate that Btk deficiency in DCs enhances their T cell-stimulatory function in vitro.
Fig. 4.
Increased in vitro and in vivo T cell stimulatory activity by btk–/– DCs. (A and B) OVA-specific naïve OTII T cells were stimulated with WT or btk–/– splenic CD11c+ cells in the presence of various concentrations of OVA for 3–4 days. (A) T cell proliferation was measured by incubating the cells with [3H]thymidine for the last 12 h of the 3-day cultures. Each value represents the mean ± SD. (B) Cytokines secreted into culture media for 4 days were measured by ELISA (mean ± SD). *, P < 0.05 (vs. the WT control; Student's t test). Similar results are reproduced four independent experiments. (C and D) OTII CD4+ (Thy1.2) cells were i.v. injected into Thy1.1 congenic B6 mice. Splenic DCs from WT and btk–/– mice were stimulated with LPS in the presence or absence of 200 μg/ml OVA for 16 h and i.v. injected into the mice 24 h after OTII CD4+ cell injection. The mice were killed 5 days later, and Thy1.2+ CD4+ T cells were enumerated by flow cytometry. Each value represents the mean ± SEM. *, P < 0.05, and ***, P < 0.0001 (vs. the DC [-OVA] control of the same genotype; two-way ANOVA); ††, P < 0.005 (vs. the WT control). Similar results are reproduced in two more independent experiments.
We next tested whether T cells receive stronger stimulation from btk–/– DCs than from WT DCs in vivo. To this end, we performed two kinds of adoptive T cell transfer experiments. First, OTII CD4+ (Thy1.2) T cells were i.v. injected to Thy1.1 congenic B6 mice. Twenty-four hours later, these mice were injected with splenic DCs from WT or btk–/– mice that had been stimulated with LPS in the presence or absence of OVA. The mice were killed 5 days after the DC injection and splenic T cells were purified. Under the conditions used, Thy1.2+ CD4+ T cell population expanded more robustly in the mice that had received OVA-loaded btk–/– DCs (Fig. 4 C and D). The proliferation of these T cells was antigen-specific, because the numbers of Thy1.2+ CD4+ cells were equivalent between the mice transferred with WT DCs and btk–/– DCs that had been incubated without OVA.
Second, 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester-labeled OTII T cells were adoptively transferred into WT and btk–/– mice, and the mice were challenged daily with OVA inhalation for three consecutive days. Proliferation of OTII T cells in lung draining lymph nodes and spleens was monitored 1 day after the last antigen challenge. OTII T cells exhibited significantly more progressive cell divisions in the draining lymph nodes of btk–/– mice than in those of WT mice (data not shown), whereas no differences in OTII T cell divisions were seen in an irrelevant organ, spleen (data not shown). Therefore, these results demonstrate that btk–/– DCs have stronger in vivo T cell-stimulatory activity than WT DCs.
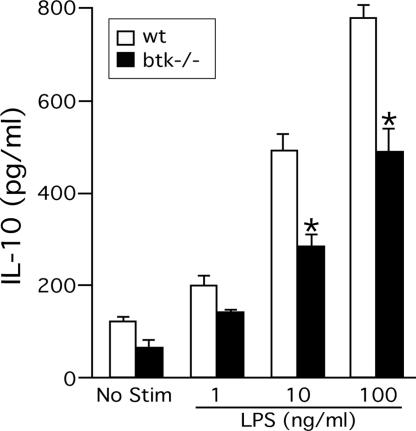
Btk Regulates IL-10 Production/Secretion in DCs. There could be potentially multiple cellular mechanisms for the increased T cell-stimulatory function of btk–/– DCs. However, uptake of fluorescently labeled OVA was not different between WT and btk–/– cells (data not shown). Furthermore, btk–/– BMDCs exhibited rather shorter survival upon GM-CSF depletion (data not shown), and bone marrow cells from btk–/– mice yielded smaller numbers (≈70% of WT mice) of BMDCs in GM-CSF than those from WT mice. Importantly, we found that secretion of IL-10 was substantially (40–50%) reduced in btk–/– BMDCs upon LPS stimulation (Fig. 5), whereas secretion of IL-6, IL-12, or TNF-α was comparable in WT and btk–/– cells (Fig. 10, which is published as supporting information on the PNAS web site). Reduced secretion of IL-10, an immunosuppressive cytokine, is consistent with the capacity of IL-10 to block maturation and function of DCs (19–21). These results indicate that Btk is required for maximal production of IL-10 in LPS-stimulated mouse DCs.
Fig. 5.
Reduced IL-10 production in btk–/– DCs. BMDCs were incubated with PBS or 1–100 ng/ml LPS for 24 h. IL-10 secreted into culture media was measured by ELISA. No detectable IL-4 was produced (data not shown). *, P < 0.05 (vs. the WT control; Student's t test). Results (mean ± SEM) shown are representative of five independent experiments.
Immunosuppressive roles for IL-10 have been extensively documented, including those in airway inflammation (22) and contact sensitivity (23). The biological significance of the reduced IL-10 production by btk–/– DCs was assessed. First, OTII T cells were stimulated with WT or btk–/– BMDCs in the presence of OVA with or without anti-IL-10 mAb. Blockade of IL-10 increased T cell proliferation induced by either WT or btk–/– BMDCs in an antigen-specific manner (Fig. 11A, which is published as supporting information on the PNAS web site). Importantly, IL-10 blockade increased T cell proliferation induced by WT BMDCs to levels similar to those induced by btk–/– BMDCs without IL-10 blockade. Second, adoptive transfer of BMDCs to B6 mice was performed with anti-IL-10 mAb or control mAb injections. Injection of anti-IL-10 mAb increased serum IgE levels in mice that had received WT, but not btk–/–, DCs (Fig. 11B). These results demonstrate that IL-10 produced by DCs is involved in suppression of in vitro T cell proliferation and that in vivo IgE responses are under the control of IL-10-mediated immunosuppression.
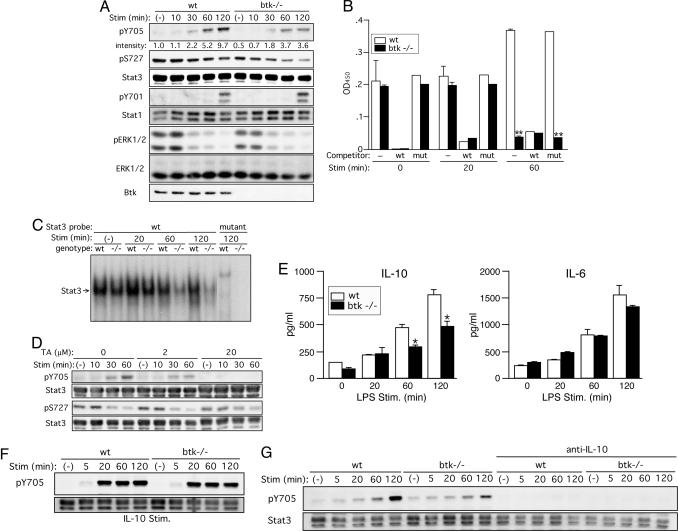
Btk Regulates Stat3 Activity in DCs in an IL-10-Dependent Manner. Stat3 signaling in APCs plays a critical role in antigen-specific tolerance (24–26). Stat3 is activated by overexpressed Bmx, a Tec family protein-tyrosine kinase, in mammalian cells (27). Furthermore, Stat3 is largely responsible for mediating IL-10 production by LPS (28). Our data, together with these studies, raised the possibility that Btk might be required for Stat3 activation to negatively regulate the immunostimulatory function of DCs. To test it, we examined phosphorylation of Stat3 in LPS-stimulated BMDCs. Immunoblotting analysis indicated that LPS induces robust phosphorylation at Tyr-705 of Stat3 in WT cells in a time-dependent manner. Importantly, this phosphorylation was substantially [78.3 ± 3.27% (n = 5) at 120 min] reduced in similarly stimulated btk–/– DCs (Fig. 6A). However, Ser-727 phosphorylation, which was constitutive and gradually decreased upon LPS stimulation, was variably, but less severely, affected in btk–/– DCs. Tyr-705 phosphorylation induces Stat3 dimerization, nuclear translocation, and DNA binding (29, 30). Consistent with the reduced Tyr-705 phosphorylation in btk–/– DCs, the DNA-binding activity of Stat3, as evaluated by ELISA-based assays (Fig. 6B) and electrophoretic mobility shift assays (Fig. 6C), was drastically reduced in these cells at 60–120 min after LPS stimulation. We next tested effects of terreic acid, a Btk/Tec inhibitor (31), on Stat3 phosphorylation in LPS-stimulated WT DCs. LPS-induced phosphorylation at Tyr-705, but not Ser-727, was inhibited by terreic acid in a dose-dependent manner (Fig. 6D). These results demonstrate that Btk is required for Stat3 activation in DCs. By contrast, phosphorylation of Stat1, ERK, and p38 was hardly affected by Btk deficiency (Fig. 6A and data not shown). Parenthetically, DNA-binding activity of p65 NF-κB was not reduced in LPS-stimulated btk–/– DCs (data not shown), although Btk is known to regulate NF-κB activity in B cells (32, 33).
Fig. 6.
Btk positively regulates Stat3 activity mainly through autocrine secretion of IL-10. (A) WT and btk–/– BMDCs were left unstimulated or stimulated with 1 μg/ml LPS for the indicated periods. The cells were lysed and analyzed by immunoblotting with anti-phospho-Stat3(Tyr-705), anti-phosho-Stat3(Ser-727), anti-phospho-Stat1(Tyr-701), anti-phospho-MAPK(Thr-202/Tyr-204), or anti-Btk. The blots were reprobed with anti-Stat3, Stat1, and anti-ERK to check for their expression. Results shown are representative of seven (Stat3) or three (others) experiments. (B) DNA-binding activities of Stat3 in BMDCs stimulated by LPS for the indicated periods were measured by ELISA-based binding assays. Specificity of Stat3 binding to the plate-bound Stat3 consensus oligonucleotide was confirmed by strong competition by free WT, but not mutant, oligonucleotide. **, P < 0.005 (vs. the WT cells; Student's t test). Similar results (mean ± SD) are reproduced in three independent experiments. (C) EMSAs were also performed by using 32P-labeled WT or mutant oligonucleotide as a probe. Position of Stat3/DNA complexes is indicated. Similar results are reproduced in three independent experiments. (D) WT BMDCs were preincubated with the indicated concentrations of terreic acid (TA) for 30 min before stimulation with LPS for the indicated periods. The cells were analyzed by immunoblotting with anti-phospho-Stat3(Tyr-705) or anti-phospho-Stat3(Ser-727). The blots were reprobed with anti-Stat3. The results shown were reproduced in two additional experiments. (E) Wt and btk–/– BMDCs were left unstimulated or stimulated with 100 ng/ml LPS for the indicated periods. Amounts of IL-10 and IL-6 secreted into medium were measured by ELISA. *, P < 0.05 (vs. the WT cells; Student's t test). Similar results (mean ± SEM) were reproduced in another experiment. (F) WT and btk–/– BMDCs were left unstimulated or stimulated with 20 ng/ml mouse recombinant IL-10 for the indicated periods. (G) WT and btk–/– BMDCs, pretreated with a neutralizing anti-IL-10 mAb for 60 min, were left unstimulated or stimulated with 1 μg/ml LPS for the indicated periods. Cell lysates were analyzed by immunoblotting for Tyr-705 phosphorylation followed by reprobing with anti-Stat3. Results shown in F and G are representative of two independent experiments.
Given the reduced IL-10 production in LPS-stimulated btk–/– DCs (Fig. 5) and that IL-10 can induce Stat3 activation (34), we tested whether the reduced Stat3 activity in LPS-stimulated btk–/– DCs is a direct consequence of an impaired response to LPS or the reduced IL-10 secretion or a combination of both. First, we measured IL-10 production at early time points after LPS stimulation. IL-10 secretion began to increase within 60 min of stimulation in both WT and btk–/– cells, and it was reduced in btk–/– DCs (Fig. 6E). By contrast, IL-6 secretion was similar at these early time points. Second, IL-10 stimulation induced Tyr-705 phosphorylation in faster kinetics compared with that induced by LPS (Fig. 6F). Tyr-705 phosphorylation induced by IL-10 was only slightly reduced in btk–/– DCs compared with WT DCs. Importantly, pretreatment with an anti-IL-10 mAb, which abrogated IL-10-induced Tyr-705 phosphorylation (data not shown), almost abrogated Tyr-705 phosphorylation in LPS-stimulated WT and btk–/– DCs (Fig. 6G). By contrast, an anti-IL-6 mAb, which abrogated IL-6-induced Tyr-705 phosphorylation, only slightly affected Tyr-705 phosphorylation in LPS-stimulated DCs (data not shown). These results indicate that LPS-induced Tyr-705 phosphorylation, thus Stat3 activation, is mediated mainly through IL-10 secretion, although other cytokines (e.g., IL-6 and IL-12) secreted from LPS-stimulated DCs can also induce Stat3 phosphorylation. Therefore, we conclude that Btk deficiency in DCs impairs LPS-induced Stat3 activation mainly by a defect in autocrine secretion of IL-10.
Discussion
Our data indicate that Btk cannot directly phosphorylate Stat3 (data not shown). The present and previous studies collectively suggest that a Btk-dependent signaling pathway leads to IL-10 secretion and subsequent activation of Stat3 and operates to negatively regulate the immunostimulatory function of DCs. In support of this model and extending upstream of this pathway, recent studies have shown that LPS stimulation activates Btk in human monocytes (35) and Btk can interact with the LPS receptor, TLR4, and several downstream signaling proteins of TLR4 in yeast two hybrid and overexpression systems (36). Thus, it is interesting to further study how Btk mediates IL-10 production.
Contrary to our present study, a recent study showed that monocyte-derived DCs from XLA patients behave similarly as those from healthy donors with respect to differentiation, maturation, and antigen presentation in mixed lymphocyte reactions (37). However, this and our present studies cannot be compared because the two studies may be dealing with different DC populations, particularly due to the fact that human DCs are much more difficult to study for their genetic heterogeneity. Furthermore, limitations on functional tests on human DCs might have hindered the researchers from uncovering differences between XLA and healthy subjects. Alternatively, this discrepancy could mean another species-specific difference in Btk function.
In conclusion, we demonstrate that Btk plays a negative regulatory role in LPS-dependent maturation in mouse DCs and negatively regulates their T cell-stimulatory functions. These Btk-dependent negative regulations appear to be mediated through autocrine secretion of IL-10 and subsequent activation of Stat3. We suggest that the enhanced T cell proliferation and Th2 cytokine production promoted by Btk-deficient DCs contribute to the exaggerated Th2-dependent immune reactions, e.g., airway inflammation and IgE responses, and that the increased Th1 cytokine production stimulated by Btk-deficient DCs contribute to an increased Th1-driven contact sensitivity. These observations not only shed insights into a long-standing enigma of high IgE responses in btk mutant mice but also imply potential application of Btk-inhibiting and -activating maneuvers of DC functions in various clinical settings.
Materials and Methods
Antibody Responses. Mice were immunized with i.p. injection of 1 μg ovalbumin mixed with 1 μg of alum or 10 μg of DNP-Asc (Ascaris suum extract conjugated with dinitrophenyl) mixed with 1 μg of alum at the indicated time points. Serum Ig levels were measured by ELISA with commercial kits (BD Biosciences Pharmingen). Antigen-specific antibodies were measured as described in ref. 38. Briefly, samples were incubated in microplate wells coated with anti-Ig antibodies. Biotinylated antigen (DNP-BSA or OVA) was added, and then peroxidase-conjugated streptavidin was added. Finally, enzyme reaction was performed.
Preparation of BMDCs, Macrophages, and Splenocytes. Mouse femur was flushed aseptically with RPMI 1460 medium. Bone marrow cells recovered were incubated in recombinant mouse GM-CSF-containing medium for 7–10 days (39). Nonadherent cells were further purified by anti-CD11c-coated MACS beads (Miltenyi Biotec, Auburn, CA). The cells thus cultured were >95% CD11c+ by flow cytometry (BMDCs). Adherent cells, i.e., macrophages, after 10 days in culture were also used in some experiments. Splenic DCs were prepared from collagenase D-digested spleens and purified by using anti-CD11c-coated MACS beads.
T Cell Stimulation. MHC class II (I-Ab)-restricted OVA-specific naïve T cells from OTII TCR transgenic mice were stimulated with WT or btk–/– splenic CD11c+ cells (I-Ab) in the presence of various concentrations of OVA for 3–4 days. T cell proliferation was measured by incubating the cells with [3H]thymidine (ICN, Irvine, CA) for the last 12 h of the 3-day cultures. Cytokines secreted into culture media for 4 days were measured by ELISA with commercial kits (BD Biosciences Pharmingen).
Immunoblotting. DCs were lysed in 1% Nonidet P-40-containing lysis buffer. Analysis of cell lysates by SDS/PAGE and blotting were performed as described in ref. 40.
Detailed information on reagents and other experimental procedures are given in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. Statistical analysis was performed by Student's t test, Mann–Whitney test, or two-way ANOVA by using a prism 4 software (GraphPad, San Diego).
Supplementary Material
Acknowledgments
We thank Shaun Chung, Beth Halteman and Ed Lemmens for technical assistance; Kirin Brewery for providing GM-CSF; and our colleagues at the La Jolla Institute for Allergy and Immunology for their insightful suggestions during the course of this study. This work was supported partly by National Institutes of Health (NIH) Grants AI61796, AI50209, and AI38348 (to T.K.). Yuko Kawakami was supported partly by NIH Grant AI51125. This work is Publication 670 from the La Jolla Institute for Allergy and Immunology.
Author contributions: Yuko Kawakami, M.C., and T.K. designed research; Yuko Kawakami, N.I., S.S.-A., J.K., H.T., K.N., Yu Kawakami, and W.X. performed research; Yuko Kawakami, N.I., S.S.-A., H.N., M.C., and T.K. analyzed data; and T.K. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: APC, antigen-presenting cell; BAL, bronchoalveolar lavage; BMDC, CD11c+ dendritic cells; DC, dendritic cell; DNFB, dinitrofluorobenzene; GM-CSF, granulocyte/macrophage colony-stimulating factor; LPS, lipopolysaccharide; OVA, ovalbumin; Th, T helper cell type; xid, X-linked immunodeficiency; XLA, X-linked agammaglobulinemia.
References
- 1.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 2.Pulendran, B., Smith, J. L., Caspary, G., Brasel, K., Pettit, D., Maraskovsky, E. & Maliszewski, C. R. (1999) Proc. Natl. Acad. Sci. USA 96, 1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldonado-Lopez, R., De Smedt, T., Michel, P., Godfroid, J., Pajak, B., Heirman, C., Thielemans, K., Leo, O., Urbain, J. & Moser, M. (1999) J. Exp. Med. 189, 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fruman, D. A., Satterthwaite, A. B. & Witte, O. N. (2000) Immunity 13, 1–3. [DOI] [PubMed] [Google Scholar]
- 5.Smith, C. I., Baskin, B., Humire-Greiff, P., Zhou, J. N., Olsson, P. G., Maniar, H. S., Kjellen, P., Lambris, J. D., Christensson, B., Hammarstrom, L., et al. (1994) J. Immunol. 152, 557–565. [PubMed] [Google Scholar]
- 6.Kishimoto, T., Shigemoto, S., Watanabe, T. & Yamamura, Y. (1979) J. Immunol. 123, 1039–1043. [PubMed] [Google Scholar]
- 7.Clough, E. R., Levy, D. A. & Cebra, J. J. (1981) J. Immunol. 126, 387–389. [PubMed] [Google Scholar]
- 8.Lebrun, P., Sidman, C. L. & Spiegelberg, H. L. (1988) J. Immunol. 141, 249–257. [PubMed] [Google Scholar]
- 9.Gaubert, S., Viana da Costa, A., Maurage, C. A., Lima, E. C., Fontaine, J., Lafitte, S., Minoprio, P., Capron, A. & Grzych, J. M. (1999) Parasite Immunol. 21, 89–101. [DOI] [PubMed] [Google Scholar]
- 10.Minoprio, P., Coutinho, A., Spinella, S. & Hontebeyrie-Joskowicz, M. (1991) Int. Immunol. 3, 427–433. [DOI] [PubMed] [Google Scholar]
- 11.Hoerauf, A., Solbach, W., Lohoff, M. & Rollinghoff, M. (1994) Int. Immunol. 6, 1117–1124. [DOI] [PubMed] [Google Scholar]
- 12.Minoprio, P., el Cheikh, M. C., Murphy, E., Hontebeyrie-Joskowicz, M., Coffman, R., Coutinho, A. & O'Garra, A. (1993) J. Immunol. 151, 4200–4208. [PubMed] [Google Scholar]
- 13.Timmers, E., de Weers, M., Alt, F. W., Hendriks, R. W. & Schuurman, R. K. (1991) Clin. Immunol. Immunopathol. 61, S83–93. [DOI] [PubMed] [Google Scholar]
- 14.Rosen, F. S., Cooper, M. D. & Wedgwood, R. J. (1995) N. Engl. J. Med. 333, 431–440. [DOI] [PubMed] [Google Scholar]
- 15.Nagai, H., Yamaguchi, S., Inagaki, N., Tsuruoka, N., Hitoshi, Y. & Takatsu, K. (1993) Life Sci. 53, PL243–PL247. [DOI] [PubMed] [Google Scholar]
- 16.Teale, J. M. (1983) J. Immunol. 130, 72–77. [PubMed] [Google Scholar]
- 17.De Smedt, T., Pajak, B., Muraille, E., Lespagnard, L., Heinen, E., De Baetselier, P., Urbain, J., Leo, O. & Moser, M. (1996) J. Exp. Med. 184, 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. (1998) Immunol. Cell Biol. 76, 34–40. [DOI] [PubMed] [Google Scholar]
- 19.Morel, A. S., Quaratino, S., Douek, D. C. & Londei, M. (1997) Eur. J. Immunol. 27, 26–34. [DOI] [PubMed] [Google Scholar]
- 20.De Smedt, T., Van Mechelen, M., De Becker, G., Urbain, J., Leo, O. & Moser, M. (1997) Eur. J. Immunol. 27, 1229–1235. [DOI] [PubMed] [Google Scholar]
- 21.Buelens, C., Verhasselt, V., De Groote, D., Thielemans, K., Goldman, M. & Willems, F. (1997) Eur. J. Immunol. 27, 1848–1852. [DOI] [PubMed] [Google Scholar]
- 22.Oh, J. W., Seroogy, C. M., Meyer, E. H., Akbari, O., Berry, G., Fathman, C. G., Dekruyff, R. H. & Umetsu, D. T. (2002) J. Allergy Clin. Immunol. 110, 460–468. [DOI] [PubMed] [Google Scholar]
- 23.Simkin, G. O., Tao, J. S., Levy, J. G. & Hunt, D. W. (2000) J. Immunol. 164, 2457–2462. [DOI] [PubMed] [Google Scholar]
- 24.Hackenmiller, R., Kim, J., Feldman, R. A. & Simon, M. C. (2000) Immunity 13, 397–407. [DOI] [PubMed] [Google Scholar]
- 25.Cheng, F., Wang, H. W., Cuenca, A., Huang, M., Ghansah, T., Brayer, J., Kerr, W. G., Takeda, K., Akira, S., Schoenberger, S. P., et al. (2003) Immunity 19, 425–436. [DOI] [PubMed] [Google Scholar]
- 26.Wang, T., Niu, G., Kortylewski, M., Burdelya, L., Shain, K., Zhang, S., Bhattacharya, R., Gabrilovich, D., Heller, R., Coppola, D., et al. (2004) Nat. Med. 10, 48–54. [DOI] [PubMed] [Google Scholar]
- 27.Saharinen, P., Ekman, N., Sarvas, K., Parker, P., Alitalo, K. & Silvennoinen, O. (1997) Blood 90, 4341–4353. [PubMed] [Google Scholar]
- 28.Benkhart, E. M., Siedlar, M., Wedel, A., Werner, T. & Ziegler-Heitbrock, H. W. (2000) J. Immunol. 165, 1612–1617. [DOI] [PubMed] [Google Scholar]
- 29.Darnell, J. E., Jr., Kerr, I. M. & Stark, G. R. (1994) Science 264, 1415–1421. [DOI] [PubMed] [Google Scholar]
- 30.Ihle, J. N. (1995) Nature 377, 591–594. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami, Y., Hartman, S. E., Kinoshita, E., Suzuki, H., Kitaura, J., Yao, L., Inagaki, N., Franco, A., Hata, D., Maeda-Yamamoto, M., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 2227–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petro, J. B., Rahman, S. M., Ballard, D. W. & Khan, W. N. (2000) J. Exp. Med. 191, 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajpai, U. D., Zhang, K., Teutsch, M., Sen, R. & Wortis, H. H. (2000) J. Exp. Med. 191, 1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finbloom, D. S. & Winestock, K. D. (1995) J. Immunol. 155, 1079–1090. [PubMed] [Google Scholar]
- 35.Horwood, N. J., Mahon, T., McDaid, J. P., Campbell, J., Mano, H., Brennan, F. M., Webster, D. & Foxwell, B. M. (2003) J. Exp. Med. 197, 1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jefferies, C. A., Doyle, S., Brunner, C., Dunne, A., Brint, E., Wietek, C., Walch, E., Wirth, T. & O'Neill, L. A. (2003) J. Biol. Chem. 278, 26258–26264. [DOI] [PubMed] [Google Scholar]
- 37.Gagliardi, M. C., Finocchi, A., Orlandi, P., Cursi, L., Cancrini, C., Moschese, V., Miyawaki, T. & Rossi, P. (2003) Clin. Exp. Immunol. 133, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirano, T., Yamakawa, N., Miyajima, H., Maeda, K., Takai, S., Ueda, A., Taniguchi, O., Hashimoto, H., Hirose, S., Okumura, K., et al. (1989) J. Immunol. Methods 119, 145–150. [DOI] [PubMed] [Google Scholar]
- 39.Lutz, M. B., Kukutsch, N., Ogilvie, A. L., Rossner, S., Koch, F., Romani, N. & Schuler, G. (1999) J. Immunol. Methods 223, 77–92. [DOI] [PubMed] [Google Scholar]
- 40.Kitaura, J., Song, J., Tsai, M., Asai, K., Maeda-Yamamoto, M., Mocsai, A., Kawakami, Y., Liu, F. T., Lowell, C. A., Barisas, B. G., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 12911–12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.