Abstract
Intracellular calcium mobilization and signaling mechanisms triggered by activation of synaptic glutamate receptors in the striatum are important modulators of neurotransmission in striatal circuits. However, the expression and functions of scaffolding proteins anchoring glutamate receptors at striatal synapses have not been addressed so far. The long-form Homer1 proteins, Homer1b/c, assemble group I metabotropic glutamate receptors (mGluR1/5) in large macromolecular complexes with sources of calcium influx and release at synapses as well as with components of the NMDA receptor complex at the neuronal cell membrane. Homer1a, the short, activity-dependent splice variant of Homer1b/c, lacks the ability of linking mGluR1/5 to synaptic proteins and functions as an endogenous negative modulator of the mGluR1/5 inositol 1,4,5-trisphosphate receptor signaling complex. We have generated transgenic mice, which overexpress Homer1a in striatal medium spiny neurons either homogenously throughout the extrastriosomal matrix (Homer1a-matrix line) or predominantly in striosomal patches (Homer1a-striosome line). Homer1a-expressing mice demonstrated normal development of striatal structure and afferent–efferent connectivity. However, motor performance in behavioral tasks and striatal responses to the psychomotor stimulant amphetamine were significantly altered in the Homer1a-striosome line. Thus, glutamate receptor scaffolding proteins of the Homer1 family critically regulate the functions of striatal medium spiny neurons in complex motor tasks and its modulation by psychomotor stimulant drugs.
Keywords: metabotropic glutamate, motor stereotypy, striosome
Group I metabotropic glutamate receptors (mGluR1/5) are linked to the activation of phospholipase C and generally mediate excitatory effects by eliciting a release of calcium from intracellular stores. mGluR1/5 are important positive modulators of synaptic function and neuronal activity and have been linked to long-term changes in synaptic function such as long-term potentiation in the hippocampal circuitry (1), long-term depression in the cerebellum (2), drug-induced plasticity in the nucleus acumbens (3), etc. In the striatum, mGluR1/5 are primarily expressed postsynaptically in striatal projection neurons and subpopulations of interneurons but can also mediate presynaptic effects on thalamostriatal and corticostriatal glutamatergic afferents as well as nigrostriatal dopaminergic afferents (4). The localization and the functions of mGluR1/5 are strongly dependent on protein–protein interactions with the Homer (Vesl) family of synaptic scaffolding proteins (5–8). In the nervous system, Homer1 proteins function as molecular bridges linking both mGluR1 and mGluR5 to the inositol 1,4,5-trisphosphate receptors (IP3Rs) on the endoplasmic reticulum (7–9) and to additional synaptic ion channels such as calcium channels (7) and transient receptor potential channels (10) as well as to components of the NMDA receptor signaling complex by virtue of their ability to form multimers (11–13). An additional short splice variant encoded by the Homer1 gene, Homer1a, is selectively expressed as an immediate early gene after synaptic activity (5–7). A striking functional feature of Homer1a is that it competes with Homer1b/c for binding to mGluRs and uncouples mGluRs from IP3Rs because of its lack of the C-terminal EVH domain, which is required for multimerization (7, 8). Several lines of evidence indicate that Homer1a overexpression in neurons leads to changes in assembly of mGluR-signaling complexes and regulates calcium signaling, synaptic trafficking, and clustering of glutamate receptors as well as spine morphogenesis (7–9, 14, 15). It has been proposed, therefore, that Homer1a functions as an endogenous antagonist of the mGluR-IP3R receptor-signaling pathway, which implies that Homer1a expression would modulate biological processes involving activation of group I mGluRs (7, 14). Indeed, manipulation of Homer1-mGluR1/5 interactions has been used a tool to address functions of group I mGluRs in diverse regions of the brain and is reported to have a major impact on neural processes involving learning and memory (16), axonal path finding (17), drug addiction (3, 18), and epileptogenesis (19). We have used overexpression of Homer1a in the striatum as a tool to address the significance of group I mGluRs and their signaling mechanisms in striatal functions in vivo. We found that mice overexpressing Homer1a in the striatal projection neurons show multiple defects in motor performance and coordination and demonstrate alterations in drug-induced stereotypy, thereby suggesting a critical role for mGluR1/5–Homer1 signaling mechanisms in the modulation of striatal output.
Results
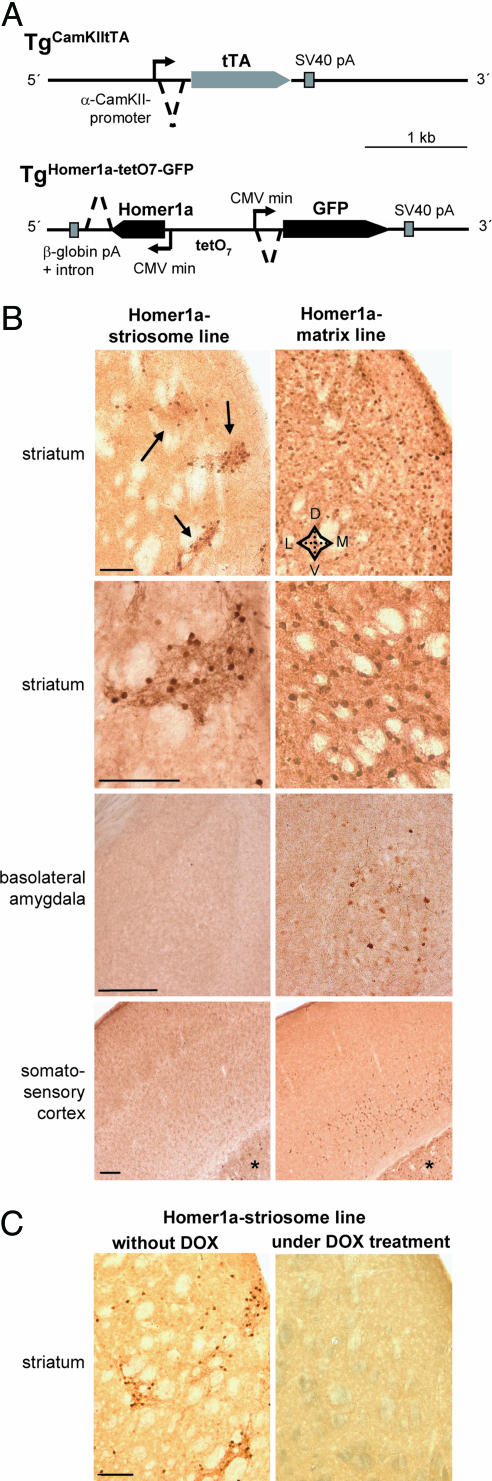
Generation and Characterization of Mouse Lines Expressing Homer1a in the Striatum. To unravel the functional significance of mGluR signaling in the forebrain, we generated mice expressing Homer1a in the forebrain using the tetracycline transactivator (tTA) system for conditional gene expression. Several transgenic founders were obtained and bred with the TgCamKIItTA line to secure tTA-induced expression of Homer1a and of EGFP. None of the transgenic lines demonstrated stable expression of EGFP. Immunohistochemical detection using an anti-myc antibody was used to detect the expression of the myc–Homer1a fusion protein (referred to as Homer1a expression henceforth).
In the double-transgenic progeny of the Homer1a and TgCamKIItTA transgenic mice, the expression of Homer1a commenced perinatally and reached its peak and stable levels at ≈3 weeks of age (data not shown). As expected from the expression profile of the fragment of the CamKIIα promoter, which is used in the TgCamKIItTA mice as described in ref. 20, Homer1a expression was restricted to the forebrain in several founder lines, and the brainstem, spinal cord, and cerebellum were entirely devoid of expression. Two mouse lines demonstrated strong expression of Homer1a in the striatum and were characterized in greater detail. In one line the expression was nearly selective for the striatum (Fig. 1B), and myc–Homer1a-expressing cells were largely concentrated in clusters in the striatum, which were reminiscent of striosomal patches (Fig. 1B, arrows). In the second line, the myc–Homer1a-expressing cells were more abundant and evenly distributed throughout the striatal matrix. These mice also showed weak expression in the basolateral amygdala and layer V of the somatosensory cortex, in addition to strong expression in the striatum (Fig. 1B). In both lines, the thalamus, the hippocampus, and other forebrain structures were devoid of Homer1a expression (data not shown).
Fig. 1.
Generation and initial characterization of transgenic mice expressing Homer1a in the forebrain. (A) Schematic representation of the targeting construct used to make mice expressing Homer1a and GFP in an inducible manner (TgHomer1a-tetO7-GFP), upon crossing with mice expressing tTA under the control of the CamKII promoter (TgCamKIItTA). pA, polyadenylation signal; CMVmin, minimal cytomegalovirus promoter; tetO7, tetracycline-binding operons. (B) Immunohistochemical determination of myc-tagged Homer1a using anti-myc antibody on forebrain sections of Homer1a-striosome mice and Homer1a-matrix mice. Arrows point to the Homer1a-positive striatal clusters resembling striosomal islands. (C) Immunohistochemical determination of myc–Homer1a using anti-myc antibody on striatal sections of Homer1a-striosome mice in the naïve state (without DOX) or 10 days after treatment with doxycycline (under DOX treatment). Doxycycline-induced sequestration of tTA leads to a loss of myc–Homer1a expression. *, striatum; D, dorsal; M, medial; L, lateral; V, ventral. (Scale bars: 200 μm in B and C.)
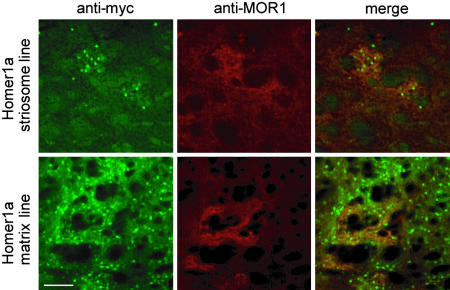
We then characterized the pattern of Homer1a expression in the striatum in greater detail (see Fig. 5, which is published as supporting information on the PNAS web site). In the adult rodent striatum, ≈95% of the neurons are medium spiny neurons (MSNs), which use GABA as a neurotransmitter (21). In both striatal mouse lines, expression of Homer1a was seen in striatal GABAergic neurons (Fig. 5A), whereas calretinin-positive (Fig. 5B), parvalbumin-positive (Fig. 5C), or choline acetyl transferase-positive (data not shown) neurons were devoid of Homer1a expression. A classical anatomical feature of the striatum is the patch–matrix distribution, which also imparts important and characteristic functional properties to the striatum. MSNs in the striatal patches and matrix differentially express calbindin: GABAergic neurons in the striatal matrix are rich in calbindin expression, whereas those found in striosomes are relatively sparse with respect to calbindin expression (22, 23). Costaining of striatal sections with anti-myc and anticalbindin antibodies revealed that, in the line with the broader expression of Homer1a (matrix line, see below), a high number of myc-positive neurons expressed calbindin (76 ± 6%), whereas, in the striatum-specific line with a patchy distribution (striosome line, see below), only 28 ± 5% of Homer1a–myc-positive neurons expressed calbindin (see typical examples of the extent of overlap of calbindin and Homer1a–myc expression in Fig. 5D). To further verify this differential predominance of myc–Homer1a-expressing neurons in the striosomes versus extrastriosomal matrix in these two mouse lines, we costained striatal sections for expression of myc–Homer1a and the μ-opioid receptor 1 (MOR1), which is known to be a marker for striatal striosomal patches (24). Myc–Homer1a-positive cells were predominantly localized in MOR1-positive islands in the striosomal line (Fig. 2, arrowheads), whereas in the matrix line they were neither particularly concentrated over nor restricted to MOR1-positive striosomes (Fig. 2). Because of the differential distribution between the striosomal and extrastriosomal matrix, the relative abundance of Homer1a–myc-positive neurons among striatal MSNs was also different in the two lines. In the matrix line, 82 ± 4% of striatal GABAergic neurons expressed myc-tagged Homer1a, whereas in the striosomal line only 23 ± 3% of striatal GABAergic neurons expressed myc-tagged Homer1a. Based on these results, we refer to the two lines hereafter as “Homer1a-striosome line” and “Homer1a-matrix line” to facilitate clarity of description of their phenotypes. However, it is important to emphasize that this nomenclature represents only the loci of predominant, but not exclusive, expression of Homer1a in these two anatomical subdomains of the striatum. In particular, the Homer1a-matrix line encompasses expression of myc–Homer1a not only in the matrix compartment but also in the striosomal neurons to a lesser extent.
Fig. 2.
Characterization of Homer1a expression in striatal subcompartments. Dual immunofluorescence with anti-myc and anti-MOR antibodies on striatal sections of Homer1a-striosome mice and Homer1a-matrix mice. In the Homer1a-striosome line, the localization of myc–Homer1a-positive neurons is largely restricted to the MOR1-positive striosomal patches, whereas in the matrix line, it is more widespread. (Scale bar: 200 μm.)
Development of the Striatum in Homer1a-Expressing Mice. To determine whether Homer1a expression during early postnatal periods influences the development of the striatum, we analyzed the striatum of adult transgenic mice for its cytoarchitecture as well as for the presence and density of the various diverse groups of striatal neurons. In both transgenic lines, the macroscopic and the microscopic structure of the striatum appeared normal (Fig. 6A, which is published as supporting information on the PNAS web site). Furthermore, immunohistochemistry for neuronal marker proteins such as calbindin, calretinin, parvalbumin, acetyl choline esterase, and choline acetyl transferase revealed a normal distribution of subpopulations of striatal neurons in both Homer1a-transgenic mouse lines in comparison with wild-type mice (see Fig. 6A for examples and Fig. 6B for quantification). Furthermore, the patch–matrix distribution in Homer1a-transgenic mice was similar to that in wild-type mice, as shown by immunostaining of patches with MOR1 (Fig. 6C). The striatal expression of mGluR5 (Fig. 6C) and mGluR1α (data not shown) also remained unchanged in both lines of Homer1a-expressing mice in comparison with wild-type mice.
mGluRs have been proposed to modulate development of nigrostriatal axons and their contacts on striatal neurons, although it is unclear whether these effects can be attributed to mGluRs situated postsynaptically on striatal neurons or presynaptically on nigrostriatal terminals (24). To address whether the afferent connectivity to the striatum from the substantia nigra develops normally in Homer1a-overexpressing mice, we performed a thorough analysis of the distribution and density of dopaminergic terminals in the dorsomedial caudate and the ventrolateral caudate (VLC) of the striatum (see Fig. 6D). Confocal analysis of striatal tyrosine hydroxylase immunofluorescence and its quantification using confocal software revealed a normal density of dopaminergic afferent terminals in the striatum of the H1a-matrix as well as H1astriosome mice upon comparison with wild-type littermates (see Fig. 6E for examples and Fig. 6F for quantification). Similarly, immunostaining with an antibody against the neuronal glutamatergic transporter vesicular glutamate transporter 1 demonstrated that the overall distribution of glutamatergic terminals was not qualitatively changed in the striatum (Fig. 6G) or cortex (data not shown) of Homer1a-expressing mice.
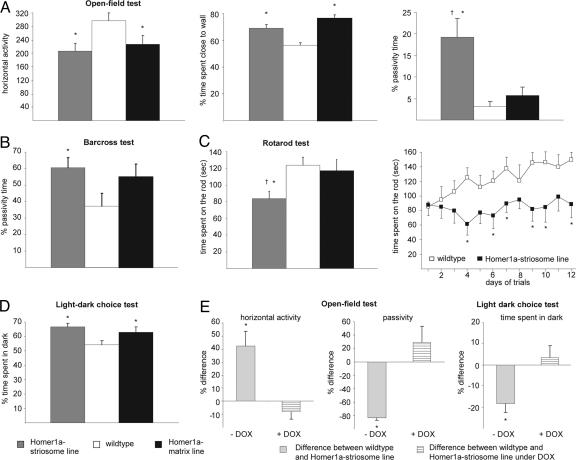
Analysis of Motor Performance in Homer1a-Expressing Mice. Homer1a-expressing mice did not demonstrate any obvious distortion of gait and posture. Footprint analysis revealed normal stride length and base width of strides (Table 1, which is published as supporting information on the PNAS web site). Analysis of locomotor activity was performed in the “open-field” test. Mice from both the Homer1a-striosome line and Homer1a-matrix line demonstrated significantly lesser locomotor activity than did wild-type littermates, which was evident as decreased horizontal activity and a significantly longer amount of time spent close to the wall of the activity box (Fig. 3A; P < 0.05 in all cases). A large increase in the passivity time was seen in the open-field test with the Homer1astriosome mice, but not with the Homer1a-matrix mice, in comparison with wild-type mice (Fig. 3A). Furthermore, when the mice were challenged in the bar-cross test, passivity time was significantly increased for the Homer1a-striosome mice as compared with wild-type mice (Fig. 3B, P < 0.003). Although the Homer1a-matrix mice displayed a tendency for increased passivity time, this tendency did not reach statistical significance. Additional parameters that were tested, such as frequency of falling, slipping, or turning around and the speed of movement across the bar, were similar across all groups (Table 1).
Fig. 3.
Behavioral analysis of motor tasks in Homer transgenic mice. Behavioral analysis of motor tasks in wild-type mice (open bars), Homer1a-striosome mice (shaded bars), and Homer1a-matrix mice (filled bars) in the open-field test (A), bar-cross test (B), rotarod test (C) and light–dark choice test (D). E represents phenotypic changes in Homer1a-striosome mice (expressed as percentage difference over wild-type mice) in the open-field test and light–dark choice test in the naïve state (–DOX, light gray bars) and upon treatment with doxycycline (+DOX, hatched bars). For the latter, both wild-type and Homer1a-striosome mice were treated with doxycycline. *, P < 0.05 upon comparison with wild-type mice (n = 7–8 mice per group in each test).
We then tested the mice on an accelerating rotarod, once per day, over a period of 12 days. The H1a-striosome mice were able to stay on the accelerating rotarod for a significantly lesser length of time than were the H1a-matrix mice (P = 0.04) or the wild-type mice (P = 0.02; Fig. 3C; represented as a mean of measurements repeated over 12 days). Interestingly, whereas wild-type mice showed an improvement in rotarod performance over 12 days, H1a-striosome mice entirely failed to show motor learning (Fig. 3C; P < 0.05 at all time points from 4 days onwards). Taken together, the H1a-striosome mice, but not the H1a-matrix mice, demonstrated clear defects in motor coordination and motor learning.
Because an increase in the amount of time spent close to wall in the open-field test as well as the increase in passivity time in the bar-cross test can be an indication of anxiety and fear in the Homer1a-expressing mice, we also tested them in the light–dark choice test. In comparison with wild-type mice, the Homer1a-striosome mice (P = 0.017) and the Homer1a-matrix mice (P = 0.046) spent significantly more time in the dark compartment of the box than in the illuminated compartment (Fig. 3D), thereby suggesting higher levels of anxiety in Homer1a-expressing mice than in wild-type mice.
We then asked whether doxycycline-induced loss of Homer1a expression could rescue motor dysfunction in Homer1a-striosome mice. Adult Homer1a-striosome mice maintained under doxycycline indeed failed to differ significantly from doxycycline-treated wild-type mice in the open-field test and light–dark choice test (Fig. 3E; P > 0.05).
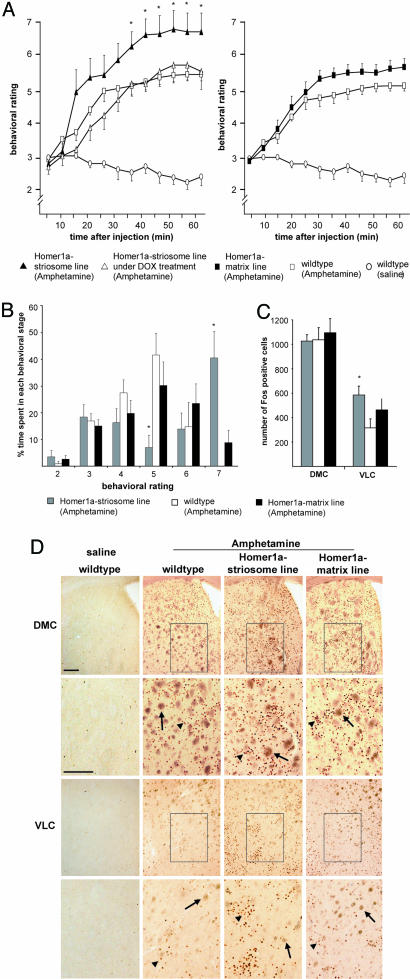
Responses to Amphetamine. Hyperactivity and stereotypy induced by the psychomotor stimulant drug amphetamine have been used as a measure for studying striatal function (23, 25, 26). To study how expression of Homer1a in striatal MSNs affects amphetamine-induced motor effects, we scored wild-type and Homer1a-expressing mice for motor responses to i.p.-injected amphetamine according to the behavioral score rating described by Mao and Wang (25). In wild-type mice, i.p. amphetamine induced progressive behavioral transition from normal level of locomotor activity (score 3) to enhanced exploratory behaviors (score 4) in a majority of mice within 20 min. Homer1a-matrix mice displayed magnitude and time course of responses to amphetamine similar to those of wild-type mice (Fig. 4 A and B). In contrast, in Homer1a-striosome mice, the amphetamine-induced increase in motor activity was more pronounced. In particular, enhanced exploratory behaviors (score 4) commenced earlier in Homer1a-striosome mice and progressed rapidly to fast, patterned, repetitive exploration with hyperactivity (score 6; Fig. 4A). Furthermore, none of the nine wild-type mice that were tested reached the score of 7 (stereotypy; Fig. 4B), whereas 75% of Homer1a-striosome mice tested (six of eight mice) demonstrated stereotypic behavior for long periods of time (Fig. 4A; P = 0.01 for all time points from 35 min onwards). Thus, Homer1a-striosome mice demonstrated exaggerated psychomotor responses to amphetamine compared with wild-type mice. In contrast, when Homer1a-striosome mice and wild-type mice were treated with doxycycline, no significant differences were observed with respect to amphetamine-induced motor responses (Fig. 4A).
Fig. 4.
Analysis of motor behavior (A and B) and expression of Fos (C and D) induced by acute i.p. administration of amphetamine or saline (control) in Homer1a-matrix mice or wild-type or Homer1a-striosome mice in the naïve state or after treatment with doxycycline. Please see the text for a detailed description of the behavioral rating scheme. A shows the behavioral score as a function of time after administration of amphetamine or saline. B represents the same data set, showing the average amount of time spent in each behavioral stage by the mice in each group as a percentage of the total observation time of 60 min. (C and D) Quantitative summary (C) and typical examples (D) of Fos immunoreactivity in the VLC or dorsomedial caudate (DMC) at 60 min after i.p. administration of saline or amphetamine in the same set of mice tested for behavioral scores shown in A and B. Boxed areas are magnified in the corresponding panels beneath them. Examples of specifically labeled, punctuate, Fos-immunoreactive cells and unspecifically labeled brown fiber bundles are indicated by arrowheads and arrows, respectively. (Scale bar: 200 μm.) *, P < 0.05 (n = 7–8 mice per group in each test).
An additional approach for addressing changes in synaptic function and activity of neurons in the striatum caused by psychostimulant drugs is to study the induction of early immediate genes in the striatum after drug administration (23, 25, 27). We therefore addressed expression of Fos, the product of the immediate early gene c-fos, in the dorsomedial caudate and VLC (25) of wild-type or Homer1a-expressing mice 1 h after amphetamine injection. Consistent with previous reports (23, 25), amphetamine treatment produced a large increase in the number of Fos-positive cells (Fig. 4C). A thorough quantitative analysis revealed that numbers of Fos-positive cells in the VLC of Homer1a-striosome mice were significantly higher than in wild-type mice (P = 0.032; see Fig. 4C for typical examples and Fig. 4D for summary). Although the Homer1a-matrix mice demonstrated a trend for an increased number of Fos-positive cells in the VLC, this trend did not reach statistical significance (P = 0.226; Fig. 4 C and D). Taken together, these results show that stereotypy and expression of plasticityrelated genes induced by acute amphetamine are significantly enhanced in Homer1a-striosome mice, but not in the Homer1amatrix mice, in comparison with wild-type littermates.
Discussion
The striatum plays a critical role in regulating the activity flow in circuits involving the cortex, the basal ganglia, and the thalamus. Deficient striatal function is implicated not only in disorders of neurological functions governing movement and posture but also in psychiatric disorders and psychostimulant addiction (23, 26–28). The components of the group I metabotropic receptor-signaling complex, such as mGluR1, mGluR5, IP3Rs receptors, and synaptic scaffolding proteins linking them together, namely Homer1/c proteins, are highly expressed in the striatum (4, 9, 29). Although mGluR1/5 have been implicated to be critical modulators of glutamatergic as well as nonglutamatergic synaptic transmission in the striatum, their contributions to the complex tasks mediated by the striatum during the execution of voluntary movements, maintenance of body posture, and complex motor tasks such as coordination, motor learning, and responses to psychostimulants are not well understood. So far, analyses of knockout mice have failed to deliver insights into functions of mGluR1 or mGluR5 in the striatum mice because of the redundancy of expression and functions of mGluR1 and mGluR5 and the lack of studies on conditional deletion of both mGluR1 and mGluR5 in the basal ganglia in general and the striatum in particular.
Here we show that striatal overexpression of Homer1a, an activity-dependent negative modulator of the mGluR1/5–Homer1b/c–IP3R signaling complex, alters complex motor tasks, including motor learning, suggesting thereby that the mGluR1/5-signaling complex is important in the endogenous regulation of striatal function. Moreover, we show that doxycycline-mediated sequestration of tTA led to a complete loss of Homer1a expression in adult Homer1a-expressing mice and to a rescue of the motor phenotype. This finding shows that Homer1a overexpression, and not other factors such as genetic background, are responsible for alterations in motor function and drug-induced stereotypy in these transgenic mouse lines. Interestingly, gait patterns and basic body posture were normal in Homer1a-expressing mice. Rather, the mice showed defects in complex movements such as locomotion on a rotating rod or an elevated bar as well as in motor learning in the rotarod test over a period of 12 days, consistent with the notion that slow, metabotropic glutamatergic signaling plays more of a modulatory role rather than mediating basic functions. In contrast to a previous in vitro study based on pharmacological blockade of mGluR1/5 in substantia nigra–striatum cocultures (24), we did not find obvious defects in nigrostriatal connectivity in Homer1a-expressing mice in vivo. This discrepancy can be explained on the basis of differences in specificity of the loci of manipulations as well as the late embryonic-to-perinatal onset of CamKIIα promoterdriven expression of Homer1a. In any case, the observation that Homer1a-expressing mice develop a normal striatal structure and afferent connectivity enabled us to analyze complex motor behavioral phenotypes in the adult stage, independent of potential artifacts that could have arisen from developmental abnormalities.
Both the matrix line and the striosome line showed significant deviations in motor behaviors in some tests (such as the open-field test) and behaviors such as grooming duration, suggesting that mGluR1/5 signaling regulates the output of striatal neurons in both compartments. However, in contrast to the Homer1a-striosome mice, Homer1a-matrix mice either failed to show changes or demonstrated only statistically insignificant trends in behavioral deviations in other tests assessing motor functions more rigorously, such as the bar-cross test or the rotarod test. This observation that the Homer1a-striosome mice displayed a stronger motor behavioral phenotype than did Homer1a-matrix mice suggests that Homer1a-induced modulation of mGluR1/5-signaling in striosomes has a greater impact on function than in the matrix. Consistent with this notion, critical signaling effectors of group 1 mGluRs such as IP3Rs, phospholipase C β, and phospholipase C γ have been reported to be enriched in MOR1-positive striosomal compartments, although neither mGluR1/5 themselves nor endogenous Homer1b/c appear to display selectivity of expression over the patch–matrix subdomain of the striatum (9, 29). Furthermore, several studies have revealed that the matrix and striosomes demonstrate a high degree of functional specificity in terms of afferent–efferent connectivity as well as processing of inputs (30, 31). Whereas the matrix neurons chiefly receive inputs from the sensorimotor neocortex and are functionally associated with the course of continuous, normal locomotor behavior in response to sensory inputs, the striosomal neurons are engaged in reentrant loops with limbic structures and are functionally associated with motivational behavior, learning, and goal-directed actions (26, 32, 33). This finding supports our observation that Homer1a-striosome mice demonstrate stronger deficits in complex motor tasks, including motor learning, than do Homer1a-matrix mice.
Limbic structures, such as the anterior cingulate cortex and the amygdala, which are important in the manifestation of fear and anxiety, have been reported to project to striatal striosomes (34). Consistent with the above, we observed increased fear-associated behavior in Homer1a-matrix mice as well as Homer1a-striosome mice, suggesting that modulating mGluR1/5-signaling complex in limbic structures (as in the Homer1a-matrix line) and in their target regions in the striatum, namely the striosomes (as in the Homer1a-striosome line), may be associated with the manifestation of fear and anxiety.
One of the most interesting aspects of this study is the observation that Homer1a expression in striosomal MSNs modulates motor hyperactivity as well as induction of plasticity-related genes in the striatum produced by acute administration of the psychostimulant amphetamine. Amphetamine elicits dose-dependent, progressive hyperlocomotion and stereotypic activity by enhancing dopaminergic neurotransmission in the nucleus acumbens and the caudate putamen (23). Furthermore, plasticity-related immediate early gene products such as Fos, zif268, Fra, and Jun get induced rapidly in the striatum by amphetamine (23, 25, 26). As with the behavioral tasks described above, amphetamine-induced stereotypy and Fos expression were selectively modulated in the Homer1a-striosome line, consistent with a concept recently proposed by Canales and Graybiel (23) that suggests that a direct link exists between motor stereotypy induced by psychomotor stimulants and the degree by which the activation of striosomes exceeds the activation of matrix.
The view that manipulating the activity of mGluR1/5-signaling complexes by changes in the expression of scaffolding proteins affects behavioral sensitization evoked by addictive drugs is also supported by studies addressing the nucleus acumbens. Recent work by Kalivas and colleagues (3, 18, 35) has shown that a cocaine administration leads to a reduced expression of the long-form Homer proteins, Homer1b/c, in the nucleus acumbens (3), which has been suggested to contribute to cocaine-induced behavioral sensitization (18, 35). It is very interesting to note that a decrease in the expression of Homer1b/c likely has mechanistic consequences similar to overexpression of Homer1a expression, i.e., down-regulation in the functional assembly of the mGluR1/5 scaffolding complex. Indeed, cocaine administration is also known to induce expression of Homer1a expression in the nucleus acumbens (5). In support of a role for striatal Homer proteins in regulating drug-induced behavior as suggested by our results, the expression of Homer1a was very recently shown to be rapidly induced in the striatum by acute administration of the psychostimulant methylphenindate (Ritalin) (36).
Our observations leave open several questions as to why and how changes in functional assembly of the anchoring and signaling apparatus of type 1 mGluRs on striosomal neurons leads to altered striatal output in complex motor tasks. However, this study has addressed one factor that has long hampered further delineating the roles of the striosomal- and matrix-based striatal systems in governing motor function and drug addiction, namely, the inability to selectively target one compartment or the other with pharmacological agents. Here, a genetic approach, which permitted selective molecular manipulations in the striosomal compartments in vivo, demonstrates that a selective change in the glutamate receptor-anchoring proteins in striosomes significantly affects motor performance, including reinforcing behavior induced by a habit-forming drug.
Materials and Methods
Generation and Maintenance of Transgenic Mice. The Homer1a-specific sequence was amplified by using AgeI- and NheI-anchored primers from a plasmid containing rat Homer1a cDNA, in which the N terminus was myc-tagged and the last 15 aa of the C terminus were deleted (kindly provided by P. Worley, Johns Hopkins University, Baltimore) and cloned into the pBI-3.tri.GFP plasmid (kindly provided by R. Sprengel, Max–Planck Institute for Medical Research, Heidelberg), which contained a TetO7 element, flanked by two bidirectional cytomegalovirus minimal promoters. The resulting myc–Homer1a–EGFP construct was used to generate transgenic mice (see detailed description in Supporting Methods, which is published as supporting information on the PNAS web site). To enable tTA-dependent expression of Homer1a, Homer1a founders were bred with the TgCaMKIItTA mouse line (20). All mouse lines generated from diverse founders were backcrossed to the C57BL/6 wild-type strain and analyzed in the fifth or sixth backcross. Littermates or age-matched wild-type mice of the same genetic background were used in all experiments. In some experiments, adult wild-type and transgenic mice were treated with doxycycline (2 g/liter of drinking water with 30% sucrose; Sigma) for 10 days. Animals were maintained and analyzed according to protocols that were approved by the local governing committee. Only adult male mice (18–27 g) were used for behavioral experiments. Mice were kept in individual cages on a 12-h light–dark cycle with constant room temperature. Behavioral tests were always conducted between 1100 hours and 1600 hours by a person who was blinded to the genotype of the mice.
Motor Function Analysis and Immunohistochemistry. For details, see Supporting Methods.
Data Analysis. All data are represented as mean ± SEM. Data were statistically analyzed by performing an ANOVA using random measures followed by Fischer's post hoc test.
Supplementary Material
Acknowledgments
We are very grateful to P. W. Worley and Rolf Sprengel for help and advice in the generation of Homer1a-transgenic mice and for sharing DNA constructs and to Peter Lepczynski, Lidija Andonovic, and Hans-Joseph Wrede for expert technical assistance. This work was supported by an Emmy Noether Program grant from the Deutsche Forschungsgemeinschaft (to R.K.).
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: VLC, ventrolateral caudate; MOR, μ-opioid receptor; mGluR, metabotropic glutamate receptor; MSN, medium spiny neuron; IP3R, inositol 1,4,5-trisphosphate receptor; tTA, tetracycline transactivator.
References
- 1.Bashir, Z. I., Bortolotto, Z. A., Davies, C. H., Berretta, N., Irving, A. J., Seal, A. J., Henley, J. M., Jane, D. E., Watkins, J. C. & Collingridge, G. L. (1993) Nature 363, 347–350. [DOI] [PubMed] [Google Scholar]
- 2.Conquet, F., Bashir, Z. I., Davies, C. H., Daniel, H., Ferraguti, F., Bordi, F., Franz-Bacon, K., Reggiani, A., Matarese, V., Conde, F., et al. (1994) Nature 372, 237–243. [DOI] [PubMed] [Google Scholar]
- 3.Swanson, C. J., Baker, D. A., Carson, D., Worley, P. F. & Kalivas, P. W. (2001) J. Neurosci. 21, 9043–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paquet, M. & Smith, Y. (2003) J. Neurosci. 23, 7659–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brakeman, P. R., Lanahan, A. A., O'Brien, R., Roche, K., Barnes, C. A., Huganir, R. L. & Worley, P. F. (1997) Nature 368, 284–288. [DOI] [PubMed] [Google Scholar]
- 6.Kato, A., Ozawa, F., Saitoh, Y., Fukazawa, Y., Sugiyama, H. & Inokuchi, K. (1998) J. Biol. Chem. 273, 23969–23975. [DOI] [PubMed] [Google Scholar]
- 7.Xiao, B., Tu, J. C. & Worley, P. F. (2000) Curr. Opin. Neurobiol. 10, 370–374. [DOI] [PubMed] [Google Scholar]
- 8.Tu, J. C., Xiao, B., Yuan, J. P., Lanahan, A. A., Leoffert, K., Li, M., Linden, D. J. & Worley, P. F. (1998) Neuron 21, 717–726. [DOI] [PubMed] [Google Scholar]
- 9.Xiao, B., Tu, J. C., Petralia, R. S., Yuan, J. P., Doan, A., Brender, C. D., Ruggiero, A., Lanahan, A. A., Wenthold, R. J. & Worley, P. F. (1998) Neuron 21, 707–716. [DOI] [PubMed] [Google Scholar]
- 10.Yuan, J. P., Kiselyov, K., Shin, D. M., Chen, J., Shcheynikov, N., Kang, S. H., Dehoff, M. H., Schwarz, M. K., Seeburg, P. H., Muallem, S. & Worley, P. F. (2003) Cell 114, 777–789. [DOI] [PubMed] [Google Scholar]
- 11.Tu, J. C., Xiao, B., Naisbitt, S., Yuan, J. P., Petralia, R. S., Brakeman, P., Doan, A., Aakalu, V. K., Lanahan, A. A., Sheng, M. & Worley, P. F. (1999) Neuron 23, 583–592. [DOI] [PubMed] [Google Scholar]
- 12.Naisbitt, S., Kim, E., Tu, J. C., Xiao, B., Sala, C., Valtschanoff, J., Weinberg, R. J., Worley, P. F. & Sheng, M. (1999) Neuron 23, 569–582. [DOI] [PubMed] [Google Scholar]
- 13.Sala, C., Piech, V., Wilson, N. R., Passafaro, M., Liu, G. & Sheng, M. (2001) Neuron 31, 115–130. [DOI] [PubMed] [Google Scholar]
- 14.Thomas U. (2002) J. Neurochem. 81, 407–413. [DOI] [PubMed] [Google Scholar]
- 15.Sala, C., Futai, K., Yamamoto, K., Worley, P. F., Hayashi, Y. & Sheng, M. (2003) J. Neurosci. 16, 6327–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klugmann, M., Wymond Symes, C., Leichtlein, C. B., Klaussner, B. K., Dunning, J., Fong, D., Young, D. & During, M. J. (2005) Mol. Cell. Neurosci. 28, 343–356. [DOI] [PubMed] [Google Scholar]
- 17.Foa, L., Rajan, I., Haas, K., Wu, G. Y., Brakeman, P., Worley, P. & Cline, H. (2003) Nat. Neurosci. 4, 499–506. [DOI] [PubMed] [Google Scholar]
- 18.Szumlinski, K. K., Dehoff, M. H., Kang, S. H., Frys, K. A., Lominac, K. D., Klugmann, M., Rohrer, J., Griffin, W., Toda, S., Champtiaux, N. P., et al. (2004) Neuron 43, 401–413. [DOI] [PubMed] [Google Scholar]
- 19.Potschka, H., Krupp, E., Ebert, U., Gumbel, C., Leichtlein, C., Lorch, B., Pickert, A., Kramps, S., Young, K., Grune, U., et al. (2002) Eur. J. Neurosci. 16, 2157–2165. [DOI] [PubMed] [Google Scholar]
- 20.Mayford, M., Bach, M. E., Huang, Y. Y., Wang, L., Hawkins, R. D. & Kandel, E. R. (1996) Science 274, 1678–1683. [DOI] [PubMed] [Google Scholar]
- 21.Ivkovic, S. & Ehrlich, M. E. (1999) J. Neurosci. 19, 5409–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De las Heras, S., Hontanilla, B., Mengual, E. & Gimenez-Amaya, J. M. (1994) J. Morphol. 221, 291–307. [DOI] [PubMed] [Google Scholar]
- 23.Canales, J. J. & Graybiel, A. M. (2000) Nat. Neurosci. 3, 377–387. [DOI] [PubMed] [Google Scholar]
- 24.Plenz, D. & Kitai, S. T. (1998) J. Neurosci. 18, 4133–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao, L. & Wang, J. Q. (2002) Brain Res. 924, 167–175. [DOI] [PubMed] [Google Scholar]
- 26.Moratalla, R., Elibol, B., Vallejo, M. & Graybiel, A. M. (1996) Neuron 17, 147–156. [DOI] [PubMed] [Google Scholar]
- 27.Canales, J. J. (2005) Neurobiol. Learn. Mem. 83, 93–103. [DOI] [PubMed] [Google Scholar]
- 28.Pierce, R. C. & Kalivas, P. W. (1997) Brain Res. Brain Res. Rev. 25, 192–216. [DOI] [PubMed] [Google Scholar]
- 29.Fotuhi, M., Dawson, T. M., Sharp, A. H., Martin, L. J., Graybiel, A. M. & Snyder, S. H. (1993) J. Neurosci. 13, 3300–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kincaid, A. E. & Wilson, C. J. (1996) J. Comp. Neurol. 374, 578–592. [DOI] [PubMed] [Google Scholar]
- 31.Bayer, S. A. (1990) Exp. Neurol. 107, 132–142. [DOI] [PubMed] [Google Scholar]
- 32.White, N. M. & Hiroi, N. (1998) Proc. Natl. Acad. Sci. USA 95, 6486–6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown, L. L., Feldman, S. M., Smith, D. M., Cavanaugh, J. R., Ackermann, R. F. & Graybiel, A. M. (2002) J. Neurosci. 22, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eblen, F.& Graybiel, A. M. (1995) J. Neurosci. 15, 5999–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghasemzadeh, M. B., Permenter, L. K., Lake, R., Worley, P. F. & Kalivas, P. W. (2003) Eur. J. Neurosci. 18, 1645–1651. [DOI] [PubMed] [Google Scholar]
- 36.Yano, M. & Steiner, H. (2005) Neuroscience 132, 855–865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.