Abstract
Objective: Atypical antipsychotic drugs have been shown to protect PC12 cells from cell death induced by a variety of stimuli in culture. Recently, it has been postulated that trophic factors, such as brain-derived neurotrophic factor (BDNF), play a role in preventing cell death. It has been shown that antipsychotic drugs attenuate the decrease in rat hippocampal BDNF that results from immobilization-induced stress. We aimed to determine whether the neuroprotective effects of antipsychotic drugs could be mediated through glial cell line–derived neurotrophic factor (GDNF). Methods: We investigated the effects of the atypical antipsychotic drugs quetiapine and clozapine and the typical antipsychotic haloperidol on the secretion of GDNF from rat C6 glioma cells. Results: All 3 drugs increased the amount of GDNF secreted from C6 glioma cells into the medium after 48-hour culture. The intracellular content of GDNF was not altered by treatment with any of the antipsychotic drugs. None of the antipsychotic drugs decreased cell number. Conclusion: This study suggests that stimulation of GDNF release from glial cells by antipsychotic drugs might underlie some of their neuroprotective properties in situ.
Medical subject headings: antipsychotic agents; cell death; glial cell line–derived neurotrophic factor; models, animal
Abstract
Objectif : On a démontré que les antipsychotiques atypiques protègent les cellules PC12 contre la mort cellulaire provoquée par divers stimuli en milieu de culture. On a posé récemment en hypothèse que des facteurs trophiques comme le BDNF (facteur neurotrophique dérivé du cerveau) jouent un rôle dans la prévention de la mort cellulaire. On a montré que les antipsychotiques atténuent la diminution des concentrations de BDNF dans l'hippocampe de rat causée par le stress provoqué par l'immobilisation. Nous voulions démontrer s'il était possible de provoquer les effets neuroprotecteurs des antipsychotiques par un facteur neurotrope dérivé de lignées de cellules gliales (GDNF). Méthodes : Nous avons étudié les effets de la quétiapine et de la clozapine, antipsychotiques atypiques, ainsi que de l'halopéridol, antipsychotique typique, sur la sécrétion de facteurs GDNF dans des cellules de gliome de rat C6. Résultats : Les trois médicaments ont augmenté la quantité de GDNF sécrétée par les cellules de gliome de rat C6 dans les milieux après une culture de 48 heures. Aucun des antipsychotiques n'a modifié la concentration intracellulaire de GDNF. Aucun des antipsychotiques n'a réduit le nombre de cellules. Conclusion : Cette étude indique que la stimulation de la libération de GDNF des cellules gliales par les antipsychotiques pourrait sous-tendre certaines de leurs caractéristiques neuroprotectrices in situ.
Introduction
Atypical antipsychotic drugs are normally used to treat schizophrenia but, more recently, their usefulness in treating other psychiatric disorders and neurodegenerative diseases, such as Alzheimer's disease, has been explored. The atypical antipsychotic drugs quetiapine, clozapine and risperidone have been reported to protect cultured PC12 cells from cell death induced by serum withdrawal.1 Olanzapine also protects PC12 cells from cell death induced by H2O2 or β-amyloid peptide treatment.2,3 Similarly, quetiapine, clozapine, olanzapine and risperidone protect PC12 cells from N-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis.4
It has been postulated that targeting the synthesis and secretion of neurotrophic factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glial cell line–derived neurotrophic factor (GDNF), might be a new approach to treating neurodegenerative and depressive disorders.5–7 NGF and BDNF have been implicated in the neuroprotective actions of antipsychotic drugs.8–12 GDNF, a trophic factor for dopaminergic and other neurons,13,14 has well-documented neuroprotective effects.14–16 Antidepressant drugs, but not the typical antipsychotic drug haloperidol, have been reported to increase GDNF release from rat C6 glioma cells.17
As outlined here, both atypical antipsychotic drugs and GDNF are neuroprotective agents. The goal of this paper was to determine whether the effects of atypical antipsychotic drugs could be mediated through GDNF. A previous study of the effects of antidepressant and antipsychotic drugs on GDNF used C6 glioma cells, a rich source of GDNF;17 therefore, in order to compare our data, we also used C6 glioma cells to study the effects of quetiapine and clozapine on GDNF secretion. Haloperidol was included for comparative purposes.
Methods
The rat C6 glioma cell line was obtained from the American type Culture Collection (Manassas, Va.). We used 2 different culture media: Dulbecco's modified Eagle's medium (DMEM) and serum-free Opti-MEM (GIBCO, Grand Island, NY). All media contained 100 U/mL of penicillin and 100 μg/ mL of streptomycin (GIBCO). Incubations were conducted at 37°C in 5% CO2 and 95% air.
The C6 cells were grown in DMEM supplemented with 2 mmol/L L-glutamine (GIBCO) and 5% fetal bovine serum (Hyclone, Logan, Utah). Cells were seeded into 6-well plates (Falcon, Franklin Lakes, NJ) at a density of 4 ∞ 105/mL in 1.0 mL of growth medium, allowed to adhere for 24 hours, and then medium was replaced with serum-free Opti-MEM (GIBCO) containing 0.5% bovine serum albumin (BSA) (Sigma Chemical Co., Louis, Mo.). The cells were then incubated for 24 hours. The medium was subsequently replaced with fresh Opti-MEM plus 0.5% BSA (1.0 mL) containing the test drug, and the cells were incubated for 48 hours.
The conditioned medium was aspirated and the GDNF content was measured, as described below. In addition, the intracellular GDNF content was measured. The cells were lysed with the 1% NP-40 lysis buffer provided with the enzyme-linked immunosorbent assay (ELISA) kit.
GDNF protein levels in cell-conditioned media and cell lysates were determined using a GDNF ELISA kit, according to the manufacturer's instructions (Promega, Madison, Wis.). Briefly, Maxisorp 96-well, flat-bottomed ELISA plates (Nunc, Roskilde, Denmark) were coated with anti-GDNF monoclonal antibody and incubated overnight at 4°C. Samples and standards were incubated at room temperature for 6 hours. The captured GDNF was incubated overnight at 4°C with chicken anti-human GDNF polyclonal antibody. After the plates were washed, horseradish peroxidase–conjugated anti-chicken immunoglobulin Y antibody was added to the plates (100 μL per well) and incubated at room temperature for 2 hours. The plates were washed, and the enzyme substrate was added at 100 μL per well. The plates were incubated for 15 minutes at room temperature in the dark. The absorbance at a wavelength of 450 nm was recorded on a microplate reader (Molecular Devices, Sunnyvale, Calif.).
The cell number was determined using a cell-counting assay kit (Dojindo, Gaithersburg, Md.). In this assay, WST-8 (2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfo-phenyl]- 2H-tetrazolium, monosodium salt) is reduced by dehydrogenases in the cells to give a soluble yellow-coloured product (formazan). The amount of formazan dye generated by the activity of dehydrogenases is directly proportional to the number of living cells. The sensitivity of the WST-8 kit is higher than that of kits using other tetrazolium salts, such as MTT, XTT or MTS. Briefly, after treatment with quetiapine, clozapine or haloperidol, 10 μL of the Cell Counting Kit-8 solution was added to each well in a 96-well plate. After incubation for 2 hours at 37°C, the absorbance of the samples at a wavelength of 450 nm was measured by a UV Max kinetic microplate reader (Molecular Devices).
The effects of the drug treatments on C6 glioma cell number and concentrations of GDNF were analyzed by 1-way analysis of variance (ANOVA); then, if a significant F value was obtained, the drug-treated samples were compared with drug vehicle control using the Tukey Honestly Significant Difference (HSD) test.
Results
The amounts of GDNF present in the cell-conditioned medium after treatment for 48 hours with vehicle or with an antipsychotic drug are shown in Figure 1. Treatment with quetiapine at concentrations of 5 μmol/L, 10 μmol/L, 15 μmol/L and 25 μmol/L significantly increased the GDNF content of the conditioned media to 128 (standard error of the mean [SEM] 9.2) pg/mL, 158 (SEM 9.6) pg/mL, 169 (SEM 5.0) pg/mL and 221 (SEM 10.0) pg/mL, respectively, compared with the control value of 45.1 (SEM 5.1) pg/mL (Fig. 1A). Similarly, clozapine at concentrations of 5 μmol/L, 10 μmol/L, 15 μmol/L and 25 μmol/L increased GDNF release significantly to 98.7 (SEM 1.3) pg/mL, 129 (SEM 5.2) pg/mL, 149 (SEM 3.9) pg/mL and 192 (SEM 8.7) pg/mL, respectively, compared with the control value of 57.1 (SEM 3.7) pg/mL (Fig. 1B). Haloperidol at concentrations of 10 μmol/L, 15 μmol/L and 25 μmol/L increased GDNF release significantly from 81.1 (SEM 1.1) pg/mL in the control condition to 126 (SEM 5.5) pg/mL, 139 (SEM 4.2) pg/mL and 219 (SEM 9.7) pg/mL, respectively (Fig. 1C).
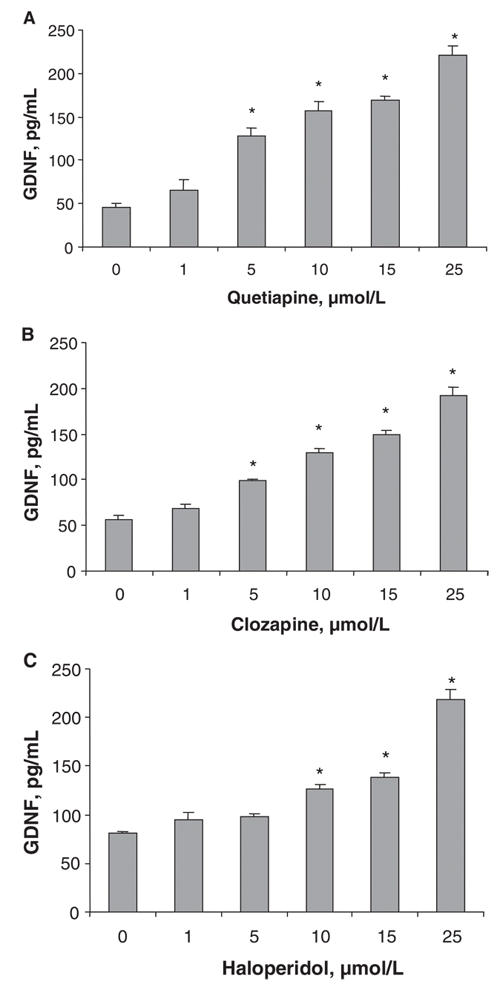
Fig. 1: The effects of quetiapine, clozapine and haloperidol on the GDNF content of conditioned media from cultured C6 cells. C6 cells were treated with different concentrations of (A) quetiapine, (B) clozapine or (C) haloperidol for 48 hours. GDNF protein levels in cell-conditioned media were detected using a GDNF ELISA kit. Values (means and standard error of the mean, n = 3) from 1 of 2 experiments are shown. *p < 0.01 compared with control. ELISA = enzyme-linked immunosorbent assay, GDNF = glial cell line– derived neurotrophic factor.
The release of GDNF by quetiapine, 25 μmol/L, was measured at different time periods up to 48 hours (Fig. 2). It can be seen that the amount of GDNF released in the presence of quetiapine was significantly increased only at the 48-hour time point compared with the appropriate control (media alone at the same time period).
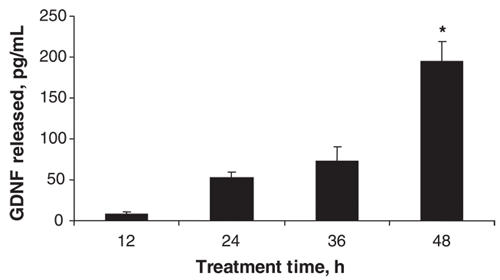
Fig. 2: The effect of quetiapine on the GDNF content of conditioned media from cultured C6 cells at various times of treatment. Cells were treated with quetiapine, 25 mmol/L, for 12, 24, 36 and 48 hours. Values (means and standard error of the mean, n = 3) from 1 of 2 experiments are shown. *p < 0.05 compared with respective control (media alone at the same time period). GDNF = glial cell line–derived neurotrophic factor.
In order to determine whether the release of GDNF was the result of leakage from damaged cells, we measured the amounts of GDNF located within the C6 cells and the effects of the antipsychotic drugs on cell number. Treating the C6 cells with concentrations of quetiapine, clozapine or haloperidol ranging from 1 μmol/L to 25 μmol/L for 48 hours did not change the amount of GDNF present in the cell lysates compared with controls (Fig. 3).
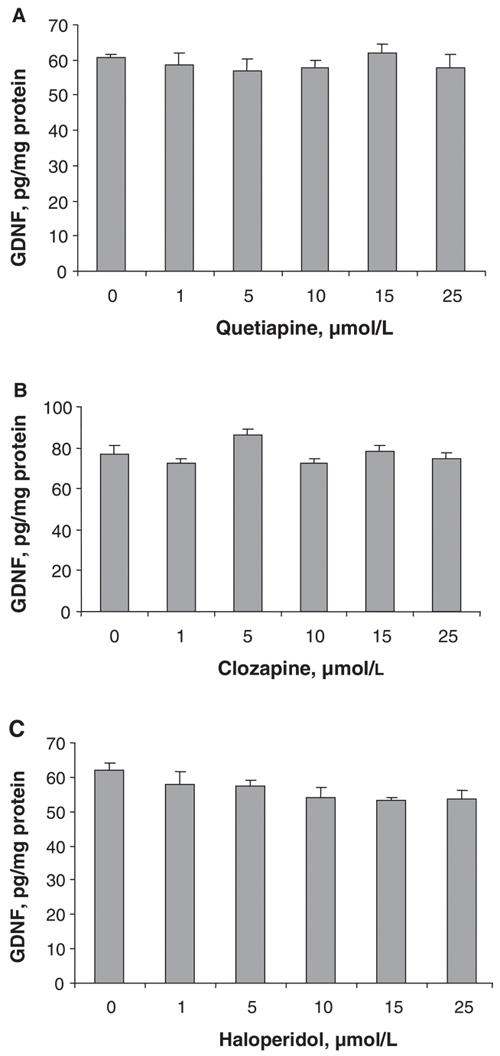
Fig. 3: The effects of quetiapine, clozapine and haloperidol on GDNF levels in cell lysates. C6 cells were cultured and treated with different concentrations of (A) quetiapine, (B) clozapine or (C) haloperidol for 48 hours. GDNF protein levels in cell lysates were detected using a GDNF ELISA kit. Values (means and standard error of the mean, n = 3) from 1 of 2 experiments are shown. ELISA = enzyme-linked immunosorbent assay, GDNF = glial cell line– derived neurotrophic factor.
Treating the cells with quetiapine, clozapine or haloperidol at concentrations ranging from 1 μmol/L to 25 μM for 48 hours in serum-free conditions had no effect on the cell number compared with vehicle controls (Fig. 4). There was neither an increase nor a decrease in the number of cells, indicating that the antipsychotic drugs had no effect on cell proliferation or cell death.
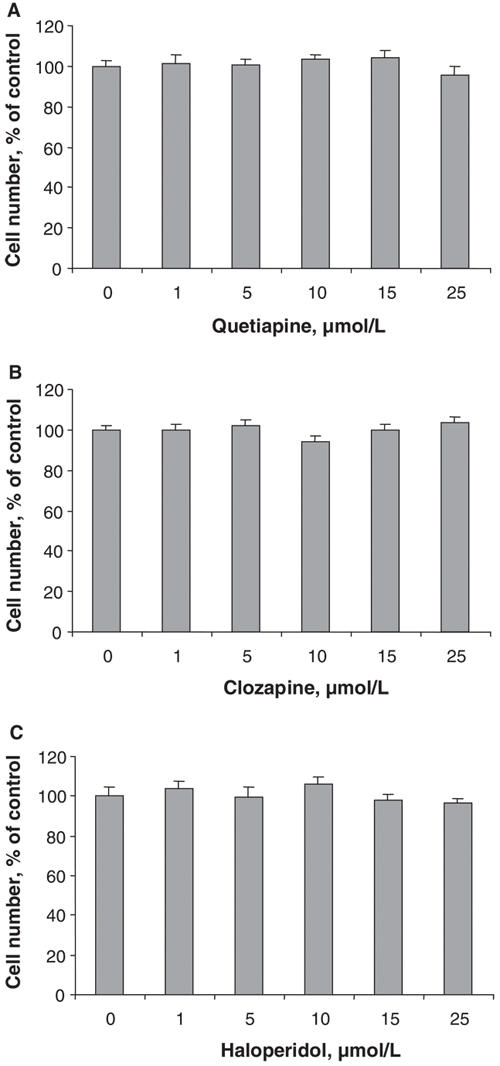
Fig. 4: The effects of quetiapine, clozapine and haloperidol on the cell growth of C6 cells cultured in a serum-free system. C6 cells were treated with (A) quetiapine, (B) clozapine or (C) haloperidol for 48 hours. Values (means and standard error of the mean, n = 3) from 1 of 2 experiments are shown.
Discussion
A previous paper investigated the effects of a variety of antidepressant drugs (amitriptyline, clomipramine, mianserin, fluoxetine and paroxetine) and other psychoactive drugs on GDNF secretion from C6 cells.17 Significant GDNF secretion occurred with antidepressant drug concentrations starting at 10 μmol/L. Similar to our results (see Fig. 1), it was found that haloperidol at a concentration of 1 μmol/L did not cause a significant increase in GDNF secretion.
Our results, however, show that concentrations of the antipsychotic drugs quetiapine, clozapine and haloperidol greater than 1 μmol/L caused relatively substantial, dose-dependent secretion of GDNF from C6 glioma cells. The lowest concentrations of the drugs that significantly increased GDNF release were 5 μmol/L for quetiapine and clozapine, and 10 μmol/L for haloperidol. Although these concentrations may seem high compared with the therapeutic plasma levels, they are similar to the concentrations of antipsychotic drugs reported to increase survival of PC12 cells in culture18 and to the concentrations of serotonin (100 μmol/L) and antidepressants (1–25 μmol/L) that cause GDNF release from C6 cells.17,19 At the highest drug concentration tested (25 μmol/L), quetiapine, clozapine and haloperidol all increased GDNF levels in cell-conditioned medium 3–5 times above control levels (Fig. 1).
The GDNF present in the conditioned medium does not appear to have been the result of leakage from damaged cells, because the drug treatments neither decreased the intracellular GDNF levels (analyzed by lysing the cells and measuring GDNF in the lysates), nor did they decrease the number of C6 cells (Fig. 3 and Fig. 4, respectively). If the GDNF secretion were simply a consequence of leakage from cells damaged by antipsychotic drug treatment, one would expect to find a decrease in intracellular GDNF levels or a decrease in the number of cells over time compared with controls, or both. Such decreases were not found. Treatment with the antipsychotic drugs at concentrations up to 25 μmol/L neither decreased the amount of GDNF present in the cell lysates, nor decreased the cell number compared with controls. Thus, the GDNF released into the conditioned media does not appear to be the result of leakage from damaged cells. Furthermore, the time course of GDNF release showed that a significant release was obtained only after a 48-hour incubation period (Fig. 2). This is the same pattern that was reported for serotonin-stimulated and for antidepressant-stimulated release of GDNF from C6 cells: significant release was observed after 48-hour drug treatment, but not at earlier times.17,19 If leakage of GDNF was occurring, a continuous release over time would be observed.
It has been reported that dopamine itself, several mixed dopamine agonists and dopamine D1 agonists increase GDNF secretion from primary cultures of rodent astrocytes and mesencephalic cells; however, a D2 agonist decreased secretion of GDNF.20–23 It is well known that antipsychotic drugs block D2 receptors. Our results, therefore, that antipsychotic drugs increase GDNF secretion, might involve blocking a D2-inhibition of GDNF secretion.
A recent study showed that serotonin, 100 μmol/L, but not dopamine or noradrenaline, increased the secretion of GDNF from rat C6 cells.19 This effect was partially blocked by inhibition of the mitogen-activated protein (MAP) kinase signalling pathway, but not by inhibition of protein kinase A or protein kinase C. However, inhibition of protein kinase A alone (i.e., not in combination with any other treatment) or the presence of dibutyrl cyclic adenosine monophosphate (cAMP) increased GDNF secretion from C6 glioma cells.24 Thus, it seems that, depending on the experimental conditions, the secretion of GDNF from C6 glioma cells can involve more than one intracellular signalling pathway. It is interesting to note that antipsychotic drugs (olanzapine, risperidone, fluphenazine, clozapine, chlorpromazine) have been reported to have direct effects via a G protein on several signalling cascades, such as those involving Akt and mitogen-activated protein kinase (MEK)/extracellular regulated kinase (ERK), that are important in cell growth and survival.18 The G-protein-coupled receptor that these drugs acted upon was not identified.18 Further studies are necessary to determine whether the antipsychotic drug–stimulated release of GDNF from C6 involves a similar direct effect on the latter pathways.
Although it has been postulated that modulation of the synthesis and secretion of trophic factors such as NGF, BDNF and GDNF by antidepressants might be a novel approach to treating depression, other psychiatric disorders and Parkinson's disease,5–7 such a possibility has only recently been considered for antipsychotic drugs.10–12 Our data show that antipsychotic drugs can increase GDNF secretion from C6 glioma cells; this points to a new pathway through which antipsychotic drugs could be neuroprotective. If antipsychotic drugs can also stimulate release of GDNF from glial cells in vivo, this might protect neurons from degeneration associated with diseases such as schizophrenia or Alzheimer's disease, or degeneration caused by toxic insults such as oxidative stress. Interestingly, R-deprenyl, a putative neuroprotective drug, has been shown to increase GDNF release from cultured mouse astrocytes.25–27
In summary, GDNF release from C6 glioma cells was stimulated by quetiapine and clozapine, at concentrations ranging from 5 μmol/L to 25 μmol/L, and by haloperidol, at concentrations ranging from 10 μmol/L to 25 μmol/L. This increase in GDNF secretion or release by antipsychotic drugs offers a novel mechanism whereby these drugs might act in the treatment of neurodegenerative disorders. Further study is necessary to explore and confirm the exact mechanisms whereby antipsychotic drugs modulate GDNF release from C6 glioma cells.
Acknowledgments
This research was supported by a Canadian Institutes of Health Research/R&D Postdoctoral fellowship to Dr. Shao (200204DFE-102742-UI-CCAA-120040) and by Saskatchewan Health. We would like to thank R. Mag-atas and G. Stegeman for technical assistance and Drs. S. Fedoroff and D. Mousseau for helpful discussion.
Footnotes
Contributors: Drs. Shao, Dyck and Li conceived and designed the study. Drs. Shao and Wang acquired and analyzed the data. Drs. Shao, Dyck and Li drafted the article, and Drs. Shao, Dyck and Wang revised it. All authors gave final approval for the article to be published.
Competing interests: None declared for Drs. Shao and Wang. Dr. Dyck has received travel assistance from AstraZeneca. Dr. Li has received a research grant, educational grants for poster presentations and travel assistance from AstraZeneca.
Correspondence to: Dr. Lillian E. Dyck, Neuropsychiatric Research Unit, University of Saskatchewan, 103 Wiggins Rd., Saskatoon SK S7N 5E4; fax 306 966-8830;Lillian.Dyck@usask.ca
References
- 1.Bai O, Wei Z, Lu W, et al. Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. J Neurosci Res 2002; 69:278-83. [DOI] [PubMed]
- 2.Wei Z, Bai O, Richardson JS, et al. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J Neurosci Res 2003;73:364-8. [DOI] [PubMed]
- 3.Wei Z, Mousseau DD, Richardson JS, et al. Atypical antipsychotics attenuate neurotoxicity of β-amyloid(25-35) by modulating Bax and Bcl-XL/S expression and localization. J Neurosci Res 2003;74:942-7. [DOI] [PubMed]
- 4.Qing H, Xu H, Wei Z, et al. The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis. Eur J Neurosci 2003;17:1563-70. [DOI] [PubMed]
- 5.Vaiyda VA, Duman RS. Depression – emerging insights from neurobiology. Br Med Bull 2001;57:61-79. [DOI] [PubMed]
- 6.Duman RS. Synaptic plasticity and mood disorders. Mol Psychiatry 2002; 7(Suppl 1):S329-34. [DOI] [PubMed]
- 7.Obara Y, Nakahata N. The signaling pathway of neurotrophic factor biosynthesis. Drug News Perspect 2002;15:290-8. [DOI] [PubMed]
- 8.Angelucci F, Mathe AA, Aloe L. Brain-derived neurotrophic factor and tyrosine kinase receptor TrkB in rat brain are significantly altered after haloperidol and risperidone administration. J Neurosci Res 2000;60:783-94. [DOI] [PubMed]
- 9.Takahashi M, Shirakawa O, Toyooka K, et al. Abnormal expression of brain-derived neurotrophic factor and receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry 2000; 5: 293-300. [DOI] [PubMed]
- 10.Chlan-Fourney J, Ashe P, Nylen K, et al. Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res 2002;954:11-20. [DOI] [PubMed]
- 11.Xu H, Qing H, Lu W, et al. Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett 2002;321:65-8. [DOI] [PubMed]
- 12.Bai O, Chlan-Fourney J, Bowen R, et al. Expression of brain-derived mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res 2003;71:127-31. [DOI] [PubMed]
- 13.Lin LF, Doherty DH, Lile JD, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993;260:1130-2. [DOI] [PubMed]
- 14.Lin LF. Glial cell line-derived neurotrophic factor (GDNF): a comprehensive review. Neural Notes 1996;11:3-7.
- 15.Lara J, Kusano K, House S, et al. Interactions of cyclic adenosine monophosphate, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor treatment on the survival and growth of postnatal mesencephalic dopamine neurons in vitro. Exp Neurol 2003;180:32-45. [DOI] [PubMed]
- 16.Ugarte SD, Lin E, Klann E, et al. Effects of GDNF on 6-OHDA-induced death on a dopaminergic cell line: modulation by inhibitors of PI3 kinase and MEK. J Neurosci Res 2003;73:105-12. [DOI] [PubMed]
- 17.Hisaoka K, Nishida A, Koda T, et al. Antidepressant drug treatments induce glial cell line-derived neurotrophic factor (GDNF) synthesis and release in rat C6 glioblastoma cells. J Neurochem 2001; 79:25-34. [DOI] [PubMed]
- 18.Lu XH, Bradley RJ, Dwyer DS. Olanzapine produces trophic effects in vitro and stimulates phosphorylation of Akt/PKB, ERK1/2, and the mitogen-activated protein kinase p38. Brain Res 2004; 1011: 58-68. [DOI] [PubMed]
- 19.Hisaoka K, Nishida A, Takebayashi M, et al. Serotonin increases glial cell line-derived neurotrophic factor release in rat C6 glioblastoma cells. Brain Res 2004;1002:167-70. [DOI] [PubMed]
- 20.Ohta M, Mizuta I, Ohta K, et al. Apomorphine up-regulates NGF and GDNF synthesis in cultured mouse astrocytes. Biochem Biophys Res Commun 2000;272:18-22. [DOI] [PubMed]
- 21.Ohta K, Kuno S, Mizuta I, et al. Effects of dopamine agonist bromocriptine, pergolide, cabergoline, and SKF-38393 on GDNF, NGF, and BDNF synthesis in cultured mouse astrocytes. Life Sci 2003;73:617-26. [DOI] [PubMed]
- 22.McNaught KS, Jenner P. Dysfunction of rat forebrain astrocytes in culture alters cytokine and neurotrophic factors. Neurosci Lett 2000; 285: 61-5. [DOI] [PubMed]
- 23.Guo H, Tang Z, Yu Y, et al. Apomorphine induces trophic factors that support fetal rat mesencephalic dopaminergic neurons in culture. Eur J Neurosci 2002;16:1861-70. [DOI] [PubMed]
- 24.Verity AN, Wyatt TL, Hajos B, et al. Regulation of glial cell line-derived neurotrophic factor release from rat C6 glioblastoma cells. J Neurochem 1998;70:531-9. [DOI] [PubMed]
- 25.Mizuta I, Ohta M, Ohta K, et al. Selegiline and desmethylselegiline stimulate NGF, BDNF, and GDNF synthesis in cultured mouse astrocytes. Biochem Biophys Res Commun 2000;279:751-5. [DOI] [PubMed]
- 26.Shimazu S, Tanigawa A, Sato N, et al. Enhancer substances: selegiline and R-(-)-1-(benzofuran-2-yl)-2-propylaminopentane [(-)-BPAP] enhance the neurotrophic factor synthesis on cultured mouse astrocytes. Life Sci 2003;72:2785-92. [DOI] [PubMed]
- 27.Tang YP, Ma YL, Chao CC, et al. Enhanced glial cell line-derived neurotrophic factor mRNA expression upon (-)-deprenyl and melatonin treatments. J Neurosci Res 1998;53:593-604. [DOI] [PubMed]


