Abstract
Objective: Dysphoria and depression have been cited as side effects of the opioid antagonist naltrexone. We aimed to assess whether depressive symptoms are a clinically relevant side effect in a population receiving naltrexone as a treatment for opioid dependence. Methods: We carried out a randomized controlled, open-label trial comparing rapid opiate detoxification under anesthesia and naltrexone treatment with continued methadone maintenance at the Alcohol and Drug Service, Royal Brisbane and Women's Hospital, Brisbane, Australia. The study subjects were patients stabilized on methadone maintenance treatment for heroin dependence who wished to transfer to naltrexone treatment. The Beck Depression Inventory, State–Trait Anxiety Inventory and Opiate Treatment Index subscales for heroin use and social functioning were used at baseline and follow-up assessments at 1, 2, 3 and 6 months. Results: Forty-two participants were allocated to receive naltrexone treatment, whereas 38 continued methadone maintenance as the control condition. Participants who received naltrexone did not exhibit worsening of depressive symptoms. In participants attending all follow-up assessments, there was a trend for those receiving naltrexone to exhibit an improvement in depression over time compared with the control group. Participants who were adherent to naltrexone treatment exhibited fewer depressive symptoms than those who were nonadherent. Conclusions: These results suggest that depression need not be considered a common adverse effect of naltrexone treatment or a treatment contraindication and that engaging with or adhering to naltrexone treatment may be associated with fewer depressive symptoms.
Medical subject headings: anxiety, clinical trials, depression, heroin dependence, methadone, mood disorders, narcotic antagonists, naltrexone, safety, treatment outcome
Abstract
Objectif : On a mentionné la dysphorie et la dépression comme effets secondaires du naltrexone, antagoniste des opiacés. Nous voulions déterminer si les symptômes dépressifs constituent un effet secondaire pertinent sur le plan clinique dans une population prenant du naltrexone comme traitement contre une accoutumance aux opiacés. Méthodes : Au service de traitement de l'alcoolisme et des toxicomanies du Royal Brisbane and Women's Hospital, à Brisbane, en Australie, nous avons procédé à un essai contrôlé randomisé ouvert pour comparer une détoxification rapide des opiacés sous anesthésie et un traitement au naltrexone conjugué à un traitement de maintien soutenu à la méthadone. Les sujets à l'étude étaient des patients stabilisés par un traitement de maintien à la méthadone pour une dépendance à l'héroïne et qui voulaient passer au traitement au naltrexone. Nous avons utilisé l'inventaire de dépression de Beck, le questionnaire sur l'anxiété chronique et réactionnelle de State–Trait et les sous-échelles de l'indice des traitements contre les opiacés pour la consommation d'héroïne et le fonctionnement social afin d'effectuer des évaluations de référence et de suivi à 1, 2, 3 et 6 mois. Résultats : On a désigné 42 participants qui suivraient le traitement au naltrexone tandis que 38 sont demeurés sur le maintien à la méthadone comme condition témoin. Les symptômes dépressifs ne se sont pas aggravés chez les participants qui ont pris le naltrexone. Parmi ceux qui se sont présentés à toutes les évaluations de suivi, ceux qui prenaient le naltrexone avaient tendance à montrer une amélioration de la dépression au fil du temps, comparativement aux sujets du groupe témoin. Les participants qui ont observé fidèlement le traitement au naltrexone montraient moins de symptômes dépressifs que ceux qui ne l'ont pas observé. Conclusions : Ces résultats indiquent qu'il ne faut pas considérer la dépression comme un effet indésirable courant du traitement au naltrexone ou comme une contre-indication au traitement, et que l'on peut établir un lien entre l'engagement avec le traitement au naltrexone ou l'observation fidèle du traitement au naltrexone et des symptômes dépressifs moins nombreux.
Introduction
Naltrexone is a competitive mu opioid receptor antagonist used in the treatment of opioid dependence. Because its affinity for mu receptors is greater than that of heroin and other opioid agonists, naltrexone is able to block the effects of other opioids.1 This may lead to extinguishing of drug-taking behaviour and may reduce opiate craving.2–4 Furthermore, administration of naltrexone to an opioid-dependent individual will trigger a withdrawal syndrome. This is the rationale for rapid opiate detoxification, a procedure often undertaken before initiating treatment with naltrexone, often in conjunction with sedation or anesthesia.5–9
There have been claims of good treatment outcomes associated with naltrexone. For example, some studies report that naltrexone treatment produces a reduction in heroin use or reductions in reincarcerations compared with placebo or other treatments.10,11 However, many reports also describe poor retention rates for individuals undergoing naltrexone treatment12–14 or low rates of uptake into naltrexone treatment,15 which may undermine its potential benefits.
Depression and dysphoria have been cited as adverse effects of naltrexone. The presence of, or concern about, these adverse effects may contribute to poor treatment uptake or retention. One of the early reports linking naltrexone with depression arose from a study of volunteers with no history of opioid or other drug use.16 Compared with placebo, a single 50-mg dose of naltrexone led to a range of unpleasant symptoms, including dysphoria. Since that time, experimental studies have described depression or dysphoria associated with the use of naltrexone in healthy volunteers17 or opioid-free former addicts.18
There is conflicting evidence as to whether depressive symptoms or dysphoria are clinically important adverse effects in patients receiving naltrexone treatment. In an uncontrolled cohort study, Miotto and colleagues19 reported that 81 patients who were receiving naltrexone for opioid dependence exhibited higher than expected rates of overdose and suicide. Despite this, depressive symptoms improved during the course of treatment. A controlled trial of naltrexone in an alcohol-dependent population20 reported that although depressive symptoms improved in naltrexone-treated and placebo-treated patients over time, a significantly higher proportion of naltrexone-treated patients had elevated depression scores at study completion compared with those receiving placebo. However, it was not established whether this difference reflected a side effect of naltrexone or greater attrition of depressed subjects from the placebo group. Few controlled studies have examined depressive symptoms in heroin users receiving naltrexone treatment for opiate dependence.
The aim of this study was to investigate the impact of naltrexone treatment on depressive symptoms in a controlled clinical study. We hypothesized that subjects taking naltrexone would experience an increase in depressive symptoms after initiation of naltrexone treatment and that the presence of depressive symptoms would be associated with a poorer treatment outcome.
Methods
This study is based on the findings of a larger research undertaking comparing rapid opiate detoxification and naltrexone with methadone maintenance as a treatment for opioid dependence. Ethics approval was obtained from the Human Research Ethics Committee of the Royal Brisbane and Women's Hospital. Recruitment and follow-up took place between March 1999 and December 2002.
We recruited 80 opioid-dependent individuals who were receiving methadone maintenance treatment who had expressed an interest in transferring to naltrexone treatment. Participants were recruited by clinical referral or self-referral through advertising in various alcohol and drug services throughout Brisbane and other relevant services. Inclusion criteria were the following: age between 18 and 60 years; a history of methadone maintenance for at least 1 year; at least 2 unsuccessful attempts to cease methadone maintenance; a stable residence (no more than 2 changes of residence in the previous 6 months); and the presence of a non-drug-using support person who would supervise naltrexone administration on a daily basis. Exclusion criteria included symptomatic chronic liver disease, active psychosis, severe sedative dependence (> 40 mg diazepam equivalents per day) or dependence on alcohol, amphetamines or cocaine. Although a history of a depressive disorder or the use of antidepressants were not a basis for exclusion, potential participants who had current severe major depressive disorder or were considered to be at high risk of suicide were excluded from the study. This was ascertained by a thorough psychiatric assessment.
After telephone screening, potentially eligible subjects were invited to attend the clinic for assessment. After they had been provided with verbal and written information about the study (including the patient information sheet), eligible participants provided written informed consent. Subsequent assessment included a physical examination, blood tests (liver function tests, hepatitis B and C, and HIV serology) and a psychiatric assessment.
Randomization took place after provision of informed consent and was carried out by an independent research facility (National Health and Medical Research Council Clinical Trials Centre). The research team provided participant IDs to the facility via a central telephone service and were then provided with details of allocation outcome. Subjects were evenly randomly allocated to receive either rapid opioid detoxification under anesthesia (RODA) followed by naltrexone maintenance for up to 12 months or continued methadone maintenance for 6 months. This study was not blinded, and researchers, clinicians and participants were aware of what intervention participants were receiving.
The treatment group continued methadone maintenance until the day of admission. On admission, all patients received a detoxification regime including clonidine and diazepam for 36 hours. Patients then underwent general anesthesia for 4 hours. Following induction of anesthesia and intubation, naltrexone (50 mg) was administered as a slurry via a nasogastric tube; a second dose (50 mg) was administered 2 hours later. Clonidine, octreotide and ondansetron were also administered during the procedure to suppress withdrawal manifestations. Patients were monitored overnight and were typically discharged the following day. Patients received 2 further 50-mg oral doses of naltrexone on the evening of the procedure and on the day of discharge. Thereafter, patients received naltrexone, 50 mg administered orally, daily for up to 12 months. Daily dosing was supervised by a non-drug-using support person, who was typically a parent. Ongoing supportive psychosocial care was provided by the clinical team throughout treatment; however, formal psychotherapy was not provided.
Participants assigned to the control condition were to continue methadone maintenance for a further 6 months. Methadone treatment was provided by the participants' existing clinic; during this time, no additional treatment was provided by the research team. All participants assigned to the control group were offered the opportunity to undergo RODA and receive naltrexone treatment after 6 months in the control condition.
Participants underwent follow-up assessments at 1, 2, 3 and 6 months after baseline. The following instruments were used. The Beck Depression Inventory (BDI)21 is a 21-item self-report questionnaire with item scores ranging from 0 to 3 and a total score of 0–63. It has been verified as a reliable and valid screening instrument to detect intensity of depression in a variety of populations and has also been employed to measure treatment response when used in a pretest and post-test study design.22
In order to control for any potential influence of anxiety on depression measures, anxiety was measured using the State–Trait Anxiety Inventory (STAI).23 The STAI is a 40-item self-report questionnaire used to measure both current anxiety or state (20 items) and anxiety as a more enduring stable personality characteristic or trait (20 items). In this study, we used only the state component of the instrument.
The Opiate Treatment Index (OTI)24 includes subscales pertaining to drug use and social functioning. Primary drug use outcomes were measured by recent frequency (number of days that heroin was used in the previous 28 days) and the monetary expenditure (Australian dollars spent on heroin in the previous 28 days). The Social Functioning subscale contains items reflecting overall social stability and support and also incorporates a measure of involvement in drug subculture.
Craving for heroin was assessed using 2 questions on frequency of thoughts about heroin (How frequently do you have thoughts about heroin?) and desire or compulsion to use heroin (How frequently do you have the desire to use heroin?). Each was assessed on a self-report, 6-point Likert scale (0 = rarely or never, 5 = very often).
Medication adherence was assessed fortnightly using self-report and corroborated by a report from a non-drug-using support person who was engaged to supervise naltrexone administration.
The sample size was calculated on the basis of the primary outcome measure of reduced heroin use in those allocated to RODA–naltrexone compared with those continuing on methadone, using an expected effect size of 0.4 standard deviation (SD) units, a power of 0.8 and an α of 0.05 to reject the null hypothesis. The expected attrition rate in both groups over 6 months was 30%.25–27 With these parameters, the required sample size for each treatment group was determined to be 37.
Treatment effects data were analyzed using a 2-way fixed-effects repeated-measures analysis of variance (ANOVA) with the between-groups factor being treatment (naltrexone or methadone) and the within-groups factor being time (0, 1, 2, 3 and 6 months). Partial h2 values (hp2) were calculated to determine effect size. The analysis examined overall changes over time and changes over time between groups (interaction of time and treatment). This was conducted on both sets of data elicited from those individuals for whom we had complete data and also on all randomly allocated subjects using an intention-to-treat analysis with missing data imputed using the last observation carried forward. As the purpose of this study was to assess the incidence of a potential adverse effect of medication, the relation between treatment adherence and depression scores was examined. Subjects were classified as treatment adherent if they received at least 80% of their naltrexone or methadone throughout the first 3 months of the study. The first 3-month time period was selected post hoc for the adherence analysis, because early treatment represents the most clinically important period for assessment of drug-related adverse effects, and very few participants were adherent to naltrexone for the entire 6-month period, making group comparisons difficult. Using 1-way ANOVA, depression scores were compared between adherent and nonadherent participants, using both existing and imputed data at 1-month, 2-month and 3-month follow-up assessments. Effect sizes were calculated using h2. Relations between continuous variables were analyzed using bivariate correlations. For normally distributed data, Pearson's product-moment correlation coefficient, r, was used; Spearman's rank-order correlation coefficient, rho, was used for data that did not meet normality assumptions. Group differences at baseline were evaluated using independent samples t tests for continuous variables and χ2 tests for categorical variables. In all analyses, the level of significance used to reject the null hypothesis was an α of 0.05.
Results
Of the 80 participants, 42 were randomly allocated to receive RODA–naltrexone; 38 were randomly allocated to the control group and continued methadone maintenance. Demographic and baseline characteristics for each group are detailed in Table 1. No baseline differences were detected between subjects receiving RODA and naltrexone treatment and those in the control group. The primary findings of this study are reported elsewhere.28 In those receiving RODA and naltrexone treatment, 74% were engaged in treatment at 10 days, and 52% remained engaged in treatment at 3 months. Participant flow through the study is outlined in Figure 1.
Table 1
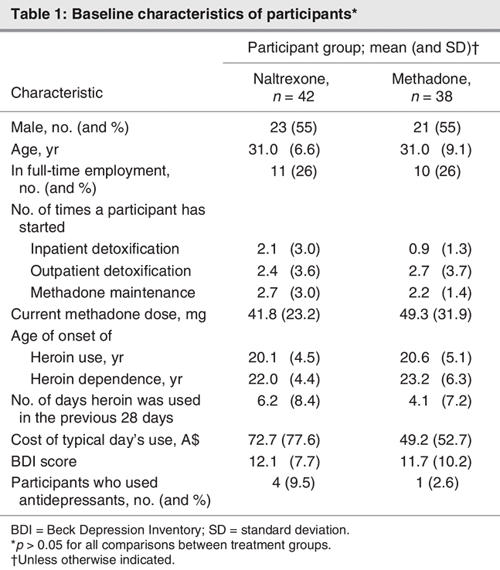
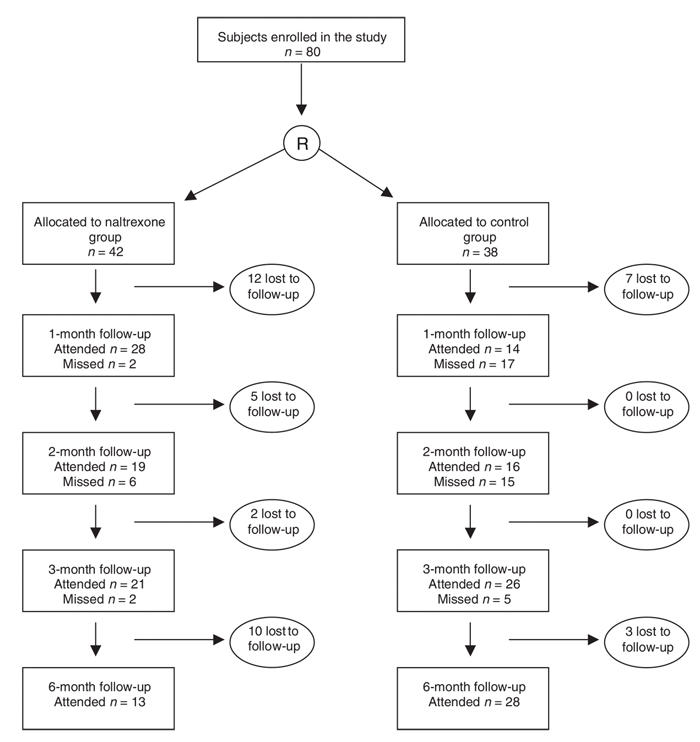
Fig. 1: Engagement in study protocol as represented by attendance at follow-up assessments throughout the study.
The mean BDI score at baseline for all participants was 11.9 (SD 8.9, range 0–37); 16% of participants had a score of 21 or greater (the accepted cut-off for moderate-to-severe depression29). BDI scores at baseline were not related to demographic variables such as sex, age, education or employment. Baseline BDI was also unrelated to drug use variables, including age of onset of heroin use or number of days heroin was used in the previous month. There was a significant positive relation between baseline depressive symptoms and baseline heroin craving measured as thoughts about heroin (Spearman r = 0.49, p = 0.010), but not when measured as desire to use heroin (Spearman r = 0.28, p = 0.16).
Treatment effects on depression and anxiety
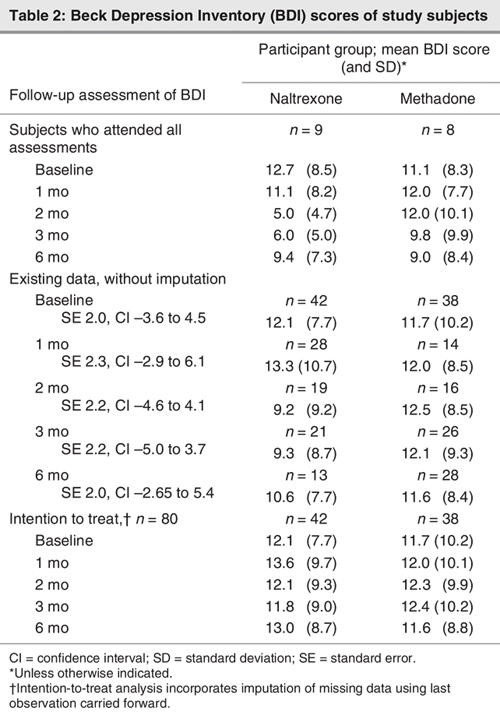
In the subset of subjects who attended all follow-up assessments (n = 17), no worsening of depression scores was observed. Indeed, there was a trend for subjects receiving naltrexone to exhibit an improvement in depression (F1,16 = 2.84, p = 0.07, hp2 = 0.49) over time compared with those on methadone maintenance. This effect was primarily the result of differences between treatment groups at 2 and 3 months; both treatment groups had similar values at 6 months. Participants who attended all follow-up assessments were fully compliant with treatment, except for the 2 receiving naltrexone who ceased naltrexone shortly before the 6-month follow-up. When analyzed on an intention-to-treat basis, there were no increases or other changes in BDI scores over time in the whole sample (F1,74 = 0.36, p = 0.83, hp2 = 0.02) and no difference between treatment groups (F1,74 = 1.30, p = 0.28, hp2 = 0.07). BDI scores are detailed in Table 2. No difference in the use of antidepressants between treatment groups was detected (naltrexone n = 16, methadone maintenance treatment n = 7), although this nonsignificant finding may have been influenced by data on antidepressant use being available for only 36% of participants.
Table 2
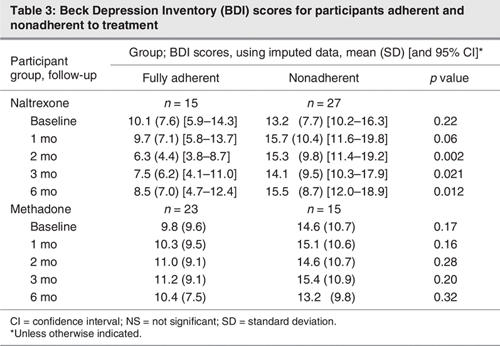
When the impact of treatment adherence on depressive symptoms was examined, the severity of symptoms was consistently lower among participants who were adherent to naltrexone treatment compared with those who were nonadherent to naltrexone treatment. This was statistically significant at month 2 (F = 11.3, p = 0.002, h2 = 0.22), month 3 (F = 5.74, p = 0.021, h2 = 0.13) and month 6 (F = 7.01, p = 0.012, h2 = 0.15), with a trend at month 1 (F = 3.91, p = 0.06, h2 = 0.09) using imputed data. There were no significant baseline differences in depressive symptoms between those adherent and nonadherent to naltrexone. There were no significant differences in depression scores between those adherent and nonadherent to methadone treatment (Table 3).
Table 3
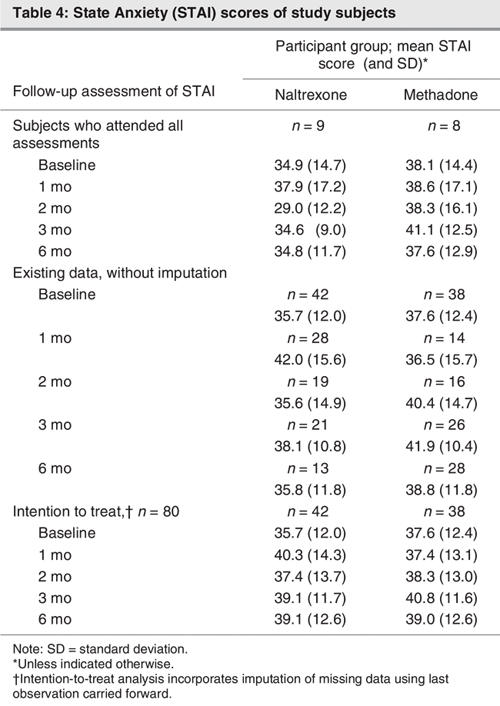
Treatment effects on anxiety were also examined. Using the intention-to-treat analysis, we observed a significant increase in anxiety symptoms in all subjects over time (F1,74 = 2.72, p = 0.037, hp2 = 0.13). There was also a trend suggesting differences between treatment groups (F1,74 = 2.27, p = 0.07, hp2 = 0.11). This was mainly because of an increase in symptoms in the naltrexone group between baseline and the 1-month assessment, whereas the control group exhibited an increase in anxiety symptoms toward the end of the 6-month period (Table 4).
Table 4
Changes in depression and anxiety — relation with treatment outcomes
Baseline depressive symptoms, as measured by the BDI, did not influence short-term detoxification outcomes including completion of detoxification (t = –1.36, p = 0.18) or abstinence during the first 7 days (t = 1.47, p = 0.15). Similarly, baseline anxiety did not predict completion of detoxification (t = –0.85, p = 0.40) or abstinence during the first 7 days (t = 0.27, p = 0.79).
There were no differences in baseline BDI scores or demographic variables between those subjects who attended all follow-up assessments and those who did not. BDI scores at a particular assessment did not predict attendance at the subsequent assessment. Those with higher anxiety scores at 2 months were less likely to attend for follow-up at 3 months (t = 2.52, p = 0.017) and 6 months (t = 2.05, p = 0.049). Anxiety at other time points had no relation with attendance.
There were no observed relations between changes in depression or anxiety scores and changes in heroin-using days or expenditure on heroin use over corresponding time periods.
There were significant relations between BDI scores and thoughts about heroin when measured at the same time point, where higher levels of depression correlated with more frequent thoughts about heroin. These relations were observed at 1-month (Spearman r = 0.51, p = 0.006), 3-month (Spearman r = 0.46, p = 0.003) and 6-month (Spearman r = 0.50, p = 0.003) assessments. Higher levels of depression also correlated with a greater desire to use heroin at 1-month (Spearman r = 0.59, p = 0.002), 3-month (Spearman r = 0.48, p = 0.002) and 6-month (Spearman r = 0.37, p = 0.034) assessments. Relations were observed between anxiety symptoms and thoughts of using heroin at 1-month (Spearman r = 0.56, p = 0.003) and 3-month (Spearman r = 0.38, p = 0.020) assessments but not at 6 months (Spearman r = 0.30, p = 0.09). Similarly, higher anxiety symptoms correlated with a greater desire to use heroin at 1-month (Spearman r = 0.63, p = 0.001) and 3-month (Spearman r = 0.37, p = 0.021) assessments but not at 6 months (Spearman r = 0.13, p = 0.46).
Improvements in BDI between 0 and 6 months correlated with improvements in social functioning over the same period as measured by the OTI (Pearson r = 0.38, p = 0.039). Changes in anxiety were unrelated to changes in social functioning (Pearson r = 0.13, p = 0.51).
Discussion
These findings describe the presence of depressive symptoms during naltrexone treatment for opiate dependence in the context of a randomized controlled trial. In contrast to our initial hypothesis, naltrexone treatment did not lead to an increase in depressive symptoms. Indeed, when we examined the subgroup of participants who were fully engaged in treatment, there was a trend for depressive symptoms to be lower while on naltrexone compared with continued methadone maintenance. In addition, participants who were adherent to naltrexone treatment had fewer depressive symptoms than those not adherent to naltrexone treatment.
These findings are in contrast to previous experimental studies that report dysphoria or depression occurring after naltrexone use.16–18 One possible explanation for this discrepancy is that the improvement in depressive symptoms observed in fully engaged or adherent subjects may reflect the attrition of those who experienced dysphoria or depressive symptoms after initiation of naltrexone. Although this possibility cannot be ruled out, changes in depression scores failed to predict attendance or compliance at subsequent assessments. Furthermore, we believe that fewer depressive symptoms in those who were adherent to naltrexone treatment indicates that depressive symptoms are not a general consequence of naltrexone exposure. The relation between depressive symptoms and poor adherence to naltrexone may be bidirectional: improving either depressive symptoms or adherence may assist in the improvement of the other.
Our findings are consistent with more recent data that report small improvements in mood associated with naltrexone treatment.19,30,31 Rea and colleagues30 compared standard doses of naltrexone (50 mg daily) with low (0.5 mg) and ultra-low (0.05 mg) doses. They report a modest reduction in depressive symptoms in those subjects attending follow-up assessments, regardless of what dose they were receiving. A large naturalistic study of alcohol dependence reported that only 1.4% (7/500) of patients receiving naltrexone reported treatment-emergent depression compared with 1.7% in a reference group not receiving naltrexone.32 A recent review of clinical studies33 concluded that naltrexone treatment does not produce dysphoria as a serious side effect.
It is difficult to engage heroin users in naltrexone treatment, and adherence rates are typically poor. As such, these current findings may be generalized only to a small proportion of the broader population receiving naltrexone for opiate dependence. In addition, it is important to note that extensive clinical research demonstrates that methadone maintenance produces superior outcomes to detoxification. Rapid detoxification is not routinely available in Australia; transferring patients stabilized on methadone maintenance to rapid detoxification or naltrexone treatment is not standard practice. Nonetheless, many patients feel that methadone maintenance is restrictive and desire more flexible treatment options. Within this context, naltrexone may be an appropriate treatment option for some patient groups.
The complexity of the relation between opiate-dependence treatment and mood state is indicated by the different trajectories for depression and anxiety. Anxiety showed an increase over time; those receiving naltrexone tended to experience their increase soon after commencement of treatment, whereas those on methadone reported their increase later. Anxiety symptoms may have been related to detoxification and naltrexone, given that those in the methadone condition were offered detoxification at the end of their 6-month follow-up, and those receiving naltrexone may have been anxious early in the treatment period, because they had started a new therapy. This is consistent with previous studies reporting high levels of anxiety both before and after detoxification.34 The relation between anxiety symptoms and lack of attendance at subsequent assessments indicates that these changes may be clinically significant. The importance of anxiety symptoms in predicting opioid-dependence treatment outcomes has not been fully established and is a subject for future research.
The timing of assessment may have influenced the observation of depressive or anxiety symptoms and may be one factor contributing to contradictory reports of depressive symptoms associated with naltrexone. Previous studies that have linked naltrexone with depression typically assessed symptoms of depression within 24 hours of exposure to a single naltrexone dose16,17 or in the early stages (weeks 1 or 2) of ongoing treatment,17,18 in contrast to this study, which did not assess depression between baseline and 1 month. Although this is a limitation of the current study, assessment of affective state soon after detoxification is difficult, because negative affective states and affective lability are frequently reported during opioid withdrawal.35–38 Even if mood was assessed at earlier stages, it would have been difficult to establish whether the observed changes were attributable to naltrexone, opioid detoxification or nonspecific factors. Nonetheless, it would be prudent to monitor depression and anxiety symptoms in patients receiving naltrexone at regular stages throughout treatment.
Another factor that may have influenced the results relates to the standard of care provided in each group. For example, methadone doses used in the control group are low (mean < 50 mg daily), and lower than doses reported to generate the full benefits of methadone treatment.26 Many participants anecdotally reported reducing their methadone dose before study enrolment, based on the expectation that this would make detoxification less challenging. However, this may have influenced results, because depressive symptoms have been associated with low methadone doses.39 Similarly, engagement in psychotherapy may have affected depressive symptoms. No specific psychotherapy was provided by this study or routinely provided by the clinics involved; engagement in psychotherapy remained the choice of the individual. A limitation of this study is that we were unable to fully determine involvement in psychotherapy and its impact on treatment outcome.
Depression scores were associated with social functioning but not measures of heroin use. This is consistent with other findings that suggest psychiatric symptoms may have a greater impact on psychosocial outcomes than substance use.40 This connection and those observed between depression, anxiety and craving may indicate that negative mood states may act as a marker for patients requiring additional support or psychological treatment. Similar associations have been reported during naltrexone treatment for alcohol use.41 It is likely that the relation between mood and drug craving is bidirectional: negative mood states may induce drug craving,42 and exposure to drug-related stimuli can increase both craving and depressed mood.43
How do these findings influence clinical decision-making regarding treatment selection for opioid dependence? It has been well established that initiation of agonist treatments is associated with improvements in mood.44,45 The capacity for antagonist treatments to elicit a similar improvement in psychological symptoms has not been convincingly demonstrated. Nonetheless, in contrast to expectations, switching from methadone maintenance to an antagonist-based treatment was not accompanied by any discernible increase in depression and may improve mood in patients fully engaged or adherent to treatment. Rounsaville and colleagues44 suggest that the volume of treatment is more important in predicting changes in mood than the particular treatment per se. The minor impact of naltrexone treatment in improving mood may be secondary to poorer rates of treatment engagement or adherence typically reported for antagonist treatments. Within this context, depressive symptoms need not be considered a contraindication for naltrexone treatment. Whatever treatment is used, it remains important to monitor individuals who are receiving opioid-dependence treatments for depressive and anxiety symptoms on a regular basis.46 The challenge for future research will be to determine which individuals are at greater risk of poorer mood outcomes and to identify effective interventions for this group.
Acknowledgments
This work was supported financially by Queensland Health. We thank numerous staff of the Alcohol and Drug Services of the Royal Brisbane Hospital, the Prince Charles Hospital Health Service Districts, Greenslopes Private Hospital and the Department of Psychiatry, University of Queensland, who assisted in many important stages of the project.
Footnotes
Contributors: Drs. Dean, Saunders, Young and Lawford and Mr. Jones conceived and designed the study. Drs. Dean, Saunders, Young, Connor and Lawford acquired the data; Drs. Dean, Saunders, Young and Connor and Mr. Jones analyzed the data. Drs. Dean, Saunders, Young and Connor and Mr. Jones drafted the article; Drs. Dean, Saunders, Young, Connor and Lawford critically revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Angela J. Dean, Kids in Mind Research, Mater Centre for Service Research in Mental Health, Mater Child and Youth Mental Health Service, Raymond Terrace, South Brisbane QLD 4001, Australia; fax 61 7 3840 1644; Angela.Dean@mater.org.au
References
- 1.Volavka J, Resnick R, Kestenbaum R, et al. Short-term effects of naltrexone in 155 heroin ex-addicts. Biol Psychiatry 1976;11:679-85. [PubMed]
- 2.Sideroff S, Charuvastra V, Jarvik M. Craving in heroin addicts maintained on the opiate antagonist naltrexone. Am J Drug Alcohol Abuse 1978;5:415-23. [DOI] [PubMed]
- 3.Judson B, Carney T, Goldstein A. Naltrexone treatment of heroin addiction: efficacy and safety in a double-blind dosage comparison. Drug Alcohol Depend 1981;7:325-46. [DOI] [PubMed]
- 4.Farren CK, O'Malley S. A pilot double blind placebo controlled trial of sertraline with naltrexone in the treatment of opiate dependence. Am J Addict 2002;11:228-34. [DOI] [PubMed]
- 5.Vining E, Kosten TR, Kleber HD. Clinical utility of rapid clonidine-naltrexone detoxification for opioid abusers. Br J Addict 1988;83:567-75. [DOI] [PubMed]
- 6.Bartter T, Gooberman L. Rapid opiate detoxification. Am J Drug Alcohol Abuse 1996;22:489-95. [DOI] [PubMed]
- 7.Brewer C. Ultra-rapid, antagonist-precipitated opiate detoxification under general anaethesia or sedation. Addict Biol 1997;2/3:291-302. [DOI] [PubMed]
- 8.O'Connor PG, Kosten TR. Rapid and ultrarapid opioid detoxification techniques. JAMA 1998;279:229-34. [DOI] [PubMed]
- 9.Tretter F, Burkhardt D, Busello-Spieth B, et al. Clinical experience with antagonist-induced opiate withdrawal under anaesthesia. Addiction 1998;93:269-75. [DOI] [PubMed]
- 10.Shufman EN, Porat S, Witztum E, et al. The efficacy of naltrexone in preventing reabuse of heroin after detoxifcation. Biol Psychiatry 1994;35:935-45. [DOI] [PubMed]
- 11.Kirchmayer U, Davoli M, Verster A. Naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2003;(2):CD001333. [DOI] [PubMed]
- 12.Tucker TK, Ritter AJ. Naltrexone in the treatment of heroin dependence: a literature review. Drug Alcohol Rev 2000;19:73-82.
- 13.D'Ippoliti D, Davoli M, Perucci CA, et al. Retention in treatment of heroin users in Italy: the role of treatment type and of methadone maintenance dosage. Drug Alcohol Depend 1998;52:167-71. [DOI] [PubMed]
- 14.San L, Pomarol G, Peri JM, et al. Follow-up after a six-month maintenance period on naltrexone versus placebo in heroin addicts. Br J Addict 1991;86:983-90. [DOI] [PubMed]
- 15.Tucker T, Ritter A, Maher C, et al. Naltrexone maintenance for heroin dependence: uptake, attrition and retention. Drug Alcohol Rev 2004;23:299-309. [DOI] [PubMed]
- 16.Mendelson JH, Ellingboe J, Keuhnle JC, et al. Effects of naltrexone on mood and neuroendocrine function in normal adult males. Psychoneuroendocrinology 1978;3:231-6. [DOI] [PubMed]
- 17.Hollister LE, Johnson K, Boukhabza D, et al. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend 1981;8:37-41. [DOI] [PubMed]
- 18.Crowley T, Wagner J, Zerbe G, et al. Naltrexone-induced dysphoria in former opioid addicts. Am J Psychiatry 1985;142:1081-4. [DOI] [PubMed]
- 19.Miotto K, McCann M, Rawson R, et al. Overdose, suicide attempts and death among a cohort of naltrexone-treated opioid addicts. Drug Alcohol Depend 1997;45:131-4. [DOI] [PubMed]
- 20.Latt N, Jurd S, Houseman J, et al. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust 2002;176:530-4. [DOI] [PubMed]
- 21.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71. [DOI] [PubMed]
- 22.Steer RA, Beck AT, Garrison B. Applications of the Beck Depression Inventory. In: Sartorius N, Bal. TA, editors. Assessment of depression. Berlin: Springer-Verlag; 1986. p. 123-42.
- 23.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory (Form Y). Palo Alto (CA): Consulting Psychologist Press Inc.; 1983.
- 24.Darke S, Hall W, Wodak A, et al. Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. Br J Addict 1992;87:733-42. [DOI] [PubMed]
- 25.Hutchinson SJ, Taylor A, Gruer L, et al. One-year follow-up of opiate injectors treated with oral methadone in a GP-centred programme. Addiction 2000;95:1055-68. [DOI] [PubMed]
- 26.Caplehorn JR, Bell J. Methadone dosage and retention of patients in maintenance treatment. Med J Aust 1991;154:195-9. [PubMed]
- 27.Rabinowitz J, Cohen H, Tarrasch R, et al. Compliance to naltrexone treatment after ultra-rapid opiate detoxification: an open label naturalistic study. Drug Alcohol Depend 1997;47:77-86. [DOI] [PubMed]
- 28.Saunders JB, Jones R, Dean A, et al. Comparison of rapid opiate detoxification and naltrexone with methadone maintenance in the treatment of opiate dependence: a randomized controlled trial. Drug Alcohol Depend 2002;66(Suppl 1):S156.
- 29.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry 1974;7:151-69. [DOI] [PubMed]
- 30.Rea F, Bell JR, Young MR, et al. A randomised, controlled trial of low dose naltrexone for the treatment of opioid dependence. Drug Alcohol Depend 2004;75:79-88. [DOI] [PubMed]
- 31.Krupitsky EM, Zvartau EE, Masalov DV, et al. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat 2004;26:285-94. [DOI] [PubMed]
- 32.Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism. Results from a multicenter usage study. The Naltrexone Usage Study Group. Arch Gen Psychiatry 1997;54:1130-5. [DOI] [PubMed]
- 33.Miotto K, McCann M, Basch J, et al. Naltrexone and dysphoria: Fact or myth? Am J Addict 2002;11:151-60. [DOI] [PubMed]
- 34.Powell JE, Taylor D. Anger, depression, and anxiety following heroin withdrawal. Int J Addict 1992;27:25-35. [DOI] [PubMed]
- 35.Handelsman L, Aronson MJ, Ness R, et al. The dysphoria of heroin addiction. Am J Drug Alcohol Abuse 1992;18:275-87. [DOI] [PubMed]
- 36.Kanof PD, Aronson MJ, Ness R. Organic mood syndrome associated with detoxification from methadone maintenance. Am J Psychiatry 1993;150:423-8. [DOI] [PubMed]
- 37.Ness R, Handelsman L, Aronson MJ, et al. The acute effects of a rapid medical detoxification upon dysphoria and other psychopathology experienced by heroin abusers. J Nerv Ment Dis 1994;182:353-9. [DOI] [PubMed]
- 38.Elman I, D'Ambra MN, Krause S, et al. Ultrarapid opioid detoxification: effects on cardiopulmonary physiology, stress hormones and clinical outcomes. Drug Alcohol Depend 2001;61:163-72. [DOI] [PubMed]
- 39.Dean AJ, Bell J, Mascord DJ, et al. A randomised, controlled trial of fluoxetine in methadone maintenance patients with depressive symptoms. J Affect Disord 2002;72:85-90. [DOI] [PubMed]
- 40.Cacciola JS, Alterman AI, Rutherford MJ, et al. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend 2001;61:271-80. [DOI] [PubMed]
- 41.Farren CK, O'Malley S. Occurence and management of depression in the context of naltrexone treatment of alcoholism. Am J Psychiatry 1999;156:1258-62. [DOI] [PubMed]
- 42.Childress AR, Ehrman R, McLellan AT, et al. Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treat 1994;11:17-23. [DOI] [PubMed]
- 43.Sideroff SI, Jarvik ME. Conditioned responses to a videotape showing heroin-related stimuli. Int J Addict 1980;15:529-36. [DOI] [PubMed]
- 44.Rounsaville BJ, Weissman MM, Crits-Christoph K, et al. Diagnosis and symptoms of depression in opiate addicts. Course and relationship to treatment outcome. Arch Gen Psychiatry 1982;39:151-6. [DOI] [PubMed]
- 45.Dean AJ, Bell J, Christie MJ, et al. Depressive symptoms during buprenorphine vs. methadone maintenance: findings from a randomised, controlled trial in opioid dependence. Eur Psychiatry 2004;19:510-3. [DOI] [PubMed]
- 46.Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry 2004;56:793-802. [DOI] [PubMed]


