Abstract
The Raf family includes three members, of which B-Raf is frequently mutated in melanoma and other tumors. We show that Raf-1 and A-Raf require Hsp90 for stability, whereas B-Raf does not. In contrast, mutated, activated B-Raf binds to an Hsp90–cdc37 complex, which is required for its stability and function. Exposure of melanoma cells and tumors to the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in the degradation of mutant B-Raf, inhibition of mitogen-activated protein kinase activation and cell proliferation, induction of apoptosis, and antitumor activity. These data suggest that activated mutated B-Raf proteins are incompetent for folding in the absence of Hsp90, thus suggesting that the chaperone is required for the clonal evolution of melanomas and other tumors that depend on this mutation. Hsp90 inhibition represents a therapeutic strategy for the treatment of melanoma.
Keywords: 17-allylamino-17-demethoxygeldanamycin, cdc37, melanoma
In the last several years, it has become clear that the Ras/Raf/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase kinase (MEK)/MAPK signaling pathway is mutationally activated in most melanomas. One member of the Ras family, N-Ras, is mutated in ≈25% of melanomas (1), whereas mutations in the H-ras and K-ras genes are rare (2). The Raf gene family (Raf-1, A-Raf, and B-Raf) encodes closely related serine/threonine protein kinases that are important effectors of Ras activation. However, no mutations in the Raf gene were found until recently, when Davies et al. (3) showed that Raf-1 and A-Raf are rarely mutated but that mutations in the B-Raf gene are common in human cancer, especially in melanoma.
Refs. 3 and 4 showed that B-Raf is mutated in ≈70% of human melanomas, 35–70% of papillary thyroid carcinomas, and less commonly in lung and colorectal carcinomas. Mutations are almost always in the B-Raf kinase domain and, in melanomas, the vast majority are V600E missense mutations (3). Marais and coworkers (5) have shown in heterologous systems that the V600E mutation leads to activation of B-Raf kinase.
The frequency and activating nature of the B-Raf mutations suggest that they have an important role in the biology of melanoma and perhaps other tumors in which they have been detected. Moreover, small interfering RNA against mutated B-Raf but not Raf-1 inhibits the transformed phenotype of melanoma cells harboring B-Raf mutations (6, 7). N-Ras and B-Raf mutations seem to be mutually exclusive in melanoma, suggesting that they make similar contributions to transformation and that activation of this pathway is a key event in the development of this disease (3, 8).
Together, these data suggest that inhibition of B-Raf/MEK/MAPK signaling could be a powerful means for treating melanomas and other tumors with B-Raf mutation. There is no validated therapy that potently inhibits mutated B-Raf function in patients. Selective inhibitors of MEK have been developed and have antitumor activity in xenograft models of melanoma (9). Putative Raf inhibitor is currently in trial but has low potency against Raf and inhibits multiple other kinases (10, 11).
The details of Raf regulation suggest another strategy for its inhibition. The protein chaperone Hsp90 is required for the conformational maturation of several key signaling proteins, including Raf-1 (12). Inhibition of Hsp90 function with natural products like geldanamycin that bind to its N terminus causes the ubiquitin-dependent degradation of Raf-1 in the proteasome (13). A geldanamycin derivative, 17-allylamino-17-demethoxygeldanamycin (17-AAG), effectively inhibits Hsp90 function in vivo at tolerable doses. Given the homology of the three members of the Raf kinase family, we reasoned that B-Raf is also likely to require Hsp90 function and that 17-AAG would induce its degradation and cause inhibition of melanoma growth. Surprisingly, we found that although Raf-1 and A-Raf are degraded in cells that are exposed to 17-AAG, WT B-Raf is not found in an Hsp90 complex and is unaffected by the inhibitor. However, mutationally activated B-Raf apparently acquires a dependence on Hsp90 for its stability; it is associated with Hsp90 and is selectively degraded in the proteasome in cells exposed to 17-AAG. Degradation of mutated B-Raf leads to MAPK inhibition, cell-cycle arrest, and apoptosis with concomitant antitumor activity in murine xenograft models.
Results
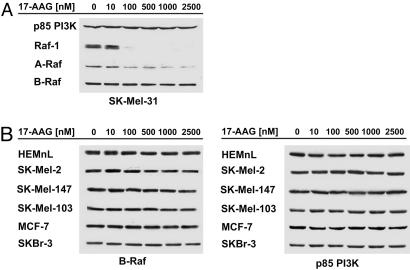
Pharmacologic Inhibition of Hsp90 Function Leads to a Decrease in the Expression of Raf-1 and A-Raf But Not B-Raf. Raf-1 (c-Raf) is a known Hsp90 client protein that binds and depends on Hsp90 chaperone function for its proper folding and stability (14). Hsp90 inhibitors such as 17-AAG disrupt the Raf1/Hsp90 association, resulting in degradation of Raf-1 via the proteasome (13). To determine whether A-Raf and B-Raf kinase are also Hsp90 client proteins, we examined the effects of 17-AAG on expression of each of the Raf family members in a panel of 16 human tumor cell lines, primarily melanomas.
As reported previously, we found that 100 nM 17-AAG causes >90% decline in Raf-1 expression levels in all tested cell lines after 24 h of treatment (Figs. 1A and 2A, and data not shown). A-Raf expression was lost with similar kinetics and sensitivity, suggesting that it is likely to be an Hsp90 client as well (Figs. 1A and 2A, and data not shown). In contrast, exposure of cells to up to 2.5 μM 17-AAG for 24 h had no significant effect on the expression of WT B-Raf in SK-Mel-31 melanoma cells or in other tumor cell lines expressing WT B-Raf (Fig. 1). Similarly, 17-AAG had no effect on expression of WT B-Raf in normal human epithelial melanocytes neonatal lightly pigmented (HEMnL) (Fig. 1B). These data suggest that B-Raf differs from A-Raf and Raf-1 in its requirement for Hsp90 for folding and stability and that WT B-Raf is unaffected by Hsp90 inhibition. In support of this idea, Hsp90 was not detected in immunoprecipitates of epitope-tagged WT B-Raf (see below and Fig. 5).
Fig. 1.
17-AAG selectively inhibits A-Raf and C-Raf but not WT B-Raf expression. (A) SK-Mel-31 cells (WT B-Raf) were treated with the indicated concentrations of 17-AAG for 24 h, and levels of A-Raf, B-Raf, C-Raf (Raf-1), and p85 PI3-kinase expression were determined by immunoblotting. 17-AAG caused a dose-dependent decline in A-Raf and C-Raf expression but had no effect on expression of WT B-Raf. (B)(Left) 17-AAG had no effect on the expression of WT B-Raf in a panel of melanoma, colon, and breast cancer cells. (Right) p85 PI3-kinase expression was assessed as a loading control.
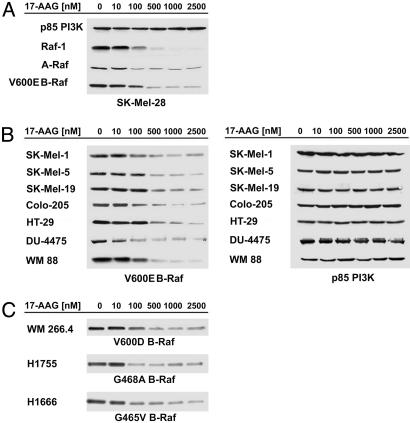
Fig. 2.
17-AAG inhibits expression of mutant B-Raf. (A) A 24-h treatment with 17-AAG caused a dose-dependent decline in A-Raf and C-Raf expression and also loss of V600E B-Raf expression in SK-Mel-28 cells. (B)(Left) The 24-h treatment with 17-AAG caused a dose-dependent down-regulation of V600E B-Raf protein levels in a panel of melanoma, colon, and breast cell lines. (Right) p85 PI3-kinase expression was assessed as a loading control. (C) A 24 h treatment with 17-AAG caused a dose-dependent down-regulation of V600D, G465V, and G468A B-Raf expression.
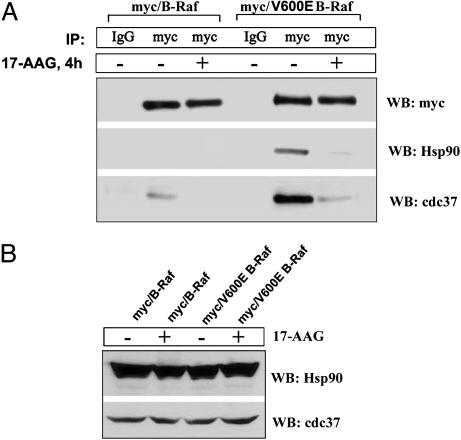
Fig. 5.
V600E B-Raf associates with Cdc37 and Hsp90. (A) Myc-tagged WT or V600E mutant B-Raf was transiently expressed in MCF-7 cells. At 24 h after transfection, cells were treated with 1 μM 17-AAG for 4 h. Lysates were assayed by IP with normal mouse IgG or 9E10 anti-myc Ab and immunoblotted for myc, Cdc37, and Hsp90. (B) 17-AAG had no effect on total expression levels of Cdc37 and Hsp90 at this time point. WB, Western blot analysis.
Activating Mutants of B-Raf Acquire Sensitivity to Hsp90 Inhibitors. Although most proteins do not require Hsp90 for stability, gain-of-function mutants, such as v-src, bcr-abl, mutant c-kit, and p53 oncogenes, may acquire dependence on Hsp90 for correct folding (15–19). In each case, the mutated oncoprotein binds to Hsp90 and is degraded in cells exposed to Hsp90 inhibitors, whereas the corresponding normal protooncogene product is much less sensitive to degradation by these drugs. This selective, mutation-dependent sensitivity to inhibition of Hsp90 turns out to be the case for mutationally activated B-Raf as well.
A V600E substitution in the activation segment of B-Raf kinase accounts for >90% of all B-Raf mutations that are found in human cancers (3). SK-Mel-28 and seven other cancer cell lines with this mutation were analyzed for B-Raf expression after 24 h of exposure to 17-AAG. In contrast to the effect on WT B-Raf, 17-AAG causes a dose-dependent loss of V600E B-Raf expression and a loss of A-Raf and Raf-1 expression in those cells (Fig. 2 A and B). Also, to determine whether the effect of 17-AAG on V600E B-Raf expression was limited to this particular B-Raf mutation, we analyzed the effect of 17-AAG in cell lines that harbor less common B-Raf mutations. We found that in WM-266.4/WM.115 melanoma cells, which express the V600D B-Raf mutated protein and in two non-small-cell lung cancer cell lines (H1666 and H1755) with mutations within the G-loop of B-Raf kinase, 17-AAG causes loss of B-Raf expression, with kinetics and sensitivity that are indistinguishable from that of V600E B-Raf (Fig. 2C).
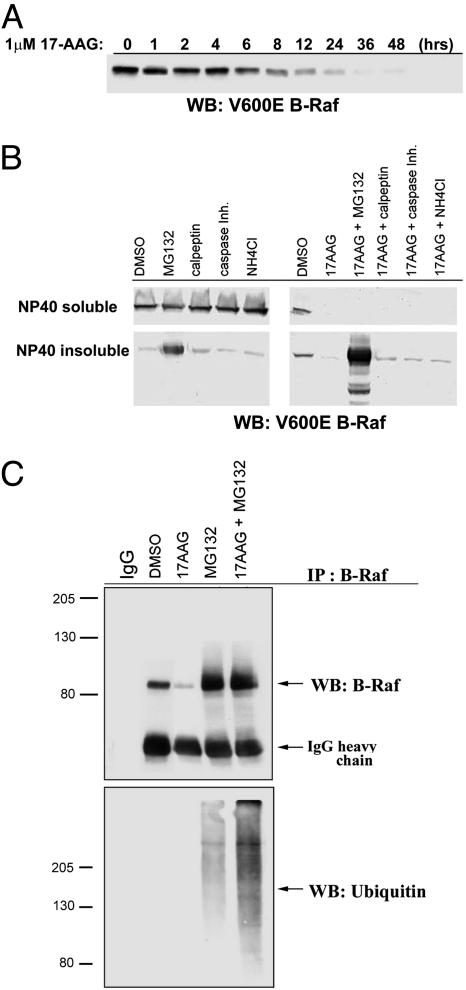
Initial loss of V600E B-Raf expression was observed as early as 12 h after exposure of SK-Mel-28 cells to 1 μM 17-AAG, whereas >90% inhibition occurred by 24 h and persisted up to 48 h later (Fig. 3A). The proteasome inhibitor MG132 prevented loss of V600E B-Raf expression, resulting in the partition into a Nonidet P-40-insoluble fraction (Fig. 3B). Lysosome, caspase, or calpain inhibitors had no effect on 17-AAG-induced loss of V600E B-Raf expression. Moreover, we found that the combination of proteasome inhibitor with 17-AAG resulted in the more pronounced formation of polyubiquitinated, higher-molecular-weight forms of V600E B-Raf, as compared with treatment of cells with the proteasome inhibitor alone (Fig. 3C). These findings are consistent with those obtained for other Hsp90 client proteins, such as HER2, AKT, and Raf-1, in which inhibition of Hsp90 leads to ubiquitination and trafficking of Hsp90 client protein to an Nonidet P-40-insoluble fraction of the proteasome where it gets degraded (13, 20, 21).
Fig. 3.
17-AAG induced ubiquitination and proteasome-mediated degradation of V600E B-Raf. (A) After SK-Mel-28 treatment with 1 μM 17-AAG, loss of V600E B-Raf expression was first observed as early as 12 h, whereas 90% inhibition was achieved by 24 h and persisted up to 48 h of treatment. (B) SK-Mel-28 cells were pretreated with 10 μM proteasome inhibitor (MG132), 30 μM calpeptin, 10 μM caspase inhibitor (Casp. Inhib.), and 20 mM ammonium chloride, followed by 24 h of exposure to 1 μM 17-AAG. Cells were lysed in Nonidet P-40 buffer, and the Nonidet P-40-insoluble fraction was solubilized in 2% SDS buffer, and B-Raf levels were then analyzed by Western blot analysis (WB). The proteasome inhibitor abrogated 17-AAG-induced loss of V600E B-Raf. The protected V600E B-Raf protein accumulated in a Nonidet P-40 insoluble fraction. (C) Total SK-Mel-28 SDS cell lysates assayed by IP with B-Raf and blotted for ubiquitin (UB) and B-Raf.
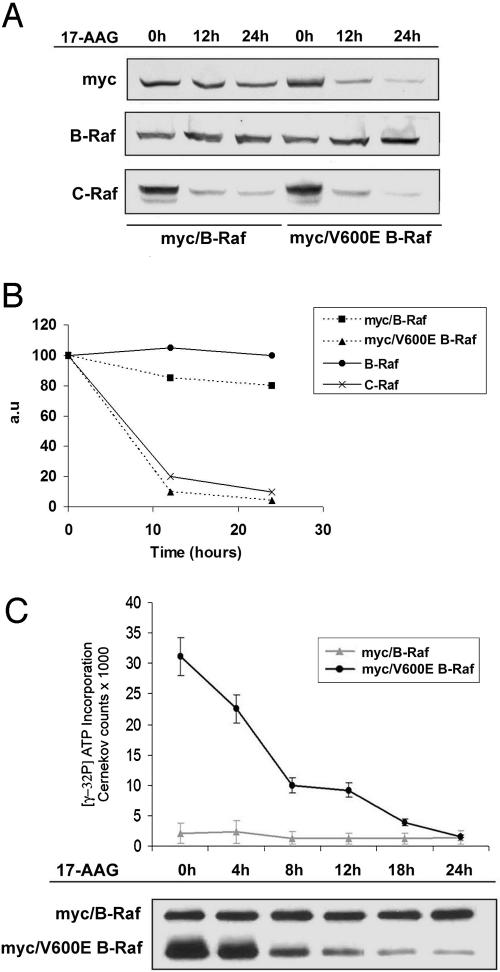
To determine whether the sensitivity of B-Raf to degradation is cell-context-dependent, MCF7 breast cancer cells were transiently transfected with myc-tagged, WT, or V600E B-Raf and subsequently exposed to 17-AAG (Fig. 4). Treatment with 1 μM 17-AAG had no effect on the expression of myc-tagged or endogenous WT B-Raf in these cells. However, 17-AAG did cause the loss of myc-tagged V600E B-Raf expression with similar kinetics and sensitivity as compared with endogenous mutated B-Raf in melanoma cells (Fig. 4 A and B). These data confirm differential sensitivity of B-Raf is a function of its mutational status. Moreover, 17-AAG caused significant inhibition of kinase activity of myc-tagged mutant B-Raf as revealed by B-Raf kinase cascade assay. The initial loss of mutant B-Raf kinase activity was observed as early as 4 h after 17-AAG treatment, whereas >70% (±8.3%) inhibition was achieved by 8 h, indicating that the loss of kinase activity of myc-tagged V600E B-Raf may occur before loss of its expression. After 24 h of exposure to 1 μM 17-AAG, a slight (31 ± 7.6%) decrease of myc-tagged WT B-Raf kinase activity was observed (Fig. 4C).
Fig. 4.
V600E B-Raf mutation confers sensitivity to 17-AAG-induced inhibition of kinase activity and protein expression. Myc-tagged WT or V600E mutant B-Raf was transiently expressed in MCF-7 cells. At 24 h after transfection, cells were treated with 1 μM 17-AAG for 12 or 24 h. (A) Expression of myc-tagged WT and V600E mutant B-Raf was analyzed by immunoblotting (Top). The effect of 17-AAG on endogenously expressed WT B-Raf (Middle) and C-Raf (Bottom) were also assessed. (B) Results of a representative experiment were quantified and are presented graphically as the relative change in expression levels of indicated proteins vs. control. (C) Kinase activity of B-Raf assayed by IP with myc after 17-AGG treatment over 24 h was measured by using a kinase assay.
V600E B-Raf Is Found in a Complex with Cdc37 and Hsp90. The data presented above suggest that Hsp90 function is required for the stability of V600E but not WT B-Raf. Serine kinases, such as Raf-1 and cdk4, that depend on Hsp90 are found in cells in a complex containing Hsp90 and its cochaperone cdc37 (14, 22). We found that Hsp90 could be detected in immunoprecipitates of myc-tagged V600E but not WT B-Raf (Fig. 5A Middle). Cdc37 was detected in myc immunoprecipitates of both WT and V600E B-Raf; however, the quantity of cdc37 that is associated with V600E B-Raf was five times greater than that associated with WT B-Raf (Fig. 5A Bottom). After 4 h of exposure to 17-AAG, neither Hsp90 nor cdc37 was found in association with V600E B-Raf. The association of cdc37 with WT B-Raf was lost as well. The 17-AAG had no effect on the expression of Hsp90 and cdc37 at this time (Fig. 5B). Loss of the association of V600E B-Raf with Hsp90 or cdc37 preceded the down-regulation of both its activity and expression, which were not clearly apparent until 12 h (compare Fig. 5A with Figs. 3A and 4C). Therefore, these results suggest that 17-AAG inhibits both the kinase activity of V600E B-Raf and V600E B-Raf/·Hsp90 heterocomplex formation.
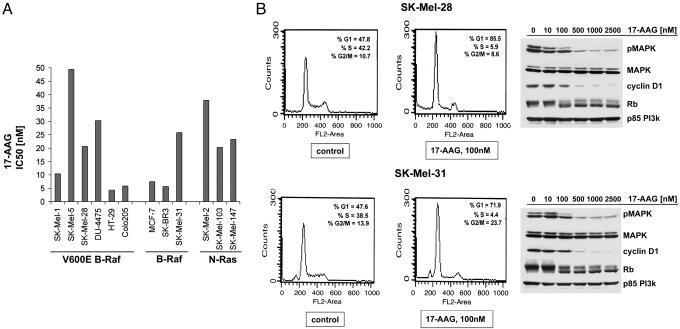
Hsp90 Inhibition Causes Inhibition of Melanoma Cell Growth, G1 Cell-Cycle Arrest, and Induction of Apoptosis. In melanoma, the frequent occurrence of either N-Ras or B-Raf mutation suggests that activation of the Ras-Raf-MAPK oncogenic signaling pathway represents a key element in melanocyte transformation (3, 8). All melanoma cells, with or without B-Raf and N-Ras mutation, were found to be sensitive to the antiproliferative effects of 17-AAG (Fig. 6A). Moreover, 17-AAG treatment causes down-regulation of MAPK activity, loss of cyclin D1 expression, hypophosphorylation of Rb, and G1 phase proliferative arrest in all tested melanoma cells (Fig. 6B). This effect is observed in parallel with A-Raf, Raf-1, and mutant B-Raf degradation, indicating that degradation of the active member of the Raf family in melanomas leads to suppression of MAPK activity. Also, 24-h and, especially, 48-h exposure to 100 nM 17-AAG causes increase in apoptosis of the SK-Mel-28 (from 5.07% to 15.14% in 48 h) and SK-Mel-31 (from 4.7% to 9.23% in 48 h), as measured by an increase in the sub-G1 fraction by FACS.
Fig. 6.
17-AAG inhibits Raf signaling and induces a G1 growth arrest. The 24-h treatment with 100 nM 17-AAG caused G1 block in SK-Mel-28 and SK-Mel-31 cell cycle and an increase in sub-G1 fraction. In parallel, 24 h of exposure to indicated concentrations of 17-AAG caused down-regulation in expression of phospho-MAPK, cyclin D1, and hypophosphorylation Rb in both cell lines and had no effect on MAPK or p85 PI3-kinase expression.
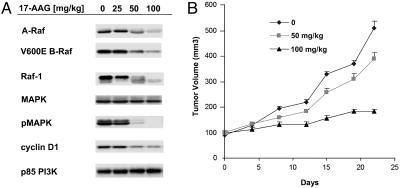
17-AAG Causes the Degradation of V600E B-Raf in Vivo and Inhibits the Growth of SK-Mel-28 Xenograft Tumors. We sought to determine whether the degradation of V600E B-Raf by 17-AAG could be elicited in xenograft tumors by 17-AAG. In SK-Mel-28 mouse xenografts, a nontoxic dose of 17-AAG caused the dose-dependent down-regulation of V600E B-Raf, A-Raf, and Raf-1 (Fig. 7A). Down-regulation of all three Raf isoforms was accompanied by inhibition of MAPK activity and a loss of cyclin D1 expression. No change was detected in the expression of several control proteins, including MAPK and p85 phosphatidylinositol 3-kinase (PI3-kinase) (Fig. 7A). Treatment of these mice with 17-AAG for 3 consecutive days each week for 4 weeks resulted in a dose-dependent inhibition of tumor growth, with 100 mg/kg causing >80% inhibition as compared with vehicle-treated mice at day 25 (Fig. 7B). The 17-AAG was well tolerated at these dose levels, with no treatment-associated mortality.
Fig. 7.
17-AAG causes degradation of mutant B-Raf and inhibited the growth of SK-Mel-28 xenografts. (A) Mice with established tumors were treated for 3 consecutive days with 17-AAG (0, 25, 50, or 100 mg/kg) or the egg phospholipid vehicle alone as a control. At 12 h after the third treatment, mice were killed and tumor tissue was homogenized in 2% SDS lysis buffer and analyzed by immunoblotting for various cellular markers as indicated. (B) 17-AAG inhibited the growth of SK-Mel-28 xenografts in a dose-dependent manner.
Discussion
The frequent activation by mutation of a specific signaling pathway in a particular tumor type generally suggests a rational strategy for mechanism-based therapy. All strategies that effectively inhibit mutationally activated protooncogene products have proven to be useful in treating the relevant cancer. Thus, pharmacologic inhibition of bcr-abl, c-kit, EGF receptor, and HER2, as well as modulation of PML-RXR have proven to be beneficial, sometimes remarkably so, in tumors in which these targets are mutated.
Recent data suggest that deregulation of Ras/Raf signaling is characteristic of melanomas and necessary for their continued proliferation. Mutational activation of N-Ras (25%) and B-Raf (60–70%) occur in high percentage of cases but not together in the same tumor (1, 3, 23, 24). The implication is that neither of the two has a selective advantage if the other is present but that activation of signaling through Raf is a common event in the development of the disease. Also, experiments in cell culture show that MAPK activation is serum- or growth-factor-dependent in melanocytes but is constitutive in tumor cells (25). These data imply that mutational activation of N-Ras or B-Raf leads to growth-factor-independent deregulation of melanoma cell proliferation and that this is a key step in tumorigenesis. In support of this inference, small interfering RNA for B-Raf but not Raf-1 inhibits the transformed phenotype (7). Suppression of oncogenic N-Ras by RNA interference induces apoptosis in human melanoma cells, suggesting that N-Ras is important for avoidance of apoptosis in melanomas that harbor the codon 61 N-Ras mutation (26).
These conclusions lead to the hypothesis that effective inhibition of signaling through the N-Ras/B-Raf pathway could be very useful in the therapy of this disease, which, when metastatic, is essentially untreatable. However, potent in vivo inhibition of neither N-Ras nor B-Raf has been accomplished. Several B-Raf inhibitors are under development, but the B-Raf inhibitor currently in clinical trial inhibits many protein kinases, is not a potent Raf inhibitor, and has little single agent activity in melanoma patients (10, 11). Its clinical antitumor activity has been attributed to its inhibition of VEGF receptor (10).
Here, we report another mechanism for inhibiting mutated B-Raf. A chaperone complex containing Hsp90, cdc37, and other cochaperones is required for the folding, conformational maturation, and stability of a subset of signaling molecules, including Raf-1 (14). Raf-1 and other client proteins are degraded in cells exposed to Hsp90 inhibitors such as 17-AAG. Here, we show that A-Raf falls into this class of proteins but that B-Raf does not. Hsp90 is not detected in B-Raf pull-down experiments and WT B-Raf is not degraded in melanocytes or tumor cells treated with 17-AAG. However, V600E B-Raf does associate with Hsp90 and this mutant is degraded in response to pharmacologic inhibition of Hsp90.
The data suggest that, unlike A-Raf and Raf-1, WT B-Raf does not require Hsp90 for stability, but mutated V600E B-Raf does. V600E is an activating mutant with kinase activity 500 times greater than WT (5). Phosphorylation of T598 within the activation loop of B-Raf is essential for B-Raf kinase activation. Structural studies by Wan et al. (5) suggest that this phosphorylation is required to disrupt the interaction between the DFG motif and the glycine-rich domain (G-loop), allowing the activation loop to adapt the catalytically active conformation. V600E and most of the other activating B-Raf mutations found in human cancers are predicted to disrupt this interaction, obviating the need for phosphorylation of T598 and accounting for constitutive activation. We show that both WT and V600E bind to the cdc37 cochaperone, but Hsp90 is detected in association only with V600E and not WT B-Raf. It is possible that, whereas WT B-Raf does not require Hsp90 for efficient folding, V600E does. Alternatively, the activated V600E conformation may require Hsp90 for stability. Induction of V600E degradation is preceded by loss of its association with Hsp90, in support of the latter idea.
It is not clear whether Hsp90 dependence is caused by the inefficient folding or instability of particular amino acid substitutions or the instability of the active conformation of the protein. V600E and V600D, which are both predicted to mimic T598 phosphorylation and disrupt activation-loop inhibition, are both sensitive to 17-AAG. Similarly, two G-loop B-Raf mutants, G465V and G468A, were also found to be sensitive to 17-AAG exposure. Although V600E, V600D, and G468A mutations generate amplified B-Raf kinase activity, G465A mutation is shown to result in impaired or lower activity of kinase as compared with WT B-Raf (5). These findings suggest that activated kinase activity is not required for sensitization to Hsp90 inhibitors. Also, we found that inhibition of intracellular V600E B-Raf kinase activity by 17-AAG begins before detection of loss of B-Raf mutant protein. Several other mutated oncoproteins and oncogenic fusion proteins, including v-src, p53, mutant c-kit, bcr-abl, and NPM-ALK, have an increased requirement for Hsp90 compared with their normal counterparts (27). A V600 B-Raf mutant, which accounts for >90% of the Raf mutants in human tumors, is included in this list. In some sense, Hsp90 may be considered necessary for the development or clonal evolution of such tumors. The dependence of some tumors on Hsp90-dependent oncoproteins may account in part for selective antitumor activity of 17-AAG in various in vivo models (28). Although the main physiologic role of Hsp90 may be to protect cells exposed to stress by refolding proteins denatured under these conditions, it also allows the selection of gain-of-function mutants that would not fold efficiently in its absence.
However, melanoma cell lines with mutant B-Raf are not more sensitive to 17-AAG than those with WT B-Raf. The above finding is perhaps not surprising, considering that Raf-1 is an Hsp90 client as well and that 17-AAG induces its degradation (13). Several lines of evidence suggest that, in melanoma, N-Ras or B-Raf mutation lead to constitutive activation of MAPK by Raf-1- or B-Raf-dependent pathways and that this activation is required for maintenance of the transformed phenotype (25). MAPK is shown to be constitutively activated in the absence of serum in melanoma cell lines (25). Marais and coworkers (5) have shown that a class of rare B-Raf mutants with reduced catalytic activity bind to and activate Raf-1 kinase and may exert their effects in tumor cells in this way. Our data show that melanoma cells are sensitive to MEK inhibition whether or not they contain the B-Raf mutation although cells with mutatant B-Raf are the most sensitive (29). Thus, Hsp90 inhibition induces the degradation of both mutated B-Raf and Raf-1 with concomitant reduction in MAPK kinase activity, decline in d-cyclin expression, G1 arrest, and an increase in apoptosis in all tested melanoma cell lines.
Together, these data suggest that induction of B-Raf and Raf-1 degradation by Hsp90 inhibitors could be an important therapeutic strategy for melanomas and other tumors with B-Raf mutation. Workman and coworkers (31) have noted significant clinical activity in several patients with melanoma in the phase-1 clinical trial of 17-AAG. Here, we show that levels of 17-AAG that are achievable in vivo without significant toxicity are sufficient to cause degradation of Raf-1, A-Raf, and mutant B-Raf, as well as marked inhibition of MAPK activity in a melanoma xenograft. These effects are associated with significant antitumor activity.
Materials and Methods
Materials. 17-AAG (330507, National Service Center, National Cancer Institute, Bethesda), MG132, calpeptin, and caspase inhibitor I (Calbiochem) were dissolved in 100% DMSO. We used the following polyclonal Abs: B-Raf, C-Raf, A-Raf, c-myc (Santa Cruz Biotechnology), p85 PI3-kinase (Upstate Biotechnology, Lake Placid, NY), cdc37 (Affinity BioReagents, Golden, CO), Hsp90 (Stressgen Biotechnologies, Victoria, Canada), and ubiquitin (Covance, Berkeley, CA). Protein G–sepharose (Amersham Pharmacia) was used for immunoprecipitation (IP).
Cell Culture. The following human melanoma cancer lines were used in this study: SK-Mel-1, SK-Mel-2, SK-Mel-5, SK-Mel-19, SK-Mel-28, WM-266.4, SK-Mel-31, SK-Mel-103, and SK-Mel-147; colon cancer lines HT-29 and Colo-205; breast cancer cell lines MCF-7 and SKBr-3 DU-4475; and non-small-cell lung cancer lines H1666 and H1755. All cells were maintained in a 1:1 mixture of DMEM/F12, except for Colo-205 (which was maintained in RPMI medium 1640) and H1755 and H1666 (which were maintained in ACL-4 supplemented with 2 mM glutamine, 50 units/ml penicillin, 50 units/ml streptomycin, and 10% heat-inactivated FBS; Gemini Bioproducts, Calabrasas, CA), and incubated at 37°C in 5% CO2/95% air. Normal human melanocytes HEMnL were purchased from Cascade Biologies (Portland, OR) and maintained at standard tissue culture conditions according to the manufacturer's instructions.
Transfections. Myc-tagged B-Raf cDNA was obtained from Walter Kolch (University of Glasgow, Glasgow, Scotland). B-Raf cDNA was inserted into the HindIII site of the pcDNA3.1 vector. The V600E-B-Raf mutant was then constructed by site-directed mutagenesis (Stragene) in which the nucleotides corresponding to amino acid 600 were changed from GTG to GAG. We transfected 2 million MCF-7 cells with 10 μg of DNA by using 20 μl of Lipofectamine reagent (Invitrogen and Life Technologies, Rockville, MD). Experiments were performed 24 h after transfection.
Protein Analysis. Cells were lysed in Nonidet P-40 buffer (50 mM Tris, pH 7.5/1% Nonidet P-40/150 mM NaCl/10% glycerol/2mM EDTA/20 mM NaF/10 mM PMSF/2.5 mM Na3VO4/1 mM β-glycerol phosphate, with 10 μM each leupeptin, aprotinin, and soybean trypsin inhibitor) and cleared by centrifugation. Nonidet P-40 insoluble fractions were lysed in 2% SDS in 50 mM Tris and boiled for 15 min. Protein concentration was determined by using BCA reagent (Pierce). Samples were separated by 7–15% SDS/PAGE, transferred to nitrocellulose, immunoblotted, and detected by chemiluminescence by using the ECL detection reagents (Amersham Pharmacia). Results were quantified by using the Gel Doc system (Bio-Rad).
IP and in Vitro Kinase Assay. MCF-7 cells were transfected with WT or V600E myc–B-Raf constructs for 24 h, subsequently incubated with 1 μM 17-AAG for various times, and lysed with Nonidet P-40 buffer. We immunoadsorbed 1,000 μg of total protein with 10 μg of 9E10 anti-myc Ab or normal anti-mouse IgG (control), followed by protein G–sepharose. The immunoadsorbed pellets were washed three times with ice-cold wash buffer (0.05% Tween 20/25 mM Tris, pH.7.5/150 mM NaCl/10 mM MgCl2/1 mM DTT) and resuspended in 2% SDS sample buffer. B-Raf kinase activity was assayed by using a B-Raf kinase cascade assay according to the manufacturer's protocols (Upstate Biotechnology).
Growth Assays. Cells were seeded in 96-well plates at 1,000 cells per well. After 24 h, cells were placed in fresh media containing drug and allowed to grow for 5 days. The cell number in treated vs. control wells was estimated after staining with Alamar blue (Informagen, Newington, NH). For cell-cycle analysis, cell nuclei were isolated as described (30) and stained with ethidium bromide, and DNA content was analyzed by using a FACS cytometer (Becton Dickinson).
Animal Studies. Experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee and institutional guidelines for the proper, humane use of animals in research were followed. To generate xenograft tumor-bearing mice, 1 × 107 SK-Mel-28 cells were mixed with Matrigel (Collaborative Research) and inoculated s.c. in the right flank of 6-week-old athymic BALB/c female mice (National Cancer Institute/Frederick Cancer Research and Development Center). As soon as tumors reached a minimum of 5 mm in diameter, mice were randomly assigned to treatment with 17-AAG 50 or 100 mg/kg by i.p. injection for 3 consecutive days each week for 4 weeks. Control mice were treated only with the egg phospholipid vehicle. Mice were weighed, and tumor volumes were calculated with the following formula: π /6 × larger diameter × (smaller diameter)2. To analyze cellular markers, mice with established tumors were treated for 3 consecutive days with 17-AAG at doses of 25, 50, or 100 mg/kg or vehicle only as control and then killed 12 h after the third dose. For immunoblotting, tumor tissue was homogenized in 2% SDS lysis buffer (pH 7.4).
Acknowledgments
We thank W. Kolch for providing myc-tagged B-Raf construct; A. Houghton and P. Chapman (both at the Memorial Sloan–Kettering Cancer Center) for the SK-Mel melanoma cell lines; and I. Osman (New York University, New York) for analysis of B-Raf and Ras mutational status in the cell lines used in this study. This work was supported by the Waxman Foundation and the Breast Cancer Research Foundation.
Author contributions: O.M.G. and A.D.B. performed research; A.S., Q.Y., and P.F. contributed new reagents/analytic tools; D.S. analyzed data; and N.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: 17-AAG, 17-allylamino-17-demethoxygeldanamycin; MAPK, mitogen-activated protein kinase; MEK, MAPK/extracellular signal-regulated kinase kinase; PI3-kinase, phosphatidylinositol 3-kinase; IP, immunoprecipitation.
References
- 1.Mercer, K. E. & Pritchard, C. A. (2003) Biochim. Biophys. Acta Rev. Cancer 1653, 25–40. [DOI] [PubMed] [Google Scholar]
- 2.Omholt, K., Karsberg, S., Platz, A., Kanter, L., Ringborg, U. & Hansson, J. (2002) Clin. Cancer Res. 8, 3468–3474. [PubMed] [Google Scholar]
- 3.Davies, H., Bignell, G. R., Cox, C., Stephens, P., Edkins, S., Clegg, S., Teague, J., Woffendin, H., Garnett, M. J., Bottomley, W., et al. (2002) Nature 417, 949–954. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, Y., Xing, M., Mambo, E., Guo, Z., Wu, G., Trink, B., Beller, U., Westra, W. H., Ladenson, P. W. & Sidransky, D. (2003) J. Natl. Cancer Inst. 95, 625–627. [DOI] [PubMed] [Google Scholar]
- 5.Wan, P. T., Garnett, M. J., Roe, S. M., Lee, S., Niculescu-Duvaz, D., Good, V. M., Jones, C. M., Marshall, C. J., Springer, C. J., Barford, D. & Marais, R. (2004) Cell 116, 855–867. [DOI] [PubMed] [Google Scholar]
- 6.Wellbrock, C., Ogilvie, L., Hedley, D., Karasarides, M., Martin, J., Niculescu-Duvaz, D., Springer, C. J. & Marais, R. (2004) Cancer Res. 64, 2338–2342. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani, S. R., Jacobetz, M. A., Robertson, G. P., Herlyn, M. & Tuveson, D. A. (2003) Cancer Res. 63, 5198–5202. [PubMed] [Google Scholar]
- 8.Reifenberger, J., Knobbe, C. B., Sterzinger, A. A., Blaschke, B., Schulte, K. W., Ruzicka, T. & Reifenberger, G. (2004) Int. J. Cancer 109, 377–384. [DOI] [PubMed] [Google Scholar]
- 9.Sebolt-Leopold, J. (2004) Curr. Pharm. Des. 10, 1907–1914. [DOI] [PubMed] [Google Scholar]
- 10.Sharma, A., Trivedi, N. R., Zimmerman, M. A., Tuveson, D. A., Smith, C. D. & Robertson, G. P. (2005) Cancer Res. 65, 2412–2421. [DOI] [PubMed] [Google Scholar]
- 11.Lyons, J. F., Wilhelm, S., Hibner, B. & Bollag, G. (2001) Endocr. Relat. Cancer 8, 219–225. [DOI] [PubMed] [Google Scholar]
- 12.Maloney, A. & Workman, P. (2002) Expert Opin. Biol. Ther. 2, 3–24. [DOI] [PubMed] [Google Scholar]
- 13.Schulte, T. W., An, W. G. & Neckers, L. M. (1997) Biochem. Biophys. Res. Commun. 239, 655–659. [DOI] [PubMed] [Google Scholar]
- 14.Grammatikakis, N., Lin, J.-H., Grammatikakis, A., Tsichlis, P. N. & Cochran, B. H. (1999) Mol. Cell. Biol. 19, 1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An, W. G., Schulte, T. W. & Neckers, L. M. (2000) Cell Growth Differ. 11, 355–360. [PubMed] [Google Scholar]
- 16.Blagosklonny, M. V., Toretsky, J., Bohen, S. & Neckers, L. (1996) Proc. Natl. Acad. Sci. USA 93, 8379–8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fumo, G., Akin, C., Metcalfe, D. D. & Neckers, L. (2004) Blood 103, 1078–1084. [DOI] [PubMed] [Google Scholar]
- 18.Whitesell, L., Mimnaugh, E. G., De Costa, B., Myers, C. E. & Neckers, L. M. (1994) Proc. Natl. Acad. Sci. USA 91, 8324–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulte, T. W. & Neckers, L. M. (1998) Cancer Chemother. Pharmacol. 42, 273–279. [DOI] [PubMed] [Google Scholar]
- 20.Mimnaugh, E. G., Chavany, C. & Neckers, L. (1996) J. Biol. Chem. 271, 22796–22801. [DOI] [PubMed] [Google Scholar]
- 21.Basso, A. D., Solit, D. B., Chiosis, G., Giri, B., Tsichlis, P. & Rosen, N. (2002) J. Biol. Chem. 277, 39858–39866. [DOI] [PubMed] [Google Scholar]
- 22.Stepanova, L., Leng, X., Parker, S. B. & Harper, J. W. (1996) Genes Dev. 10, 1491–1502. [DOI] [PubMed] [Google Scholar]
- 23.Brose, M. S., Volpe, P., Feldman, M., Kumar, M., Rishi, I., Gerrero, R., Einhorn, E., Herlyn, M., Minna, J., Nicholson, A., et al. (2002) Cancer Res. 62, 6997–7000. [PubMed] [Google Scholar]
- 24.Pollock, P. M., Harper, U. L., Hansen, K. S., Yudt, L. M., Stark, M., Robbins, C. M., Moses, T. Y., Hostetter, G., Wagner, U., Kakareka, J., et al. (2003) Nat. Genet. 33, 19–20. [DOI] [PubMed] [Google Scholar]
- 25.Satyamoorthy, K., Li, G., Gerrero, M. R., Brose, M. S., Volpe, P., Weber, B. L., Van Belle, P., Elder, D. E. & Herlyn, M. (2003) Cancer Res. 63, 756–759. [PubMed] [Google Scholar]
- 26.Eskandarpour, M., Kiaii, S., Zhu, C., Castro, J., Sakko, A. J. & Hansson, J. (2005) Int. J. Cancer 115, 65–73. [DOI] [PubMed] [Google Scholar]
- 27.Neckers, L. & Ivy, S. P. (2003) Curr. Opin. Oncol. 15, 419–424. [DOI] [PubMed] [Google Scholar]
- 28.Workman, P. (2003) Curr. Cancer Drug Targets 3, 297–300. [DOI] [PubMed] [Google Scholar]
- 29.Solit, D. B., Garraway, L. A., Pratilas, C. A., Sawai, A., Getz, G., Basso, A., Ye, Q., Lobo, J. M., She, Y., Osman, I., et al. (2005) Nature, in press. [DOI] [PMC free article] [PubMed]
- 30.Nusse, M., Beisker, W., Hoffmann, C. & Tarnok, A. (1990) Cytometry 11, 813–821. [DOI] [PubMed] [Google Scholar]
- 31.Banjeri, U., O'Donnel, A., Scurr, M., Pacey, S., Stapleton, S., Asad, Y., Simmons, L., Maloney, A., Raynaud, F., Campbell, M., et al. (2005) J. Clin. Oncol. 23, 4152–4161. [DOI] [PubMed] [Google Scholar]